Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chiwei Lan | -- | 3394 | 2023-02-02 07:33:25 | | | |
| 2 | Camila Xu | Meta information modification | 3394 | 2023-02-02 07:53:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ng, H.S.; Wan, P.; Kondo, A.; Chang, J.; Lan, J.C. Production and Recovery of Ectoine. Encyclopedia. Available online: https://encyclopedia.pub/entry/40754 (accessed on 08 February 2026).
Ng HS, Wan P, Kondo A, Chang J, Lan JC. Production and Recovery of Ectoine. Encyclopedia. Available at: https://encyclopedia.pub/entry/40754. Accessed February 08, 2026.
Ng, Hui Suan, Pik-Ki Wan, Akihiko Kondo, Jo-Shu Chang, John Chi-Wei Lan. "Production and Recovery of Ectoine" Encyclopedia, https://encyclopedia.pub/entry/40754 (accessed February 08, 2026).
Ng, H.S., Wan, P., Kondo, A., Chang, J., & Lan, J.C. (2023, February 02). Production and Recovery of Ectoine. In Encyclopedia. https://encyclopedia.pub/entry/40754
Ng, Hui Suan, et al. "Production and Recovery of Ectoine." Encyclopedia. Web. 02 February, 2023.
Copy Citation
Ectoine (CAS No.: 96702-03-3), chemically known as 1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid, is a heterocyclic amino acid derivative of aspartate with a zwitterionic structure. Ectoine was first identified and isolated from a halophilic bacterium, Ectothiorhodospira halochloris.
aqueous biphasic system
ectoine
fermentation
microbial
1. Introduction
Ectoine (CAS No.: 96702-03-3), chemically known as 1,4,5,6-tetrahydro-2-methyl-4-pyrimidinecarboxylic acid, is a heterocyclic amino acid derivative of aspartate with a zwitterionic structure. Ectoine was first identified and isolated from a halophilic bacterium, Ectothiorhodospira halochloris [1]. In later years, ectoine was discovered in several naturally occurring aerobic chemoheterotrophic bacteria [2]. Ectoine’s biosynthetic gene was cloned and functionally expressed for the first time in Escherichia coli by Louis and Galinski in 1997 and later by He et al. (2006) with the advancement of genetic engineering, which has marked a great breakthrough in the sustainable production of ectoine [3][4].Ectoine is synthesized by halophilic bacteria via the amino acid biosynthesis of the aspartate family with the enzymatic reactions of three main enzymes: l-2, 4-diaminobutyrate transaminase (ectB), l-2, 4-diaminobutyrate acetyltransferase (ectA), and ectoine synthase (ectC) [5][6]. Several halophilic bacteria inclusive of Chromohalobacter salexigens and Halomonas elongata tend to synthesize hydroxyectoine via a hydroxylation reaction in the presence of the enzyme ectoine hydroxylase (ectD) [7]. A high-salt environment often triggers the expression of these ectoine-synthesizing enzymes, resulting in the synthesis of high concentrations of ectoine.
Ectoine, with its strong stabilizing capability, often acts as a stress and thermal protectant for halophilic bacteria such as C. salexigens and Virgibacillus pantothenticus against extreme freezing, drying, and heating conditions [8]. Ectoine provides osmotic balance to microbial cells without disrupting the cellular metabolic processes of the microorganisms. The zwitterionic ectoine exerts a negligible effect on the ionic strength of the cytoplasm of the microorganism; therefore, there is no imperative modification on the intracellular enzymes and organelles [9][10]. The concentration of the ectoine accumulated intracellularly is often dependent upon the surrounding environmental osmolarity [10]. For instance, a hypo-osmotic environment induces the synthesis of ectoine through the unique efflux system which regulates the internal osmolarity of microorganisms by increasing the intracellular solutes pool and vice versa [10].
Ectoine is among the most extensively used compatible solutes for various commercial applications due to the intensely powerful stabilizing capability shown [11]. Ectoine is an excellent stabilizer for intracellular macromolecules enzymes, nucleic acids, and various proteins [10][12]. Ectoine alters the solvent properties by preferentially excluding the solutes from the enzyme surface, resulting in a more compact conformation of the enzyme [13]. In addition, the accumulation of ectoine also enhances the cellular hydration and the turgor maintenance under unfavorable conditions. In the context of stabilization of the nucleic acid structure, ectoine acts to trigger the structural change of the nucleic acids which can restrict the cleavage reactions by most of the endonucleases [10].
2. Extracellular Ectoine Production
The recovery of extracellular ectoine from the fermentation medium is relatively simple when compared to the recovery of intracellular ectoine from microbial cells. The production cost of industrial-scale extracellular ectoine production can be reduced with minimal usage of high salt concentrations and elimination of additional steps to extract intracellular ectoine from microbial cells [14]. However, there are finite ectoine-excreting bacterial strains known to date, and extracellular ectoine is usually produced in small quantities in fermentation broth which often demands subsequent downstream processes to concentrate and purify the ectoine produced [15].
Halomonas salina DSM 5928 is an innate ectoine-excreting strain which can synthesize ectoine extracellularly with a low salt concentration of 0.5 M NaCl [16]. The presence of glucose and yeast extract in the fermentation medium adversely affected the production yield of the extracellular ectoine. A negative correlation between the yield of the extracellular ectoine and NaCl concentration in the fermentation medium was observed, which is in contrast to most of the bacterial strains that synthesized ectoine intracellularly [16]. A combined batch-fermentation approach utilizing both growing and resting cells of H. salina DSM 5928T was developed for enhanced extracellular ectoine production (11.75 g/L) [16]. The growing cells were cultured in the optimal fermentation medium containing monosodium glutamate as the primary carbon and nitrogen source. Upon reaching the late exponential growth phase, the cells were fed with phosphate-limited medium for optimum ectoine production.
Advances of Integrated Approaches for Ectoine Fermentation
Following the advances in fermentation technology, several integrated approaches on ectoine fermentation were introduced in order to enhance the production yields of ectoine. The advances in metabolic engineering also enhance the production of ectoine in microbial fermentation [17][18]. For instance, Van-Thuoc et al., 2010, have successfully integrated the two-step fed-batch fermentation and bacterial milking of H. boliviensis LC1T cells which resulted in enhanced yields of ectoine in the second fed-batch fermentation after a high cell density was recorded in the first fed-batch H. boliviensis LC1T cell cultivation [19]. Ectoine was produced in the fermentation broth with a rate of 9.1 g/L per day in this research. However, the overall ectoine fermentation process was dependent upon the selection of the salt medium because a relatively high salt concentration of 2.55 M NaCl was demanded for the cultivation of bacterial cells [19].
A cascade of two continuously operating bioreactors for intracellular ectoine production from the moderately halophilic C. salexigens was constructed later in 2010 by Fallet et al. The first bioreactor was designed for accumulation of a high cell density of C. salexigens with an extreme pH and salt concentration, whereas, a second coupled bioreactor was engineered for the synthesis of ectoine through the osmotic and thermal downshock methods [20]. The overall ectoine yield recovered was improved by the high cell density of 61 g/L using a cross-flow ultrafiltration. A total of 540 mg ectoine/g of dry cell weight was recorded, which has outperformed the ectoine yield demonstrated by the ectoine biofactory developed by Sauer and Galinski, 1998 (155 mg ectoine/g of dry cell weight).
The yield of ectoine can be further enhanced by uncoupling the high osmolarity from the ectoine biosynthesis pathway of microorganisms via the metabolic engineering approach. Heterologous producers of ectoine have been developed by incorporating the ectoine-synthesis gene into a non-halophilic microorganism. Ectoine can be synthesized and secreted extracellularly by the heterologous ectoine producers while maintaining their biological properties when they are exposed to a new abiotic stress-resistance environment [10]. The ectoine-synthesis gene cluster sourced from a variety of halophiles such as Marinococcus halophilus and C. salexigens has been transferred to Escherichia coli in most of the metabolic engineering studies [21].
In 2007, Schubert et al. successfully transferred the ectABC gene cassette from C. salexigens to E. coli DH5α host cells using the pASK-IBA7 vector. The resulting recombinant E. coli produced extracellular ectoine in low-salinity broth, which alternately simplified the overall ectoine recovery process during industrial-scale production [22]. Becker et al., 2013, later successfully developed a recombinant Gram-positive Corynebacterium glutamicum strain by integrating the ectABCD gene cluster sourced from halophilic Pseudomonas stutzeri A1501 into the genome of C. glutamicum. In contrast to the existing halophilic extracellular ectoine-producing microbial strains, the resulting recombinant C. glutamicum excreted ectoine into the fermentation medium irrespective of the high salt concentration in the medium [23].
With the recent advances in metabolic engineering, over-expression of the ectoine gene cluster ectABCD, which is sourced from H. elongata cells, was initiated in E. coli K12 BW25113 [24]. The recombinant E. coli produced a remarkably high yield of ectoine in a fermenter, as compared to the shake-flask cultures. Repetitive cycles of ectoine fermentation using whole-cell biocatalysts were feasible by using this transgenic E. coli strain in view of the high consistency of ectoine yields recorded at the second cycle (84%) and third cycles (69%) when compared to the first ectoine-production cycle [23].
Tanimura et al., 2013, further investigated the effects of different types of sugars as carbon sources on engineered H. elongata strains for enhanced ectoine production. H. elongata mutants could serve as potential hosts for ectoine synthesis with biomass-derived lignocellulose and rice straw hydrolysate as carbon sources [24]. Inedible lignocellulose is widely available worldwide; the utilization of the lignocellulose-derived sugars as carbon sources replacing glucose in the fermentation media has facilitated a biomass-oriented sustainable society [19]. In addition, the study revealed that the yield of ectoine could be enhanced by applying rice straw hydrolysate, xylose, cellobiose, and a mixture of glucose and xylose as the carbon source. The deletion of the ectD gene from the H. elongata mutant further promoted ectoine production [24].
Following their success in 2013, Tanimura et al., 2016, further improved the production of ectoine by over-expressing the sugar transporters in the H. elongata mutant. The sugar-consumption rate of the H. elongata cells was reduced during the ectoine-producing stage to maintain osmotic balance. The majority of the sugars was consumed during the bacterial growth phase after ectoine production and a higher rate of ectoine production was recorded in the ectoine-producing phase [25]. Ectoine productivity was enhanced with the facilitation of the sugar transport from the medium into the cells.
In the latest literature, a potential synthetic cell factory for ectoine production by transferring the ectoine-synthesis gene cluster, ectABC, from halophilic H. elongata to a non-halophilic E. coli host was established [26]. The performance of E. coli in ectoine production was further improved by the deletion of the thrA gene encoding bifunctional aspartokinase (Ask) to inhibit the competitive l-threonine branch along the ectoine biosynthetic pathway. To complement the enzyme deficiency due to thrA deletion, feedback resistance of lysC sourced from Corynebacterium glutamicium was incorporated to synthesize Ask [26]. The final engineered E. coli strain could produce a total of 25.1 g/L of ectoine with a productivity of 0.84 g/L per hour through fed-batch fermentation with low salinity. However, further research on improving the metabolic network and fermentation conditions is required, because only 17% of the predicted maximum ectoine yield was obtained in this research [26].
3. Recovery and Downstream Processes of Ectoine
The ectoine fermentation process is often coupled with the production of other bioproducts such as PHB and hydroxyectoine [27]. Moreover, ectoine is preferably synthesized in the intracellular compartment of microorganisms, which further complicates the downstream processes of ectoine production. Additional multi-step separation, concentration, and clarification downstream processes are required to obtain ectoine with high yield and purity. The current state of and biotechnological advances in the recovery and downstream processes of ectoine are discussed in the following sections.
3.1. Coupled Cation-Exchange Resin and Crystallization
Sauer and Galinski, 1998, first attempted the purification of ectoine using a two-step purification strategy which consisted of a coupled cation-exchange chromatography and crystallization. Ectoine and hydroxyectoine were successfully recovered in their cationic states at a pH of 1.4 using this purification strategy. Ectoine was purified via a cation-exchange resin (Na+ form). Due to differences in ionic charges, the contaminants were removed, while the ectoine was bound to the cation-exchange resin and recovered with the addition of sodium hydroxide (NaOH). Further separation of ectoine and hydroxyectoine were achieved using methanol evaporation, followed by a water crystallization step for the recovery of ectoine [28].
3.2. Ethanol Crystallization
The recovery and purification of intracellular ectoine from the fermentation broth of Brevibacterium epidermis was extended to a two-step hypo-osmotic extraction of ectoine using water and ethanol, respectively. However, further downstream processing was required to remove the impurities and contaminants such as the remaining NaCl and glutamate to obtain a high purity of ectoine. The crude ectoine was then concentrated using evaporation with a reduced pressure until a brownish precipitate was obtained. Ethanol was later used to re-crystallize the recovered ectoine [29].
Ethanol crystallization was applied as the initial purification step to recover ectoine. Wei et al., 2011, presented an ectoine recovery extraction method by mixing ethanol, chloroform, and water at a ratio of 1:1:1 (v/v/v) to eliminate most of the chloroform-soluble and lipid-soluble materials from the ectoine-containing bacterial cultures. The Marinococcus sp. ECT1 cells were harvested and centrifuged prior to the addition of the solvent at the above-mentioned ratio to the cell pellets. The mixture was then centrifuged again, and the pellet was subjected to oven drying. Ethanol was used to re-dissolve the dried pellet, and ectoine was purified using activated carbon to absorb the ectoine. The ethanolic ectoine extract was then crystallized by drying at 100 °C to obtain the purified ectoine [30]. The use of large amounts of organic solvents such as ethanol and chloroform are hazardous to the environment in industrial-scale ectoine production.
3.3. Ultrafiltration with Hollow Fibers, Ethanol Precipitation, and Ion-Exchange Chromatography
Multiple combined purification steps inclusive of hollow-fiber ultrafiltration, ethanol precipitation, and ion-exchange chromatography have been applied in the purification of extracellular ectoine from H. salina DSM 5928T fermentation broth [16]. Hollow-fiber ultrafiltration was performed to remove the bacterial cells and contaminant proteins present in the broth. The resultant mixture was then concentrated by depression vaporization. Ethanol precipitation was used to remove impurities such as monosodium glutamate and NaCl. Next, the supernatant was concentrated and crystallized again with depression vaporization. Ectoine was purified with ion-exchange chromatography using highly acidic hydrochloric acid. Notably, the rates of recovery (≥86%) and purity of ectoine (97.6%) obtained after a series of multiple steps of purification and recovery were quite promising [16]. However, this purification strategy can be further improved to reduce the time-consuming steps and overall production cost of ectoine.
The yield and purity of ectoine, overall production costs, and time consumption are often the major concerns for the industrial-scale production of ectoine. Ectoine production equipment is vulnerable to corrosion due to the high-salinity ectoine fermentation media and low yields of ectoine resulting from prolonged fermentation processes are among the factors contributing to the increase in the overall production cost of ectoine. Heterologous ectoine producers can overcome the drawbacks mentioned above; however, the biological activities and morphology of the host bacteria are not well-conserved in this context [14]. Furthermore, there is an urgent need to develop a novel and cost-effective alternative for industrial-scale ectoine production, with limited enhanced integrated approaches for the production and recovery of ectoine being reported to date. The aqueous biphasic system (ABS) can serve as a potential tool to integrate the recovery, concentration, clarification, and purification of ectoine into a single-step operation. In subsequent sections, the potential of using the ABS as an integrated approach to recover ectoine with high yields is discussed.
3.4. Applications of Aqueous Biphasic System in Recovery of Ectoine from Microbial Fermentation
The aqueous biphasic system (ABS) is a well-established separation method that is widely applied in the separation and purification of various biomaterials, including low-molecular-weight bioactive compounds [31], proteins [32], metabolites [33], antibodies [34], and cell organelles. The versatile applications of the ABS provide a promising alternative for the production and recovery of ectoine from microbial sources.
The ABS is an ideal liquid–liquid extraction technology that efficiently purifies and separates labile biomaterials by partitioning the target bioproducts between two distinct aqueous phases, based on their physiochemical interaction with the phase-forming components [35]. The basis of the separation of the target bioproduct in the ABS is associated with the types, hydrophobicity, concentration, and molecular weight of the phase-forming components, as well as the surface properties, sizes, shapes, charges, and specific binding sites of the target bioproduct [36]. Therefore, different ABS parameters such as pH, temperature, and concentration of the phase-forming components are often the main factors in determining the partition behavior of the target bioproduct in an ABS. The exclusive partition of a target bioproduct in an ABS can be enhanced by the addition of certain adjuvants such as neutral salt (e.g., NaCl) [37] and ionic liquids [38]. These additives can alter the resultant physiochemical interactions between the target bioproduct and the phase-forming components, and, therefore, enhance the exclusive partition of the target bioproduct to one specific phase of the ABS. An ABS with a high-water content (70–80%) provides a mild and biocompatible environment for the recovery of biomaterials and the growth of microorganisms. A green ABS is often coupled with non-toxic, biodegradable, and non-flammable phase-forming components [39]. Furthermore, the low interfacial surface tension between the two phases facilitates the efficient mass transfer of molecules in the ABS with a minimal loss of bioactivity.
Ectoine is a zwitterionic molecule which is anticipated to exhibit different partition behavior in different types of ABS owing to the different physiochemical properties featured by different types of ABS. Bacterial milking of ectoine is often associated with high salinity, which will result in corrosion. An ABS is regarded as a promising strategy for the downstream processing of ectoine, which can prevent the use of high salt concentrations in the fermentation process and the direct recovery of ectoine by immediate removal of ectoine via extractive fermentation. Moreover, the practical implementation of an ABS for large-scale industrial application is easy and simple to operate, with great potential for continuous and automated operation with minimal overall production costs and energy inputs [40].
The immediate removal of ectoine from the fermentation broth or cell mixture upon osmotic downshock is often demanded because the ectoine will be depleted over time to supplement the microbial cell growth [37]. The recent study by Wan et al. (2018) demonstrated the one-step recovery of intracellular ectoine from an H. salina DSM 5928T cell mixture using a poly(propylene) glycol (PPG)/sulphate ABS. Crude intracellular ectoine was extracted from the fermentation broth of H. salina DSM 5928T after the osmotic downshock. The ABS was later applied to recover the ectoine from the H. salina DSM5 928T cell mixture by optimizing the concentrations of the phase-forming components, amount of loaded crude, pH of the system, and the concentration of neutral salts added into the ABS. A successful extractive ABS purification of ectoine was established in which ectoine was recovered in the salt-rich bottom phase of the PPG/sulphate ABS by exclusive partition with a partition coefficient (KE) of 150.21 ± 6.04. A total ectoine yield of 94.7% with a final purity of 87.03% and enrichment factor of 1.7 were obtained in this research, demonstrating the feasibility of the ABS to separate and recover ectoine from the H. salina cell mixture in a one-step operation [37]. In a later study, ectoine was recovered using an ionic liquids-based ABS, which further enhanced the yields and purity of the ectoine recovered with green solvent [41].
The ABS can integrate the upstream and downstream processes of ectoine into a one-step unit operation and thereby reduces the product’s inhibitory effects and the cost of the overall processes [42]. Extractive ABS fermentation is exceptionally efficient for the industrial manufacturing of ectoine without any additional investment for sophisticated equipment setup. This strategy is well suited for the recovery of extracellular ectoine sourced from the microbial fermentation of ectoine-excreting bacterial strains.
The proposed extractive ABS fermentation for ectoine is first inoculated with the desired microbial culture broth into the phase system at the optimum partition conditions. Extractive fermentation of extracellular ectoine using the ABS is achieved when the microbial cells and other impurities are partitioned to one phase, whereas ectoine was accumulated in another phase after the phase separation (Figure 1). Continuous cultivation of microbial cells and sequestration of extracellular ectoine can be attained with this integrated continuous operation by retaining the microbial cells in one phase and harvesting the ectoine from the cell-free phase. Preliminary studies on the stability of the microbial cell growth in the designed ABS are important for the development of the extractive ABS fermentation of the ectoine recovery, despite the ABS parameters for enhanced yield of ectoine.
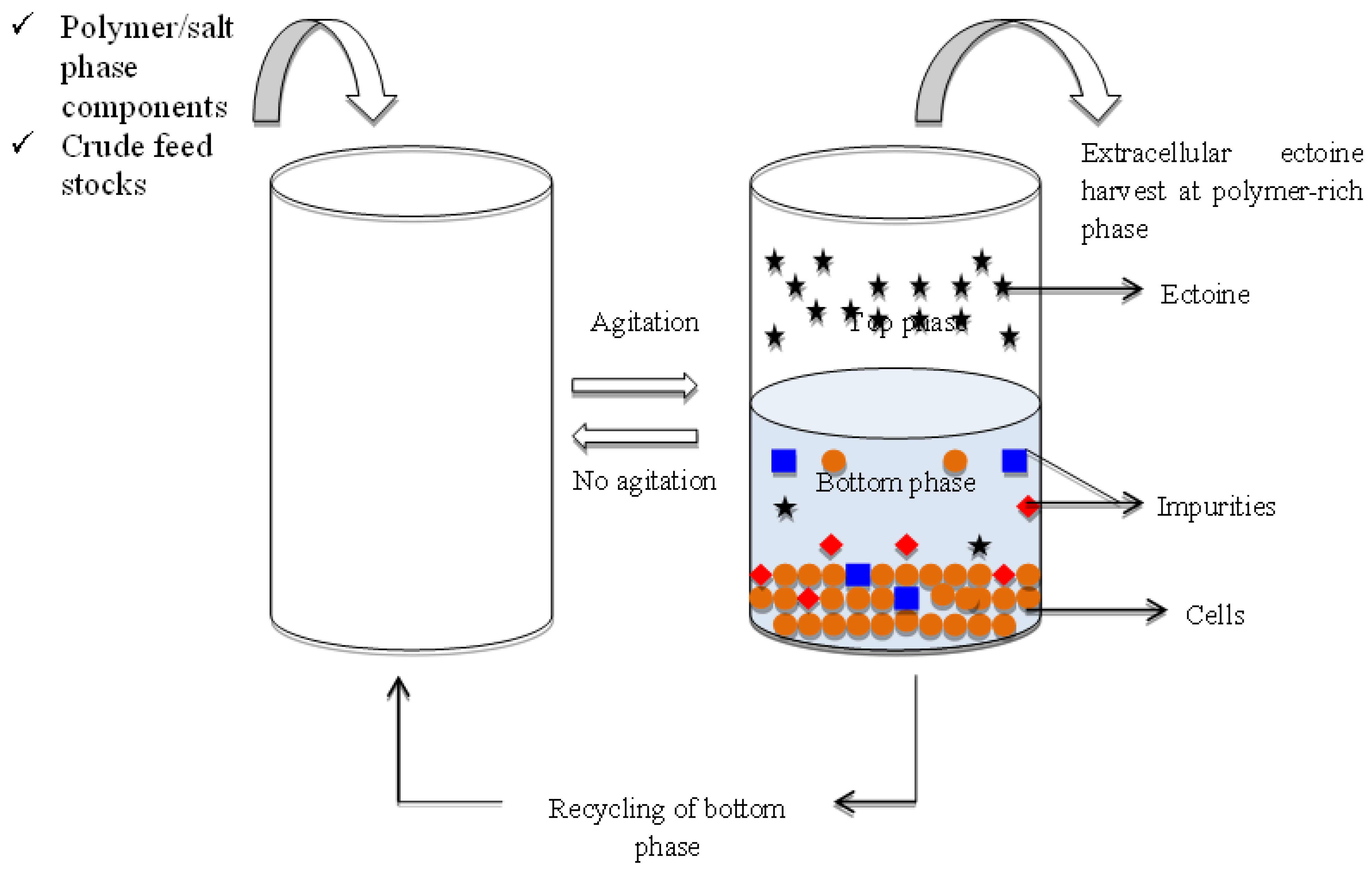
Figure 1. Schematic diagram on the proposed extractive fermentation of microbial ectoine with ABS. A process scheme was illustrated for continuous repetitive batch of extractive ABS fermentation for recovery of ectoine. The microbial cells is represented by “🟠”, the ectoine is denoted by “🟊” and the impurities are denoted with “▄” and “◆”.
In addition, simultaneous microbial cell disruption and ectoine recovery can be achieved by integrating the ABS into the extractive disruption of intracellular ectoine. The extractive ABS disruption is feasible by incorporating the ultrasonication, homogenization, or chemical cell disruption into the ABS recovery of the intracellular ectoine. Since most of the microorganisms tend to synthesize ectoine intracellularly, extractive ABS disruption is regarded as a promising recovery strategy to integrate the cell disruption and ectoine recovery into a one-unit operation with enhanced yield and great cost efficiency. The schematic representation of the continuous, repetitive-batch extractive disruption of the intracellular ectoine-producing strain using the ABS is illustrated in Figure 2.
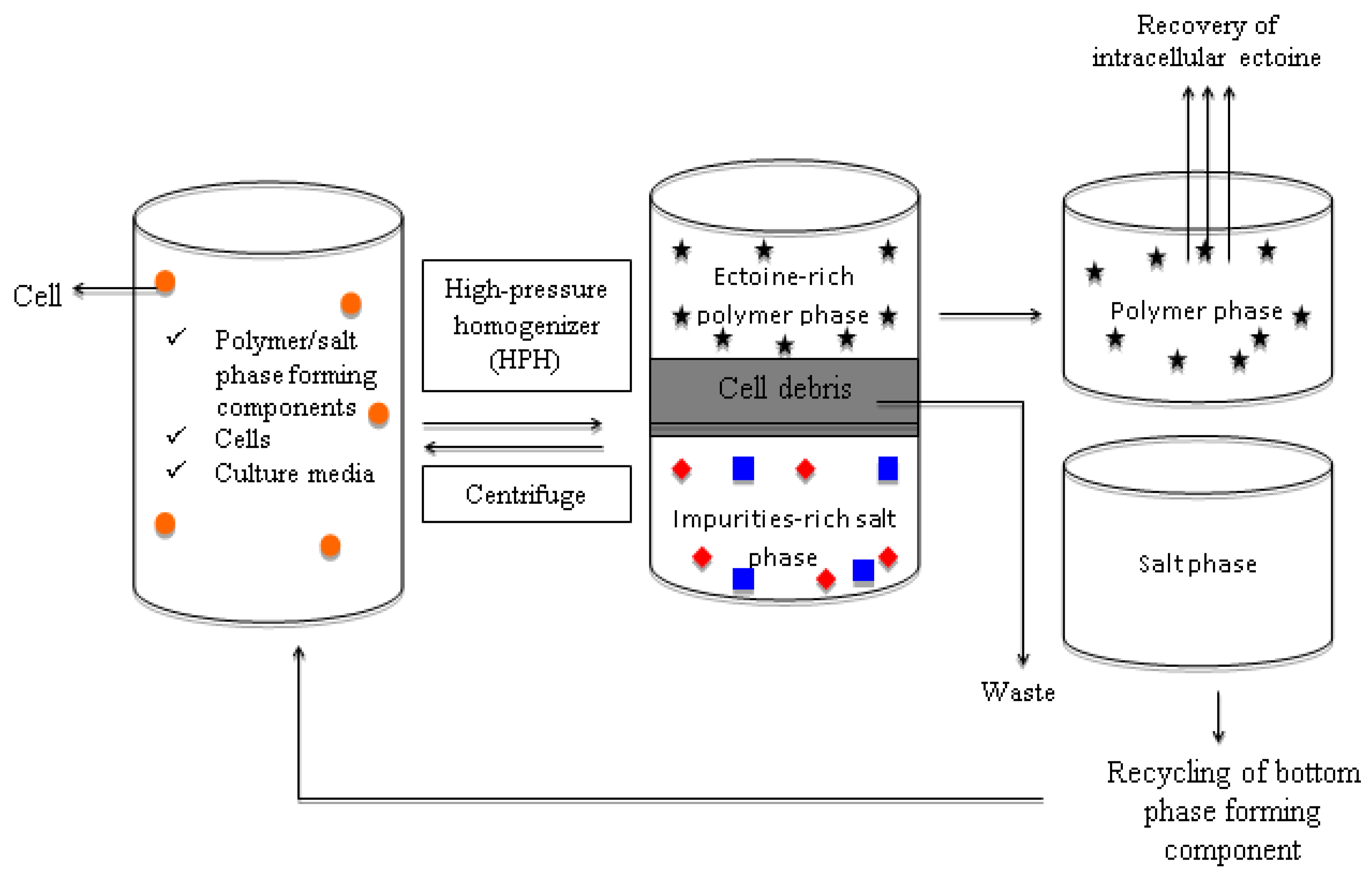
Figure 2. Schematic diagram on the proposed extractive disruption of microbial ectoine with ABS. A process scheme was illustrated for continuous repetitive batch of extractive ABS disruption for recovery of ectoine. The microbial cells is represented by “🟠”, the ectoine is denoted by “🟊” and the impurities are denoted with “▄” and “◆”.
In the proposed ABS (Figure 2), the disrupted bacterial cells are retained in the salt-rich bottom phase of the polymer/salt ABS and the extracted ectoine was partitioned to the top phase of the ABS. However, further studies and validations are required in developing an ideal ABS for the direct recovery of ectoine via this extractive disruption approach.
References
- Galinski, E.A.; Pfeiffer, H.P.; Trüper, H.G. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid: A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur. J. Biochem. 1985, 149, 135–139.
- Galinski, E.A. Osmoadaptation in bacteria. Adv. Microb. Physiol. 1995, 37, 273–328.
- Louis, P.; Galinski, E.A. Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 1997, 143, 1141–1149.
- He, J.; Huang, X.; Gu, L.; Jiang, J.; Li, S. Cloning of the ectoine biosynthesis gene ectABC from Halomonas sp. BYS-1 and salt stressed expression in Escherichia coli. Wei Sheng Wu Xue Bao Acta Microbiol. Sin. 2006, 46, 28–32.
- Becker, J.; Schafer, R.; Kohlstedt, M.; Harder, B.J.; Borchert, N.S.; Stoveken, N.; Bremer, E.; Wittmann, C. Systems metabolic engineering of Corynebacterium glutamicum for production of the chemical chaperone ectoine. Microb. Cell Factories 2013, 12, 110.
- Bestvater, T.; Louis, P.; Galinski, E.A. Heterologous ectoine production in Escherichia coli: By-passing the metabolic bottle-neck. Saline Syst. 2008, 4, 12.
- Bursy, J.; Pierik, A.J.; Pica, N.; Bremer, E. Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J. Biol. Chem. 2007, 282, 31147–31155.
- Shivanand, P.; Mugeraya, G. Halophilic bacteria and their compatible solutes–osmoregulation and potential applications. Curr. Sci. 2011, 100, 1516–1521.
- Oren, A. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 1999, 63, 334–348.
- Pastor, J.M.; Salvador, M.; Argandoña, M.; Bernal, V.; Reina-Bueno, M.; Csonka, L.N.; Iborra, J.L.; Vargas, C.; Nieto, J.J.; Cánovas, M.J. Ectoines in cell stress protection: Uses and biotechnological production. Biotechnol. Adv. 2010, 28, 782–801.
- Lippert, K.; Galinski, E.A. Enzyme stabilization be ectoine-type compatible solutes: Protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 1992, 37, 61–65.
- Kolp, S.; Pietsch, M.; Galinski, E.A.; Gütschow, M.J. Compatible solutes as protectants for zymogens against proteolysis. Biochim. Biophys. Acta BBA Proteins Proteom. 2006, 1764, 1234–1242.
- Göller, K.; Galinski, E.A. Protection of a model enzyme (lactate dehydrogenase) against heat, urea and freeze-thaw treatment by compatible solute additives. J. Mol. Catal. B Enzym. 1999, 7, 37–45.
- Zhang, L.H.; Lang, Y.J.; Nagata, S. Efficient production of ectoine using ectoine-excreting strain. Extrem. Life Under Extrem. Cond. 2009, 13, 717–724.
- Yao, C.-L.; Lin, Y.-M.; Mohamed, M.S.; Chen, J.-H. Inhibitory effect of ectoine on melanogenesis in B16-F0 and A2058 melanoma cell lines. Biochem. Eng. J. 2013, 78, 163–169.
- Lang, Y.J.; Bai, L.; Ren, Y.N.; Zhang, L.H.; Nagata, S. Production of ectoine through a combined process that uses both growing and resting cells of Halomonas salina DSM 5928T. Extrem. Life Under Extrem. Cond. 2011, 15, 303–310.
- Jiang, A.; Song, Y.; You, J.; Zhang, X.; Xu, M.; Rao, Z. High-yield ectoine production in engineered Corynebacterium glutamicum by fine metabolic regulation via plug-in repressor library. Bioresour. Technol. 2022, 362, 127802.
- Wang, D.; Chen, J.; Wang, Y.; Du, G.; Kang, Z. Engineering Escherichia coli for high-yield production of ectoine. Green Chem. Eng. 2021, in press.
- Van-Thuoc, D.; Guzmán, H.; Quillaguamán, J.; Hatti-Kaul, R. High productivity of ectoines by Halomonas boliviensis using a combined two-step fed-batch culture and milking process. J. Biotechnol. 2010, 147, 46–51.
- Fallet, C.; Rohe, P.; Franco-Lara, E. Process optimization of the integrated synthesis and secretion of ectoine and hydroxyectoine under hyper/hypo-osmotic stress. Biotechnol. Bioeng. 2010, 107, 124–133.
- Anbu Rajan, L.; Joseph, T.C.; Thampuran, N.; James, R.; Ashok Kumar, K.; Viswanathan, C.; Bansal, K.C. Cloning and heterologous expression of ectoine biosynthesis genes from Bacillus halodurans in Escherichia coli. Biotechnol. Lett. 2008, 30, 1403–1407.
- Schubert, T.; Maskow, T.; Benndorf, D.; Harms, H.; Breuer, U. Continuous synthesis and excretion of the compatible solute ectoine by a transgenic, nonhalophilic bacterium. Appl. Environ. Microbiol. 2007, 73, 3343–3347.
- He, Y.-Z.; Gong, J.; Yu, H.-Y.; Tao, Y.; Zhang, S.; Dong, Z.-Y. High production of ectoine from aspartate and glycerol by use of whole-cell biocatalysis in recombinant Escherichia coli. Microb. Cell Factories 2015, 14, 55.
- Tanimura, K.; Nakayama, H.; Tanaka, T.; Kondo, A. Ectoine production from lignocellulosic biomass-derived sugars by engineered Halomonas elongata. Bioresour. Technol. 2013, 142, 523–529.
- Tanimura, K.; Matsumoto, T.; Nakayama, H.; Tanaka, T.; Kondo, A. Improvement of ectoine productivity by using sugar transporter-overexpressing Halomonas elongata. Enzym. Microb. Technol. 2016, 89, 63–68.
- Ning, Y.; Wu, X.; Zhang, C.; Xu, Q.; Chen, N.; Xie, X. Pathway construction and metabolic engineering for fermentative production of ectoine in Escherichia coli. Metab. Eng. 2016, 36, 10–18.
- Guzman, H.; Van-Thuoc, D.; Martin, J.; Hatti-Kaul, R.; Quillaguaman, J. A process for the production of ectoine and poly(3-hydroxybutyrate) by Halomonas boliviensis. Appl. Microbiol. Biotechnol. 2009, 84, 1069–1077.
- Sauer, T.; Galinski, E.A. Bacterial milking: A novel bioprocess for production of compatible solutes. Biotechnol. Bioeng. 1998, 57, 306–313.
- Onraedt, A.E.; Walcarius, B.A.; Soetaert, W.K.; Vandamme, E.J. Optimization of ectoine synthesis through fed-batch fermentation of Brevibacterium epidermis. Biotechnol. Prog. 2005, 21, 1206–1212.
- Wei, Y.H.; Yuan, F.W.; Chen, W.C.; Chen, S.Y. Production and characterization of ectoine by Marinococcus sp. ECT1 isolated from a high-salinity environment. J. Biosci. Bioeng. 2011, 111, 336–342.
- Simental-Martínez, J.; Montalvo-Hernández, B.; Rito-Palomares, M.; Benavides, J. Application of Aqueous Two-Phase Systems for the Recovery of Bioactive Low-Molecular Weight Compounds. Sep. Sci. Technol. 2014, 49, 1872–1882.
- Ng, H.S.; Wang, C.-C.; Tan, J.S.; Lan, J.C.-W. Primary recovery of recombinant human serum albumin from transgenic Oryza sativa with a single-step aqueous biphasic system. J. Taiwan Inst. Chem. Eng. 2018, 84, 60–66.
- Gu, B.H.; Zheng, P.; Yan, Q.; Liu, W. Aqueous two-phase system: An alternative process for recovery of succinic acid from fermentation broth. Sep. Purif. Technol. 2014, 138, 47–54.
- Campos-Pinto, I.; Espitia-Saloma, E.; Rosa, S.A.S.L.; Rito-Palomares, M.; Aguilar, O.; Arévalo-Rodríguez, M.; Aires-Barros, M.R.; Azevedo, A.M. Integration of cell harvest with affinity-enhanced purification of monoclonal antibodies using aqueous two-phase systems with a dual tag ligand. Sep. Purif. Technol. 2017, 173, 129–134.
- Tan, Z.; Li, F.; Xu, X. Isolation and purification of aloe anthraquinones based on an ionic liquid/salt aqueous two-phase system. Sep. Purif. Technol. 2012, 98, 150–157.
- Asenjo, J.A.; Andrews, B.A. Aqueous two-phase systems for protein separation: A perspective. J. Chromatogr. A 2011, 1218, 8826–8835.
- Wan, P.K.; Lan, J.C.-W.; Chen, P.-W.; Tan, J.S.; Ng, H.S. Recovery of intracellular ectoine from Halomonas salina cells with poly(propylene) glycol/salt aqueous biphasic system. J. Taiwan Inst. Chem. Eng. 2018, 82, 28–32.
- Aziz, N.F.H.A.; Abbasiliasi, S.; Ng, H.S.; Phapugrangkul, P.; Bakar, M.H.A.; Tam, Y.J.; Tan, J.S. Purification of β-mannanase derived from Bacillus subtilis ATCC 11774 using ionic liquid as adjuvant in aqueous two-phase system. J. Chromatogr. B 2017, 1055, 104–112.
- Ruiz, C.S.; van den Berg, C.; Wijffels, R.H.; Eppink, M.H.M. Rubisco separation using biocompatible aqueous two-phase systems. Sep. Purif. Technol. 2018, 196, 254–261.
- Ng, H.S.; Ooi, C.W.; Mokhtar, M.N.; Show, P.L.; Ariff, A.; Tan, J.S.; Ng, E.-P.; Ling, T.C. Extractive bioconversion of cyclodextrins by Bacillus cereus cyclodextrin glycosyltransferase in aqueous two-phase system. Bioresour. Technol. 2013, 142, 723–726.
- Ng, H.S.; Wan, P.K.; Ng, T.-C.; Lan, J.C.-W. Primary purification of intracellular Halomonas salina ectoine using ionic liquids-based aqueous biphasic system. J. Biosci. Bioeng. 2020, 130, 200–204.
- Ooi, C.W.; Hii, S.L.; Kamal, S.M.M.; Ariff, A.; Ling, T.C. Extractive fermentation using aqueous two-phase systems for integrated production and purification of extracellular lipase derived from Burkholderia pseudomallei. Process Biochem. 2011, 46, 68–73.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
02 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




