Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food Protein-Derived Antioxidant Peptides. Encyclopedia. Available online: https://encyclopedia.pub/entry/40753 (accessed on 08 February 2026).
Zhu Y, Lao F, Pan X, Wu J. Food Protein-Derived Antioxidant Peptides. Encyclopedia. Available at: https://encyclopedia.pub/entry/40753. Accessed February 08, 2026.
Zhu, Yongsheng, Fei Lao, Xin Pan, Jihong Wu. "Food Protein-Derived Antioxidant Peptides" Encyclopedia, https://encyclopedia.pub/entry/40753 (accessed February 08, 2026).
Zhu, Y., Lao, F., Pan, X., & Wu, J. (2023, February 02). Food Protein-Derived Antioxidant Peptides. In Encyclopedia. https://encyclopedia.pub/entry/40753
Zhu, Yongsheng, et al. "Food Protein-Derived Antioxidant Peptides." Encyclopedia. Web. 02 February, 2023.
Copy Citation
The antioxidant activity of protein-derived peptides was one of the first to be revealed among the more than 50 known peptide bioactivities to date. The exploitation value associated with food-derived antioxidant peptides is mainly attributed to their natural properties and effectiveness as food preservatives and in disease prevention, management, and treatment. An increasing number of antioxidant active peptides have been identified from a variety of renewable sources, including terrestrial and aquatic organisms and their processing by-products. This has important implications for alleviating population pressure, avoiding environmental problems, and promoting a sustainable shift in consumption.
bioactive peptides
food sources
antioxidant activity
molecular mechanism
1. Introduction
Rapid and uncontrolled urbanization, the globalization of unhealthy lifestyles, and environmental and climatic degradation resulting from human development activities have contributed to the high prevalence of non-communicable chronic diseases (NCDs) worldwide. According to statistics, premature deaths due to NCDs exceed 41 million per year, equivalent to 71% of total global deaths [1]. There is growing evidence that oxidative stress caused by the disturbance of redox homeostasis in living organisms is involved in the pathogenesis and development of many chronic diseases, such as cancer, atherosclerosis, and diabetes [2][3]. Reactive oxygen species (ROS) are a class of free radical species produced mainly by the mitochondrial respiratory chain and are involved in oxidative stress signalling in normal cells. However, if the accumulation of ROS exceeds the capacity of the cellular free radical scavenging system, these reactive species trigger uncontrolled reactions with non-target biomolecules (lipids, proteins, and DNA) and cells, and mediate the subsequent activation of pro-inflammatory or pro-apoptotic pathways. This situation requires additional supplementation to regulate the balance of antioxidants and oxidants in biological tissues. Since the beginning of this century, the World Health Organization has been calling for an increase in the consumption of dietary sources of antioxidants worldwide, as food is a natural and sustainable source of these compounds [4]. The application of antioxidant active peptides in the prevention and management of oxidative damage and related pathologies in the body has been extensively studied over the past decades. As of June 2022, 772 peptide sequences with biological antioxidant functions were registered in the BIOPEP database, second only to angiotensin-converting enzyme-inhibiting peptides, reflecting their great commercial exploitation value. The sources of these active peptides cover a wide range of human edible biological resources on earth, including animals, plants, and algae. They can be produced from low economic-value catches or crops, or various edible or non-edible by-products of food processing, even involving some refined products. Most biofunctional peptides are produced mainly through enzymatic hydrolysis of proteins, either in vivo during gastrointestinal digestion, controlled degradation using appropriate exogenous proteases, or during specific food processing (e.g., ham and milk fermentation) [5] (Figure 1). Traditionally, the characterization of peptides follows a standardized procedure, which simply includes the selection of the original protein, enzymatic hydrolysis, isolation, purification, and identification, and after the last step, information on the activity, amino acid sequence, structure and corresponding functional properties of the candidate peptide can be largely determined [6]. However, this approach is expensive and time-consuming, and more importantly, it does not meet the requirements of industrial scale-up production. In recent years, the establishment of emerging bioinformatics analysis systems (in silico) has provided a new possibility for the study of biopeptides including antioxidant peptides. In addition, besides their potential as therapeutic agents, the research value of antioxidant peptides is also reflected in their applications as food additives, nutritional fortification in health foods, and anti-aging and photoprotective components in cosmetics [7][8]. Numerous experiments have shown that the addition of food-based antioxidant protein hydrolysates or peptides as antioxidants can effectively inhibit lipid peroxidation during food transportation and storage, thus maintaining the stability of food flavour and nutritional quality (vitamins and essential unsaturated fatty acids) [9][10]. Therefore, the development of natural antioxidant peptides from food or other readily available sources as alternative food preservatives may help to alleviate consumer concerns about the potential toxicity risks associated with the widespread use of synthetic antioxidants in current food formulations.

Figure 1. The main food-based sources of antioxidant active peptides.
2. Digestive Stability and Bioavailability of Antioxidant Peptides
As an ideal alternative to synthetic antioxidants at this stage, the stability and accessibility of functional protein hydrolysates or peptide derivatives in the complex and demanding digestive environment of the human body are undoubtedly decisive aspects in the biological validation of known and novel food-derived antioxidant peptides. However, the reality is that bioactive peptides, including antioxidant peptides, are still far from clinical application due to the lack of mature delivery and bioavailability support and the fact that the necessary biological analysis is still mostly at the in vitro cellular level. Like drug molecules and other functional components, in addition to their direct physiological effects in the intestinal wall, which effectively induce antioxidant defense mechanisms in the body, peptide molecules as therapeutic agents or health-promoting supplements must enter the portal circulation in their active form and exert systemic or local antioxidant effects.
To achieve this expectation, the bioactive peptides after oral administration need to be subjected to modification or degradation triggered by proteolytic enzymes in the gastrointestinal (GI) tract, while the peptide activity and function are also subjected to the possible impact of the toxic environment in the GI lumen such as potentially damaging secretions (including bile salts, acids and other digestive enzymes such as elastase) and various food-derived oxidants and toxins [11]. Peptides that survive gastrointestinal digestion or their released fragments must also overcome further hydrolysis by brush border peptidases and/or cell membrane peptidases of the intestinal membrane epithelium before they can be absorbed into the internal circulation by intestinal wall cells; there are four main mechanisms for the trans-cortical flux of peptides in this process as shown in Figure 2, including active transport by H+-coupled PepT1 and PepT2 transporters, Na+-coupled oligopeptide transport systems SOPT1 and SOPT2, passive bypass diffusion by intercellular tight junctions (TJs), and trans-cellular action in the form of endocytosis, depending on molecular size and structural properties such as hydrophobicity of peptides [12][13][14]. Finally, these cell-penetrating peptides also need to escape the breakdown of vascular endothelial tissue peptidases and soluble plasma peptidases, as well as the first-pass effect in the liver [15]. In fact, due to peptidase activity, most exogenous peptides have low stability and fast clearance in plasma [7]. In conclusion, in the face of the metabolic activity of peptidases in the gastrointestinal tract and plasma and the low permeability of the intestinal barrier, many therapeutic peptides have difficulty in maintaining their full activity or reaching the target site in very low amounts (1~2%) and are less likely to elicit a pharmacological response outside the GI tract [16]. Considering the strict ethical regulations of animal studies and the high cost and resource intensity of human trials, the evaluation, and integration of information on the digestive permeation behavior of bioactive molecules based on in vitro digestion and intestinal absorption models may provide valuable guidance for their in vivo effects and the subsequent development of co-delivery and bioavailability strategies.
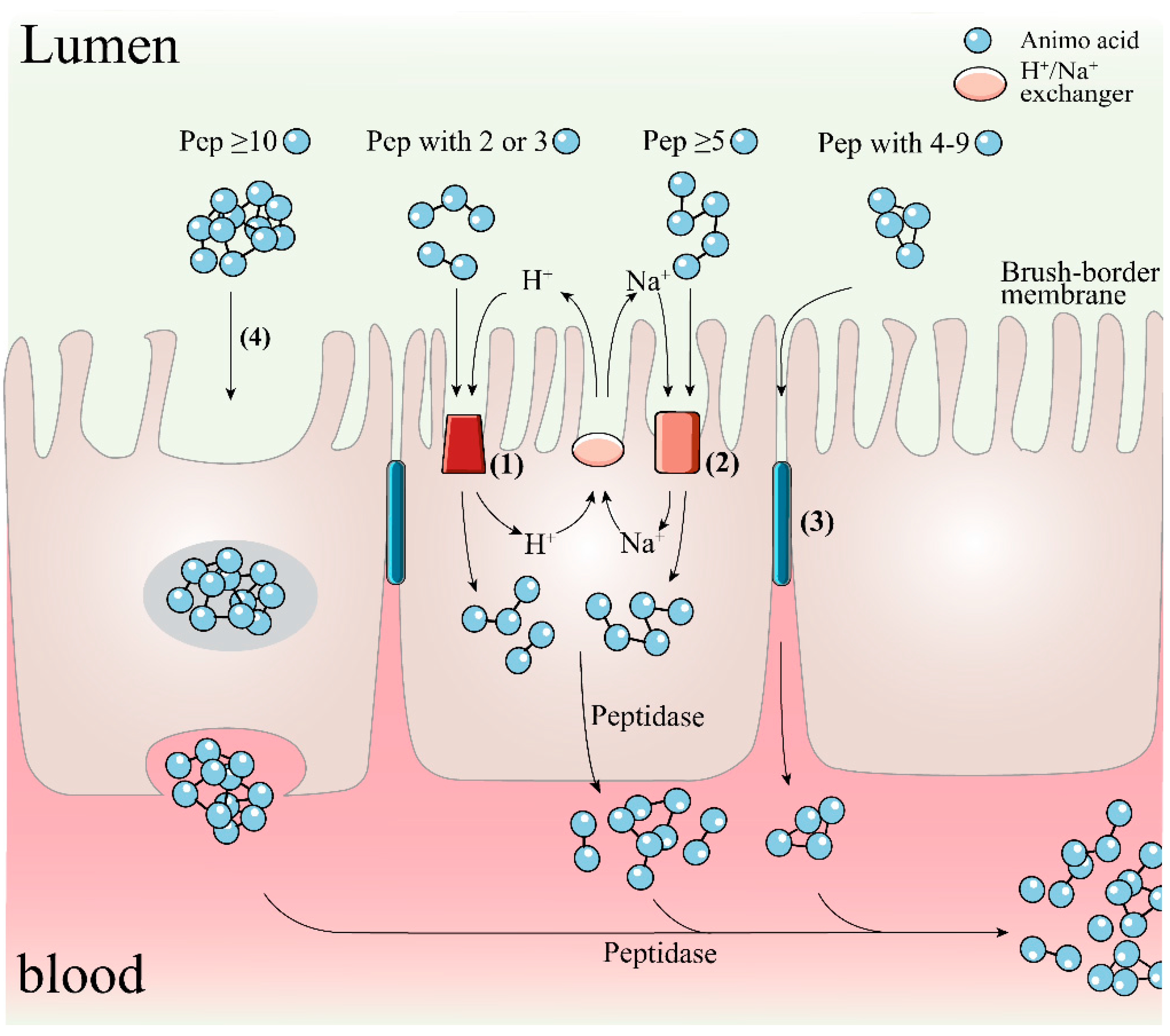
Figure 2. Underlying mechanisms of transcortical transport of peptides with diverse sizes. (1) H+-coupled PepT1 and 2; (2) Na+-coupled SOPT1 and 2; (3) paracellular; (4) transcytosis. Pep, peptide.
To exert their biological activity, hydrolysates or peptides must be evaluated for digestibility and subsequent release of bioactive peptides in relevant in vitro intestinal models and the in vivo GI tract lumen. In vitro methods using cultures such as monolayers of human intestinal Caco-2 epithelial cells and in vivo models to determine permeability can aid in the prediction of oral bioavailability. The selective permeability of the intestinal barrier to candidate peptides is based on an understanding of the structural and chemical properties of the active compounds, the interactions in the gastrointestinal tract, and knowledge of the physiology of the GI tract [17]. As previously mentioned, it has long been known that hydrolysates with many short peptides, especially dipeptides and tripeptides, can lead to their better absorption and are more efficient than free amino acids and larger precursor peptide molecules [18]. If the MW of the molecule increases above 500 Da, the oral bioavailability decreases dramatically [15]. For example, the bioavailability of fractions of casein-derived peptides less than 500 Da was 16.23%, compared to 9.54% for fractions in the molecular weight range of 500–1000 Da [19]. Also of interest is that the length of the peptide chain provides a clue as to whether a transdermal transporter is involved. Specifically, dipeptides and tripeptides have been described as substrates for PepT1 binding and transport, which is a peptide transporter with a transmembrane electrochemical protein gradient located on the apical membrane of enterocytes [20]. In contrast, the TJs-mediated paracellular pathway is responsible for the translocation of oligopeptides containing four to nine amino acid residues [21][22]. This conclusion is corroborated by the work of the group of Xu et al. [23]. In an assay evaluating the uptake mechanism and bioavailability of rapeseed protein-extracted peptide WDHHAPQLR (RAP) in Caco-2 cell monolayers, they found that RAP is degraded by brush border peptidases, and then longer fragments of RAP, DHHAPQLR and WDHHAP are transported via the paracellular pathway, while tripeptide QLR is taken up via PepT1. In addition, through pharmacokinetic tests, they found that the absolute bioavailability of RAP (100 mg/kg BW) could reach 3.56% in rats after oral gavage, although the effective permeation rate of the basal side of Caco-2 monolayer measured in the preliminary screening test was only 1% at most, which was not sufficient to exert antioxidant effects. These results suggest that RAP may be developed as an efficient radical scavenging peptide. An earlier study by the same group showed that 65.57% of YWDHNNPQIR was resistant to hydrolysis by brush border peptidase and could be absorbed by the intestinal epithelium intact. More importantly, the absorbed peptides could still exhibit favorable antioxidant activity [24]. Wang et al. [25] reported an interesting work to screen and identify antioxidant peptides with digestive stability and bioaccessibility in muscle hydrolysates of silver carp. Two digestion products, i.e., viniferase and papain-induced hydrolysate fractions with molecular weight less than 1 kDa after SGID, showed 9.21% and 11.45% permeability by trans-cortical transport analysis of the Caco-2 monolayer. Then further identified by LC-MS/MS, the fragmented peptide LVPVAVF in the permeate showed the strongest DPPH• cleavage (EC50 0.65 mg/mL) and ROS quenching activity (27.23% at 50 µg/mL). Similarly, Feng and Betti [26] reported that digestion products of bovine collagen hydrolysate could reach up to 21.4% transport efficiency across Caco-2 cell monolayers. These studies support the idea that protein digests screened by in vitro permeability assays to obtain highly permeable fractions have greater potential as natural resistance components in food and drug systems than single or purified peptides. However, further studies focusing on the relationship between intestinal absorption of antioxidant peptides and subsequent in vivo metabolism are needed.
In conclusion, given the properties of antioxidant peptides, such as molecular weight, charge, hydrogen bond potential and hydrophobicity sensitivity to peptidase and intestinal transport, and the correlation between intestinal epithelial transport of peptides and peptidase catalysis, most oligopeptides exhibiting in vitro antioxidant activity rarely maintain their integrity or activity after transmembrane transport and subsequently affect their bioavailability, even though small amounts of target peptides may be detected in plasma. The presence of small amounts of the target peptide may be detected in plasma. As recently novel antioxidant peptides LHSMK [27], YFCLT and GLLLPH [22], WDHHAPQLR [23], GNPDIEHPE, SVIKPPTDE and VIKPPTDE [28] were reported as such. Therefore, to maximize health benefits, future work needs to shift more towards the development of promotion strategies for the stability and bioavailability of bioactive peptides.
3. Application of Antioxidant Peptides in Food Systems
Thanks to the natural properties and generally high biological activity of antioxidant protein hydrolysates or peptides, the use of these high value-added products as functional and nutritional fortification ingredients in specialty formulations is an area of increasing interest. Currently, the application of these active compounds is focused on four main areas: (1) as matrix enhancers, preservatives, or nutritional supplements in food systems; (2) as therapeutic agents to be incorporated into pharmaceutical systems; (3) as feed additives to improve animal immunity; and (4) development of anti-aging and photoprotective pharmaceuticals. Unlike living organisms, the quality features of food products suffer from irreversible decay from the date of production, which can be postponed from a few hours to months or even years if appropriate strategies are adopted. In this case, it turned out to be a common trend for the food industry to prioritize the use of synthetic antioxidants such as BHA (butylated hydroxyanisole), BHT (dibutylated hydroxytoluene), and TBHQ (tert-butylated hydroquinone) to promote food stability and extend shelf life [29]. However, given the potential health hazards of synthetic antioxidants, the current consumer trend is a dramatic increase in demand for ‘clean label’ foods and functional foods enriched with natural active ingredients. This trend has reinforced the demand of the food industry and researchers to obtain and apply food additives of natural origin that also exert bioactivity to prevent the development of NCDs. In this respect, antioxidant peptides have acted, at the laboratory level, as potential food additives. Few studies have evaluated the effects of antioxidant peptides in real food matrices, which could support their potential use as additives (Table 1). Meat products are a more studied food system as they are susceptible to lipid oxidation and require exogenous antioxidants to scavenge the active substances. For example, Shen et al. [30] reported that the addition of silver carp protein hydrolysate (2 and 4%, w/w) to surimi attenuated the formation of myofibrillar protein carbonyls, inhibited the reduction of free sulfhydryl content, and slowed down the formation of peroxidized lipid MDA and the rate of change of flavor compounds. Similarly, Lin et al. [31] proved that the incorporation of bighead carp gills hydrolysate (1 and 2%, w/w) treated with neutral protease to surimi increased the concentration of sulfhydryl and salt-soluble proteins, enhanced Ca2+-ATPase activity, reduced disulfide bonds, carbonyls, and hydrophobicity, and improved gel strength and texture. Nowadays, rarely commercial foods containing antioxidant active peptides exist on the market, despite the considerable literature on them. The possible reason is the lack of sufficient evidence for the biological effectiveness, processing and matrix stability, and toxicological safety of most antioxidant peptides. The stability of peptides during food processing and storage is critical for their application as functional ingredients, as peptides are vulnerable to chemical modifications of the backbone or side chains. These chemical reactions involve disulfide bond formation, dehydration, glycation, and aromatic ring oxidation, inducing changes in the structure and bioactivity of peptides [32][33]. The interaction between peptides and food matrix components such as proteins, lipids, and polysaccharides during food processing and storage could trigger a number of physicochemical reactions, such as hydrophobic interactions, disulfide interactions, and Maillard reaction, thereby favorably or adversely impacting the biological activity, solubility, sensory profiles, and color and texture parameters of peptide-based functional foods [32][34]. Over the decades, remarkable progress has been made in exploring the role of peptides in textural, sensory, and health aspects. Previous studies have focused on how peptides shape textural and technical functional properties, such as how mackerel gelatine hydrolysate affects flavor, color, emulsion activity and stability, and foaming properties and stability of carbonated beverage [35]. However, understanding how and to what extent peptides affect the functional properties of foods requires a comprehensive consideration of multiple complexities (e.g., peptide amphipathicity, solubility, and gelation capacity, food composition and ingredient distribution, and food processing and storage conditions) for better tailoring the type of hydrolysate in the formulated product to obtain the desired functional properties. Thus, further studies are requested to assess the impact of antioxidant bioactive peptides on the technical properties and consumer acceptance of final products, even though promising outcomes have been recorded in literatures (especially for minced meat and beverages).
Table 1. the use of antioxidant protein hydrolysates (peptides) in food systems.
| Source | Extraction Tool | Hydrolysate/ Peptide |
Product | Effects | Ref. |
|---|---|---|---|---|---|
| Silver carp | Protamex | Silver carp protein hydrolysate (2 and 4%, w/w) |
Surimi | Delayed the formation of MDA and unfavorable flavor volatiles; inhibited the oxidation of free sulfhydryl and the formation of carbonyls in myofibrillar proteins | [30] |
| Bovine | Pepsin | TSKYR (0.1 and 0.5%, w/w) |
Ground beef | 0.5% TSKYR provided similar protection against lipid oxidation as 0.1 and 0.5% BHT | [36] |
| Bighead carp | Flavourzyme Alcalase Neutral protease Papain |
Bighead carp gill protein hydrolysate (1 and 2%, w/w) |
Surimi | Increased sulfhydryl and salt-soluble protein concentrations, enhanced Ca2+-ATPase activity, reduced disulfide bonds, carbonyls and hydrophobicity, and improved gel strength and texture | [31] |
| Barred mackerel | Alcalase and actinidin | Barred mackerel gelatine hydrolysate (<3 kDa) |
Carbonated beverage | No adverse effects on emulsification activity and stability, foam expansion and stability, color, and flavor | [35] |
| Faba bean seed | Pepsin Trypsin Alcalase |
Faba bean hydrolysate (1%, w/v) | Apple juice | No adverse effects on organoleptic acceptability | [9] |
| Capelin | Alcalase Neutrase Papain |
Capelin protein hydrolysate (0.5–3.0%, w/w) | Ground pork | Inhibited 17.7–60.4% of TBARS production; increased cooking yield (up to 4% at 3.0%) | [37] |
| Goby | Grey triggerfish proteases | Goby protein hydrolysate (0.01–0.2%, w/w) | Turkey meat sausage | 0.02% and 0.04% hydrolysate showed higher MDA inhibition capacity than 0.2% vitamin C | [38] |
References
- WHO. Non-Communicable Diseases. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 26 June 2022).
- Chen, M.; Ning, P.; Jiao, Y.; Xu, Z.; Cheng, Y. Extraction of antioxidant peptides from rice dreg protein hydrolysate via an angling method. Food Chem. 2021, 337, 3–8.
- Wang, M.; Sun, X.; Luo, W.; Božović, S.; Gong, C.; Ren, J. Characterization and analysis of antioxidant activity of walnut-derived pentapeptide PW5 via nuclear magnetic resonance spectroscopy. Food Chem. 2021, 339, 128047.
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases. Available online: http://apps.who.int/iris/bitstream/handle/10665/42665/WHO_TRS_916.pdf (accessed on 26 June 2022).
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 3–8.
- Barati, M.; Javanmardi, F.; Mousavi Jazayeri, S.M.H.; Jabbari, M.; Rahmani, J.; Barati, F.; Nickho, H.; Davoodi, S.H.; Roshanravan, N.; Mousavi Khaneghah, A. Techniques, perspectives, and challenges of bioactive peptide generation: A comprehensive systematic review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1488–1520.
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci. Technol. 2019, 86, 399–411.
- Lafarga, T.; Sánchez-Zurano, A.; Villaró, S.; Morillas-España, A.; Acién, G. Industrial production of spirulina as a protein source for bioactive peptide generation. Trends Food Sci. Technol. 2021, 116, 176–185.
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Martini, S.; Gotti, R.; Themelis, T.; Tesini, F.; Gianotti, A.; Gallina Toschi, T.; Babini, E. Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba, L.) seed protein hydrolysates and fortified apple juice. Food Chem. 2020, 330, 127120.
- Al-Shamsi, K.A.; Mudgil, P.; Hassan, H.M.; Maqsood, S. Camel milk protein hydrolysates with improved technofunctional properties and enhanced antioxidant potential in in vitro and in food model systems. J Dairy Sci. 2018, 101, 47–60.
- Ketnawa, S.; Wickramathilaka, M.; Liceaga, A.M. Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food Chem. 2018, 254, 36–46.
- Zhang, X.; He, H.; Xiang, J.; Li, B.; Zhao, M.; Hou, T. Selenium-containing soybean antioxidant peptides: Preparation and comprehensive comparison of different selenium supplements. Food Chem. 2021, 358, 129888.
- Fernández-Tomé, S.; Sanchón, J.; Recio, I.; Hernández-Ledesma, B. Transepithelial transport of lunasin and derived peptides: Inhibitory effects on the gastrointestinal cancer cells viability. J. Food Compost. Anal. 2018, 68, 101–110.
- Zhu, B.; He, H.; Hou, T. A comprehensive review of corn protein-derived bioactive peptides: Production, characterization, bioactivities, and transport pathways. Compr Rev Food Sci Food Saf. 2019, 18, 329–345.
- Abeer, M.M.; Trajkovic, S.; Brayden, D.J. Measuring the oral bioavailability of protein hydrolysates derived from food sources: A critical review of current bioassays. Biomed. Pharmacother. 2021, 144, 112275.
- Antosova, Z.; Mackova, M.; Kral, V.; Macek, T. Therapeutic application of peptides and proteins: Parenteral forever? Trends Biotechnol. 2009, 27, 628–635.
- Vij, R.; Reddi, S.; Kapila, S.; Kapila, R. Transepithelial transport of milk derived bioactive peptide VLPVPQK. Food Chem. 2016, 190, 681–688.
- Singh, U.; Kaur, D.; Mishra, V.; Krishania, M. Combinatorial approach to prepare antioxidative protein hydrolysate from corn gluten meal with dairy whey: Preparation, kinetics, nutritional study and cost analysis. Lebensm. Wiss. Technol. 2022, 153, 112437.
- Wang, B.; Li, B. Effect of molecular weight on the transepithelial transport and peptidase degradation of casein-derived peptides by using Caco-2 cell model. Food Chem. 2017, 218, 1–8.
- Xue, L.; Yin, R.; Howell, K.; Zhang, P. Activity and bioavailability of food protein-derived angiotensin-I-converting enzyme-inhibitory peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1150–1187.
- Vig, B.S.; Stouch, T.R.; Timoszyk, J.K.; Quan, Y.; Wall, D.A.; Smith, R.L.; Faria, T.N. Human PepT1 pharmacophore distinguishes between dipeptide transport and binding. J. Med. Chem. 2006, 49, 3636–3644.
- Ding, L.; Wang, L.; Zhang, T.; Yu, Z.; Liu, J. Hydrolysis and transepithelial transport of two corn gluten derived bioactive peptides in human Caco-2 cell monolayers. Food Res. Int. 2018, 106, 475–480.
- Xu, F.; Zhang, J.; Wang, Z.; Yao, Y.; Atungulu, G.G.; Ju, X.; Wang, L. Absorption and metabolism of peptide WDHHAPQLR derived from rapeseed protein and inhibition of HUVEC apoptosis under oxidative stress. J. Agric. Food Chem. 2018, 66, 5178–5189.
- Xu, F.; Wang, L.; Ju, X.; Zhang, J.; Yin, S.; Shi, J.; He, R.; Yuan, Q. Transepithelial transport of YWDHNNPQIR and its metabolic fate with cytoprotection against oxidative stress in human intestinal Caco-2 cells. J. Agric. Food Chem. 2017, 65, 2056–2065.
- Wang, K.; Han, L.; Hong, H.; Pan, J.; Liu, H.; Luo, Y. Purification and identification of novel antioxidant peptides from silver carp muscle hydrolysate after simulated gastrointestinal digestion and transepithelial transport. Food Chem. 2021, 342, 128275.
- Feng, M.; Betti, M. Transepithelial transport efficiency of bovine collagen hydrolysates in a human Caco-2 cell line model. Food Chem. 2017, 224, 242–250.
- Liu, M.; Zhang, T.; Liang, X.; Yuan, Q.; Zeng, X.; Wu, Z.; Pan, D.; Tao, M.; Guo, Y. Production and transepithelial transportation of casein-derived peptides and identification a novel antioxidant peptide LHSMK. Lebensm. Wiss. Technol. 2021, 151, 112194.
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305.
- Lorenzo, J.M.; Munekata, P.E.S.; Gomez, B.; Barba, F.J.; Mora, L.; Perez-Santaescolastica, C.; Toldra, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147.
- Shen, X.; Li, T.; Li, X.; Wang, F.; Liu, Y.; Wu, J. Dual cryoprotective and antioxidant effects of silver carp (Hypophthalmichthys molitrix) protein hydrolysates on unwashed surimi stored at conventional and ultra-low frozen temperatures. Lebensm. Wiss. Technol. 2022, 153, 112563.
- Lin, J.; Hong, H.; Zhang, L.; Zhang, C.; Luo, Y. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868.
- Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 2011–2027.
- Udenigwe, C.C.; Fogliano, V. Food matrix interaction and bioavailability of bioactive peptides: Two faces of the same coin? J Funct. Foods 2017, 35, 9–12.
- Lacou, L.; Leonil, J.; Gagnaire, V. Functional properties of peptides: From single peptide solutions to a mixture of peptides in food products. Food Hydrocoll. 2016, 57, 187–199.
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Kim, Y.; Eun, J. Antioxidant mechanism, antibacterial activity, and functional characterization of peptide fractions obtained from barred mackerel gelatin with a focus on application in carbonated beverages. Food Chem. 2021, 342, 3–8.
- Przybylski, R.; Firdaous, L.; Châtaigné, G.; Dhulster, P.; Nedjar, N. Production of an antimicrobial peptide derived from slaughterhouse by-product and its potential application on meat as preservative. Food Chem. 2016, 211, 306–313.
- Shahidi, F.; Han, X.; Synowiecki, J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem. 1995, 53, 285–293.
- Nasri, R.; Younes, I.; Jridi, M.; Trigui, M.; Bougatef, A.; Nedjar-Arroume, N.; Dhulster, P.; Nasri, M.; Karra-Châabouni, M. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: Effect on meat lipid oxidation. Food Res. Int. 2013, 54, 552–561.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
02 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




