Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chang, C.; Tang, X.; Woodley, D.T.; Chen, M.; Li, W. Hsp90α and Hsp90β. Encyclopedia. Available online: https://encyclopedia.pub/entry/40735 (accessed on 04 March 2026).
Chang C, Tang X, Woodley DT, Chen M, Li W. Hsp90α and Hsp90β. Encyclopedia. Available at: https://encyclopedia.pub/entry/40735. Accessed March 04, 2026.
Chang, Cheng, Xin Tang, David T. Woodley, Mei Chen, Wei Li. "Hsp90α and Hsp90β" Encyclopedia, https://encyclopedia.pub/entry/40735 (accessed March 04, 2026).
Chang, C., Tang, X., Woodley, D.T., Chen, M., & Li, W. (2023, February 01). Hsp90α and Hsp90β. In Encyclopedia. https://encyclopedia.pub/entry/40735
Chang, Cheng, et al. "Hsp90α and Hsp90β." Encyclopedia. Web. 01 February, 2023.
Copy Citation
Hsp90α and Hsp90β are both ubiquitously expressed in all cell types, but assigned for distinct and irreplaceable functions. Hsp90β is essential during mouse development and Hsp90α only maintains male reproductivity in adult mice. Neither Hsp90β nor Hsp90α could substitute each other under these biological processes. Hsp90β alone maintains cell survival in culture and Hsp90α cannot substitute it. Hsp90α also has extracellular functions under stress and Hsp90β does not.
heat shock protein-90
Hsp90-alpha
Hsp90-beta
1. Cytosolic Hsp90 Isoforms
In vertebrates, two distinct Hsp90 genes encode the cytosolic Hsp90α (HSPCAL4, 14q32.31, MIM# 140571) and Hsp90β (HSPCB, 6p21.1, MIM# 140572), respectively. In addition, two organelle-residing isoforms, Grp94 and TRAP1, also belong to the Hsp90 protein superfamily [1]. As shown in Figure 1A, the human Hsp90α and Hsp90β share approximately 86% amino acid homology and differ at a total of 99 amino acid residues along their full-length 732- (Hsp90α) and 724- (Hsp90β) amino acid sequences. The differences in amino acids include 58 conservative substitutions, 41 non-conservative substitutions and 12 (variable numbers of amino acid) deletions in Hsp90β. The substitutions or deletions are not evenly distributed along the two proteins. It is noticeable that there are 21 amino acid substitutions and three amino acid deletions within the 32 amino acid sequences of the linker region (LR), resulting in a reduced homology between Hsp90α and Hsp90β of 40%. Accordingly, as shown in Figure 1B, the recombinant Hsp90α protein migrates slightly slower than Hsp90β in SDS gel electrophoresis. For unknown reasons, the recovery of recombinant Hsp90β from cultured bacteria, even using various improved strains, could only reach 10–20% of recombinant Hsp90α protein production [2]. In the absence of extracellular stress, the steady-state levels of Hsp90α and Hsp90β appear to be similar in cultured cell lines [3]. However, their levels of expression vary dramatically in different mouse organs [4][5][6]. As shown in Figure 1C, while the expression of Hsp90β remains relatively constant among different mouse organs (Note: it is known that beta-actin is uncharacteristically lower in heart tissue), the variations in Hsp90α expression, lowest in the heart and highest in the testis, have as much as a 20-fold difference. While the overall ATPase activity (note, ATP hydrolysis, instead of ATP/ADP exchange) of the purified Hsp90 proteins is uncharacteristically lower than other ATPases under similar testing conditions, there has been no report on the relative ATPase activity between Hsp90α and Hsp90β. The ATPase activity of purified Hsp90α and Hsp90β proteins may nevertheless prove biologically irrelevant, since the ATPase activity of Hsp90s has been shown to be also regulated by interaction with specific co-chaperones. Moreover, the ATP-dependent client protein activation by Hsp90 has been localized to so-called ‘mature’ complexes [7]. Therefore, the physiological ATPase activity of client protein- and/or co-chaperone-bound Hsp90 inside living cells could be surprisingly more volatile.
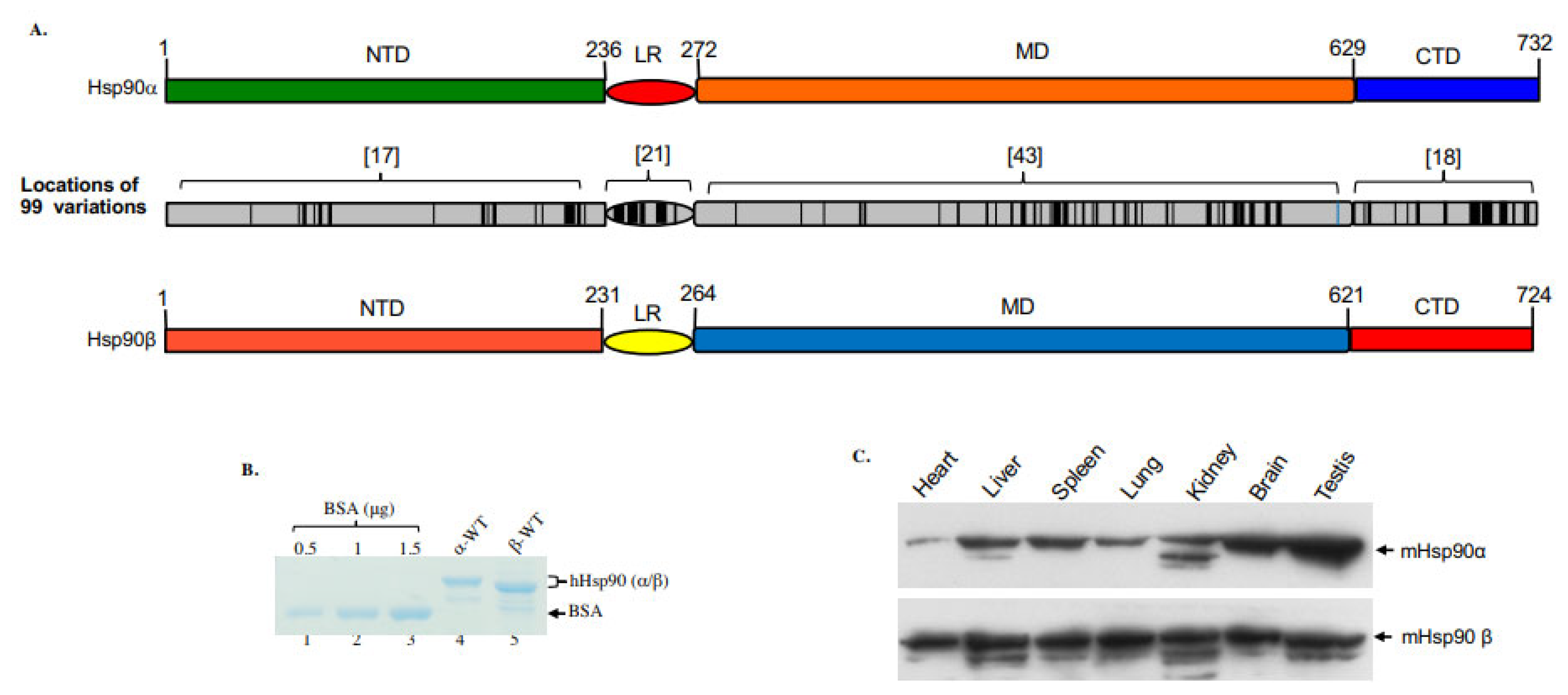
Figure 1. Variations in location, molecular mass and expression in mouse organs between Hsp90α and Hsp90β. (A) Locations (black colored) of the 99 amino acid substitutions and deletions between human Hsp90α and Hsp90β, in which the LR shows the highest percentage of the mutations. (B) The molecular mass of human recombinant Hsp90α and Hsp90β on an SDS-PAGE gel. (C) The relative expression of Hsp90α and Hsp90β in indicated mouse organs in reference to equalized total proteins of the various organs.
2. Studies of Hsp90α and Hsp90β Genes in Lower Organisms
Convincing evidence for the importance of Hsp90 genes is their critical roles during the germline or tissue-specific development of various animal models throughout their evolution. The deletion of the Escherichia coli Hsp90 homolog HptG was viable but showed growth disadvantages as the environmental temperature increased [8], indicating the requirement for HptG to deal with environmental stress. However, much of the pioneering investigations into the machinery of Hsp90 was conducted in yeasts. In the budding yeast Saccharomyces cerevisiae, cells with homozygous mutations for both HSP82 (Hsp90α) and HSC82 (Hsp90β) die at any temperature, in which higher levels of Hsp90 correlated with a higher tolerance for cell growth [9]. While HSP82 and HSC82 share 97% amino acid identity, purified HSP82 and HSC82 proteins exhibit variable ATPase activities, distinct client interactions and different sensitivities of single isoform-expressing cells in growth in response to stress [10]. In Schizosaccharomyces pombe, the Wee1 tyrosine kinase requires Hsp90 (swo1–26) to regulate the cell cycle [11]. In Drosophila, mutations in the only known Hsp90-related gene, Hsp83 (E(sev)3A), are lethal. The cDNA sequence of E(sev)3A shows 72% and 74% similarities to those of human Hsp90α and Hsp90β, respectively. Interestingly, the amino acid sequence identities of the Drosophila Hsp83 (717 a.a.) to the human Hsp90α (732 a.a.) and Hsp90β (724 a.a.) are both exactly 78.5%. Cutforth and Rubin showed that the Drosophila Hsp90 (E(sev)3A) was required for downstream signaling by the tyrosine kinase receptor, the sevenless receptor, leading to R7 photoreceptor neuron differentiation [12], apparently via the Raf kinase pathway [13]. More interestingly, Yue and colleague reported a critical function for Drosophila Hsp90 (E(sev)3A) during spermatogenesis, which appeared to be most sensitive to the loss of E(sev)3A [14].
3. Studies of Hsp90α vs. Hsp90β in Mammalian Cells
A dozen studies compared Hsp90α vs. Hsp90β side-by-side in various cellular functions. Kuo et al. reported that Hsp90β is involved in CpG-B ODN signaling but did not carry out experiments to address the specific role of Hsp90α [15]. Using a similar approach, Bouchier-Hayes and colleagues showed that Hsp90α is a key negative regulator of heat shock-induced caspase-2 activation, suggesting a possible mechanism for Hsp90α involvement in anti-apoptosis [16]. This study did not clarify whether Hsp90α acts alone or still requires the co-participation of Hsp90β, i.e., whether the downregulation of Hsp90β affects the status of caspase-2. Houlihan et al. compared Hsp90α and Hsp90β for MHC class II presentation and reported that the disruption of either Hsp90α or Hsp90β expression inhibited the class II presentation of the exogenous and endogenous GAD Ag [17]. Chatterjee et al. recently showed that Hsp90β plays a more important role than Hsp90α in the control of multiple myeloma cell survival [18]. A very interesting but previously overlooked study by Passarino and colleagues reported a healthy Caucasian human bears a missense mutation in the Hsp90α gene that impairs the translation of the protein product, suggesting that Hsp90α is unessential for life [19]. Taherian et al. compared the binding of Hsp90α and Hsp90β to client proteins and co-chaperones in mammalian cells and Xenopus oocytes and reported that Hsp90α and Hsp90β exhibit similar interactions with co-chaperones, but significantly different behaviors with client proteins under stress conditions, inconsistent with the theory of co-chaperones determining client binding specificity [20]. Cortes-González et al. showed that Hsp90α enhances, whereas Hsp90β reduces, NO and O(2)(–) generation by eNOS via modulating eNOS conformation and the phosphorylation state [21]. The KCNQ4 channel plays a critical role in DFNA2, a subtype of deafness with progressive sensorineural hearing loss. Gao and colleagues compared Hsp90α and Hsp90β for controlling KCNQ4 homeostasis and found that overexpressed Hsp90β could restore KCNQ4 surface expression, although it was insufficient to rescue the function of KCNQ4 channels [22]. When skin is injured and blood vessels clot, the local environment becomes ischemic. Jayaprakash and colleagues demonstrated that Hsp90α and Hsp90β work together to promote cell motility in wounded skin and accelerate wound closure, in which Hsp90β binds to the cytoplasmic tail of the LDL receptor-related protein-1 (LRP-1) and stabilizes the receptor at the cell surface. Hsp90α, however, is secreted by the cell into the extracellular space, where it binds and signals through the LRP-1 receptor to promote cell motility, leading to wound closure [23]. Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease characterized by apoptosis-resistant myofibroblasts and the excessive production of ECMs overtaking the normal lung tissue space. Consistent with the findings of Jaraprakash and colleagues during skin wound healing, Bellaye et al. showed that the intracellular form of HSP90β stabilizes the LRP-1 receptor to amplify the functionality of eHsp90α’s extracellular reparative function [24]. Recently, Zou et al. used CRISPR/Cas9 gene-editing technology to knock out Hsp90α and Hsp90β in MDA-MB-231 breast cancer cells. They found that Hsp90α knockout had little effect on the survival and doubling time of the cancer cells, but specifically nullified the cells’ ability to migrate and invade in the absence of serum support in vitro and form tumors in nude mice. More surprisingly, the lost ability of migration and invasiveness in vitro and tumorigenicity in vivo in the Hsp90α knockout cancer cells could be fully rescued by extracellular supplementation or injection to circulation with human recombinant Hsp90α, but not Hsp90β, protein. In contrast, a similar attempt to knock out Hsp90β failed to obtain any viable cell colonies during drug selection [2]. These authors went on to demonstrate that the major difference that distinguish Hsp90α from Hsp90β with regard to having extracellular functions or not is a dual lysine motif, K270/K277. This motif is only present in Hsp90α and substituted with G262/T269 in Hsp90β, as schematically shown in Figure 2A. Swapping between K270/K277 in Hsp90α and G262/T269 in Hsp90β completely abolishes the extracellular functions of Hsp90α and, in reverse, grants Hsp90β the extracellular functions of Hsp90α. This extracellular function-determining lysine motif is evolutionarily conserved in members of the Hsp90α subfamily (Figure 2B) and is substituted in members of the Hsp90β subfamily (Figure 2C). Similarly, it is of great interest to identify the key amino acid motifs that distinguish the specificity of the intracellular chaperone functions between Hsp90α and Hsp90β, which could serve as the targets for Hsp90α- and Hsp90β-specific inhibitors.
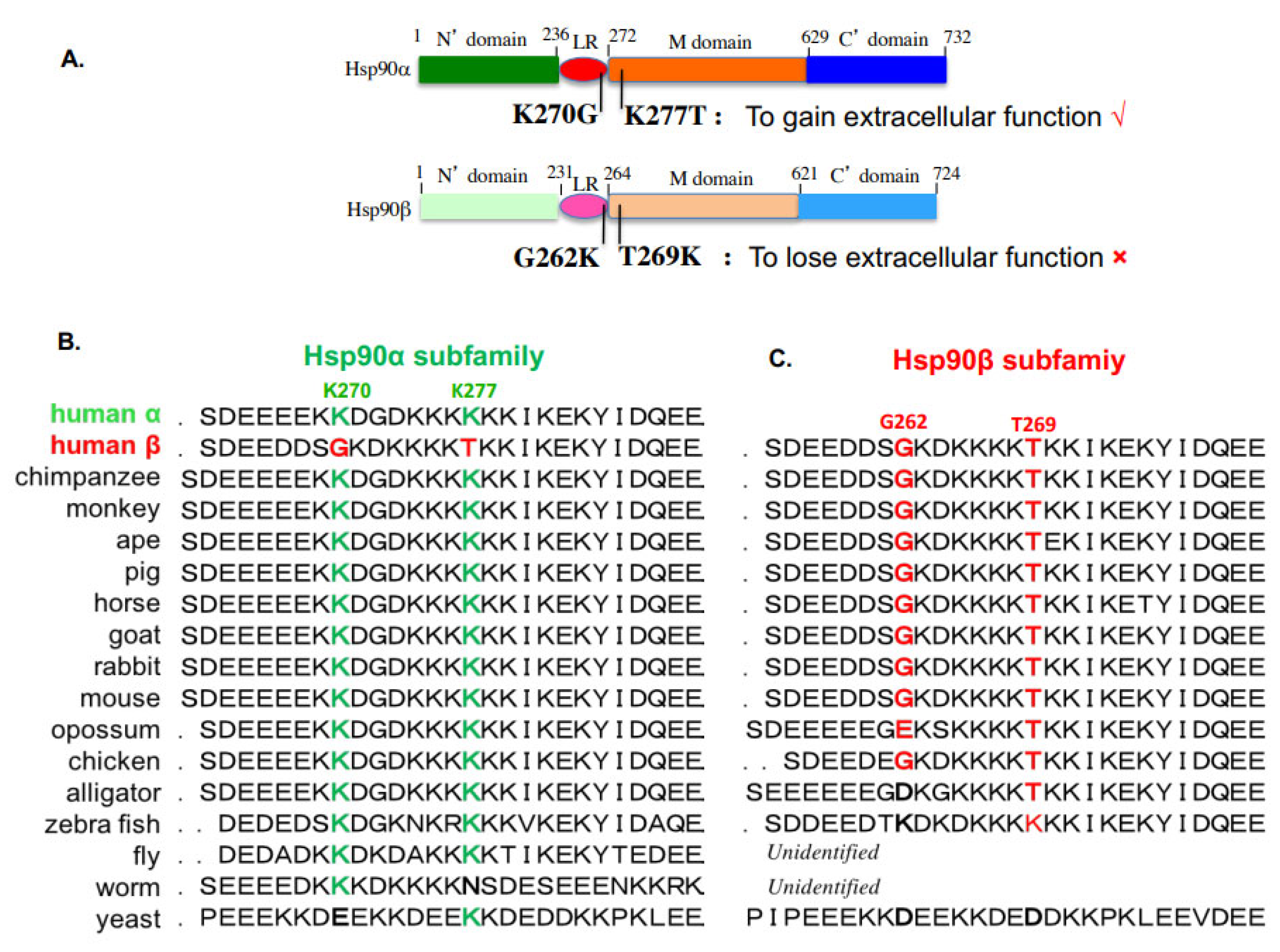
Figure 2. Evolutionarily conserved lys-270 and lys-277 distinguish Hsp90α from Hsp90β for having important extracellular functions. (A) The dual lysine motif is essential for the extracellular and ATPase-independent functions of eHsp90α. Substitutions of G262 and T269 in Hsp90β with lysine grant Hsp90β similar extracellular functions to eHsp90α. The dual K270/K277 motif in human Hsp90α (B) and the corresponding G262/T260 motif in human Hsp90β (C) are evolutionarily conserved in all the Hsp90α subfamily and the Hsp90β subfamily members, respectively.
4. Role of Hsp90α and Hsp90β during Mouse Development
Since 2000, several mouse genetic studies have provided perhaps the strongest evidence that Hsp90α and Hsp90β, albeit an 86% amino acid identity, have distinct and non-compensating functions during mouse development. Voss et al. tried to generate Hsp90β mutant mice by gene trap insertion into the exon 9 and reported that heterozygous Hsp90β mutant mice were normal, whereas the homogenous Hsp90β mutant embryos remained normal by E9.0/9.5 but died a day later due to a developmental defect in the placental labyrinth formation, even in the presence of a normal level of Hsp90α [25]. Grad and colleagues showed that mice with C-terminal 36-amino acid-deleted Hsp90α, theoretically preventing the protein to form a dimer, had little phenotypic difference from their wild-type counterparts. However, the lack of the Hsp90α chaperone function, even in the presence of a higher level of Hsp90β, specifically paralyzed the production of sperm in male mice [4]. Interestingly, an earlier study by Yue and colleagues reported that a reduced level of Hsp82 (E(sev)3A) was associated with a specific defect in spermatogenesis in Drosophila [14]. In addition to a defect in spermatogenesis, Imai and colleagues reported that the destruction of Hsp90α’s chaperone function caused defect in extracellular antigen translocation across the endosomal membrane into the cytosol in both male and female mice [5]. A recent study provided a likely explanation for why Hsp90α is specifically involved in spermatogenesis. While most tissues keep oxygen pressure between 2% and 9% (the oxygen levels in mouse circulation), the testis is known to have a constant oxygen pressure lower than 1.5%, as well as a temperature two degree lower than the rest of the body [26]. Accordingly, the low oxygen pressure causes a constitutive expression of hypoxia-inducible factor-1alpha (HIF-1α) in the premeiotic cells of the mouse testis [27][28] and in human sperm [29]. Tang et al. showed that CRISPR-cas9-mediated knockout of Hsp90α destabilized HIF-1α in mouse testis, resulting in infertility in male mice [6]. More interestingly, the requirement of Hsp90α for supporting spermatogenesis has been shown during the adult life of mice as well [30]. Researchers have recently found that the testis is the only organ in mice that constitutively expresses a higher level of HIF-1α, providing a mechanism for why Hsp90α knockout specifically affects the testis’ function. Researchers speculate that, beside the specific role of Hsp90α in stabilizing HIF-1 during spermatogenesis, the evolutionarily conserved duty for Hsp90α, both intracellularly and extracellularly, is to deal with stress-related pathophysiological conditions, such as wound healing, tissue inflammation and tumor progression. In contrast, Hsp90β serves as the critical housekeeper inside the cells.
5. The Chaperoning Data In Vitro Do Not Match the Biology In Vivo between Hsp90α and Hsp90β
Even though the role for Hsp90α and Hsp90β during mouse development dramatically varies, with Hsp90α knockout only affecting spermatogenesis and Hsp90β knockout causing death, similar degrees of difference at the molecular level, such as the profiles of their client proteins and co-chaperones, have never been established. In contrast, the results of the limited studies that compared the profiles of Hsp90α- vs. Hsp90β-associated signaling molecules have consistently shown much fewer variations than similarities. Taking the most critical PI-3K—Akt pathway that regulates cell metabolism and the EGFR-Ras-Raf-MEK-ERK signaling pathway that controls cell growth, Taherian et al. showed that Hsp90α and Hsp90β equally bind to Raf-1 and MEK1 under physiological conditions [20]. Tang and colleagues knocked out Hsp90α or knocked down Hsp90β or both in MDA-MB-231 cells and measured the steady-state stability of EGFR, Akt1, Akt2, Erk1/2 and cyclin D1. Their results show that the absence of either Hsp90α or Hsp90β alone did not cause any significant degradation of these signaling molecules, except that the absence of Hsp90β affected the stability of Akt2, Erk1/2 and cyclin D1 more than Hsp90α absence, whereas the absence of both Hsp90α and Hsp90β caused a catastrophic degradation of all the signaling molecules of the pathway [31]. If these results were interpretated as Hsp90α and Hsp90β compensating for each other’s absence, it would not explain the dramatically different outcomes of Hsp90α knockout and Hsp90β knockout in mice. If the interpretation were instead that Hsp90β, but not Hsp90α, chaperones a yet unidentified factor independent of the EGFR-Ras-Raf-MEK-ERK signaling pathway and critical for mouse development, it would be of a great interest to identify such a factor(s).
References
- Sreedhar, A.S.; Kalmár, E.; Csermely, P.; Shen, Y.F. Hsp90 isoforms: Functions, expression and clinical importance. FEBS Lett. 2004, 562, 11–15.
- Zou, M.; Bhatia, A.; Dong, H.; Jayaprakash, P.; Guo, J.; Sahu, D.; Hou, Y.; Tsen, F.; Tong, C.; O’Brien, K.; et al. Evolutionarily conserved dual lysine motif determines the non-chaperone function of secreted Hsp90alpha in tumour progression. Oncogene 2017, 36, 2160–2171.
- Dong, H.; Zou, M.; Bhatia, A.; Jayaprakash, P.; Hofman, F.; Ying, Q.; Chen, M.; Woodley, D.T.; Li, W. Breast Cancer MDA-MB-231 Cells Use Secreted Heat Shock Protein-90alpha (Hsp90α) to Survive a Hostile Hypoxic Environment. Sci. Rep. 2016, 6, 20605.
- Grad, I.; Cederroth, C.R.; Walicki, J.; Grey, C.; Barluenga, S.; Winssinger, N.; De Massy, B.; Nef, S.; Picard, D. The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS ONE 2010, 5, e15770.
- Imai, T.; Kato, Y.; Kajiwara, C.; Mizukami, S.; Ishige, I.; Ichiyanagi, T.; Hikida, M.; Wang, J.Y.; Udono, H. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 16363–16368.
- Tang, X.; Chang, C.; Hao, M.; Chen, M.; Woodley, D.T.; Schönthal, A.H.; Li, W. Heat shock protein-90alpha (Hsp90α) stabilizes hypoxia-inducible factor-1α (HIF-1α) in support of spermatogenesis and tumorigenesis. Cancer Gene Ther. 2021, 28, 1058–1070.
- Prodromou, C.; Siligardi, G.; O’Brien, R.; Woolfson, D.N.; Regan, L.; Panaretou, B.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 1999, 18, 754–762.
- Bardwell, J.C.; Craig, E.A. Ancient heat shock gene is dispensable. J. Bacteriol. 1988, 170, 2977–2983.
- Borkovich, K.A.; Farrelly, F.W.; Finkelstein, D.B.; Taulien, J.; Lindquist, S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol. Cell. Biol. 1989, 9, 3919–3930.
- Girstmair, H.; Tippel, F.; Lopez, A.; Tych, K.; Stein, F.; Haberkant, P.; Schmid, P.W.N.; Helm, D.; Rief, M.; Sattler, M.; et al. The Hsp90 isoforms from S. cerevisiae differ in structure, function and client range. Nat. Commun. 2019, 10, 3626.
- Aligue, R.; Akhavan-Niak, H.; Russell, P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994, 13, 6099–6106.
- Cutforth, T.; Rubin, G.M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell 1994, 77, 1027–1036.
- van der Straten, A.; Rommel, C.; Dickson, B.; Hafen, E. The heat shock protein 83 (Hsp83) is required for Raf-mediated signalling in Drosophila. EMBO J. 1997, 16, 1961–1969.
- Yue, L.; Karr, T.L.; Nathan, D.F.; Swift, H.; Srinivasan, S.; Lindquist, S. Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics 1999, 151, 1065–1079.
- Kuo, C.C.; Liang, C.M.; Lai, C.Y.; Liang, S.M. Involvement of heat shock protein (Hsp)90 beta but not Hsp90 alpha in antiapoptotic effect of CpG-B oligodeoxynucleotide. J. Immunol. 2007, 178, 6100–6108.
- Bouchier-Hayes, L.; Oberst, A.; McStay, G.P.; Connell, S.; Tait, S.W.; Dillon, C.P.; Flanagan, J.M.; Beere, H.M.; Green, D.R. Characterization of cytoplasmic caspase-2 activation by induced proximity. Mol. Cell 2009, 35, 830–840.
- Houlihan, J.L.; Metzler, J.J.; Blum, J.S. HSP90alpha and HSP90beta isoforms selectively modulate MHC class II antigen presentation in B cells. J. Immunol. 2009, 182, 7451–7458.
- Chatterjee, M.; Andrulis, M.; Stühmer, T.; Müller, E.; Hofmann, C.; Steinbrunn, T.; Heimberger, T.; Schraud, H.; Kressmann, S.; Einsele, H.; et al. The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica 2013, 98, 1132–1141.
- Passarino, G.; Cavalleri, G.L.; Stecconi, R.; Franceschi, C.; Altomare, K.; Dato, S.; Greco, V.; Luca Cavalli Sforza, L.; Underhill, P.A.; de Benedictis, G. Molecular variation of human HSP90alpha and HSP90beta genes in Caucasians. Hum. Mutat. 2003, 21, 554–555.
- Taherian, A.; Krone, P.H.; Ovsenek, N. A comparison of Hsp90alpha and Hsp90beta interactions with cochaperones and substrates. Biochem. Cell Biol. 2008, 86, 37–45.
- Cortes-González, C.; Barrera-Chimal, J.; Ibarra-Sánchez, M.; Gilbert, M.; Gamba, G.; Zentella, A.; Flores, M.E.; Bobadilla, N.A. Opposite effect of Hsp90α and Hsp90β on eNOS ability to produce nitric oxide or superoxide anion in human embryonic kidney cells. Cell. Physiol. Biochem. 2010, 26, 657–668.
- Gao, Y.; Yechikov, S.; Vazquez, A.E.; Chen, D.; Nie, L. Distinct roles of molecular chaperones HSP90α and HSP90β in the biogenesis of KCNQ4 channels. PLoS ONE 2013, 8, e57282.
- Jayaprakash, P.; Dong, H.; Zou, M.; Bhatia, A.; O’Brien, K.; Chen, M.; Woodley, D.T.; Li, W. Hsp90α and Hsp90β together operate a hypoxia and nutrient paucity stress-response mechanism during wound healing. J. Cell Sci. 2015, 128, 1475–1480.
- Bellaye, P.S.; Shimbori, C.; Yanagihara, T.; Carlson, D.A.; Hughes, P.; Upagupta, C.; Sato, S.; Wheildon, N.; Haystead, T.; Ask, K.; et al. Synergistic role of HSP90α and HSP90β to promote myofibroblast persistence in lung fibrosis. Eur. Respir. J. 2018, 51, 1700386.
- Voss, A.K.; Thomas, T.; Gruss, P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development 2000, 127, 1–11.
- Gruber, M.; Mathew, L.K.; Runge, A.C.; Garcia, J.A.; Simon, M.C. EPAS1 Is Required for Spermatogenesis in the Postnatal Mouse Testis. Biol. Reprod. 2010, 82, 1227–1236.
- Takahashi, N.; Davy, P.M.; Gardner, L.H.; Mathews, J.; Yamazaki, Y.; Allsopp, R.C. Hypoxia Inducible Factor 1 Alpha Is Expressed in Germ Cells throughout the Murine Life Cycle. PLoS ONE 2016, 11, e0154309.
- Marti, H.H.; Katschinski, D.M.; Wagner, K.F.; Schäffer, L.; Stier, B.; Wenger, R.H. Isoform-specific expression of hypoxia-inducible factor-1alpha during the late stages of mouse spermiogenesis. Mol. Endocrinol. 2002, 16, 234–243.
- Depping, R.; Hägele, S.; Wagner, K.F.; Wiesner, R.J.; Camenisch, G.; Wenger, R.H.; Katschinski, D.M. A dominant-negative isoform of hypoxia-inducible factor-1 alpha specifically expressed in human testis. Biol. Reprod. 2004, 71, 331–339.
- Kajiwara, C.; Kondo, S.; Uda, S.; Dai, L.; Ichiyanagi, T.; Chiba, T.; Ishido, S.; Koji, T.; Udono, H. Spermatogenesis arrest caused by conditional deletion of Hsp90α in adult mice. Biol. Open 2012, 1, 977–982.
- Tang, X.; Chang, C.; Mosallaei, D.; Woodley, D.T.; Schönthal, A.H.; Chen, M.; Li, W. Heterogeneous Responses and Isoform Compensation the Dim Therapeutic Window of Hsp90 ATP-Binding Inhibitors in Cancer. Mol. Cell. Biol. 2022, 42, e0045921.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
02 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





