Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sarah Spitz | -- | 2063 | 2023-02-01 13:54:56 | | | |
| 2 | Sirius Huang | Meta information modification | 2063 | 2023-02-02 02:42:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Spitz, S.; Ko, E.; Ertl, P.; Kamm, R.D. The Glymphatic System and Neuropathologies. Encyclopedia. Available online: https://encyclopedia.pub/entry/40729 (accessed on 07 February 2026).
Spitz S, Ko E, Ertl P, Kamm RD. The Glymphatic System and Neuropathologies. Encyclopedia. Available at: https://encyclopedia.pub/entry/40729. Accessed February 07, 2026.
Spitz, Sarah, Eunkyung Ko, Peter Ertl, Roger D Kamm. "The Glymphatic System and Neuropathologies" Encyclopedia, https://encyclopedia.pub/entry/40729 (accessed February 07, 2026).
Spitz, S., Ko, E., Ertl, P., & Kamm, R.D. (2023, February 01). The Glymphatic System and Neuropathologies. In Encyclopedia. https://encyclopedia.pub/entry/40729
Spitz, Sarah, et al. "The Glymphatic System and Neuropathologies." Encyclopedia. Web. 01 February, 2023.
Copy Citation
The lack of a conventional lymphatic system that permeates throughout the entire human brain has encouraged the identification and study of alternative clearance routes within the cerebrum. In 2012, the concept of the glymphatic system, a perivascular network that fluidically connects the cerebrospinal fluid to the lymphatic vessels within the meninges via the interstitium, emerged. Although its exact mode of action has not yet been fully characterized, the key underlying processes that govern solute transport and waste clearance have been identified.
AQP4
glymphatic system
neurodegeneration
1. The Glymphatic System
In the peripheral tissues, the continuous exchange of interstitial fluid, an essential prerequisite to tissue homeostasis, is carried out by a coordinated interplay of the vascular and the lymphatic system. High arterial pressures generate a flux of fluid across the arterioles and capillaries of the vascular system into the surrounding tissues, generating a flow of interstitial fluid that either re-enters the circulation into the venules or flows to nearby lymphatic vessels, which ultimately return the fluid into the venous circulation. Thereby, nutrients are transported into the extracellular space while waste products are simultaneously cleared [1]. Interestingly, within the central nervous system, where imbalances in cerebral tissue homeostasis can have detrimental effects ranging from disturbances in synaptic cell signaling to the onset of neurodegenerative diseases, this conventional lymphatic network is restricted to the meninges, an arrangement of three membranes that envelopes the human cerebrum [2][3].
In vivo, the human brain is pervaded by a dense network of blood vessels comprising arteries, arterioles, capillaries, venules, and veins that, in addition to transporting nutrients and removing waste products, protect the nervous tissue from neurotoxic factors. To that end, the capillary network is equipped with a strictly regulated barrier, the blood–brain barrier (BBB), that, in contrast with the peripheral blood circulation, is composed of three distinct cell types: brain endothelial cells, pericytes, and astrocytes [4]. The high integrity of the BBB is conferred by a combination of tight endothelial cell–cell connections, ensheathing the pericytes, and the terminal end-feet of astrocytes positioned within the abluminal space of the blood vessels. To enable selective molecular transport across this tight barrier, endothelial cells are equipped with a range of transporter proteins, facilitating the transport of molecules into the brain parenchyma (influx) and vice versa (efflux) [4].
While the cerebrospinal fluid (CSF), a clear ultrafiltrate of plasma that surrounds the human brain, has long been thought to aid in the clearance of cerebral interstitial fluid, it was the identification of the meningeal lymphatics by MRI that made the presence of additional clearance routes increasingly evident [3]. The concept of an alternate clearance system was first proposed in 2012 by researchers from the Medical Center at the University of Rochester (Nedergaard Lab), who demonstrated the convective pathways of the CSF through the brain using two-photon imaging [5]. In detail, by intracisternal injection of fluorescently labeled CSF in mice, Iliff et al. monitored the subarachnoid influx of the CSF into the Virchow–Robin spaces along arterioles (1), followed by a directional influx of CSF into the parenchyma, where it mixed with the interstitial fluid (ISF) (2), before a subsequent efflux of the CSF/ISF mixture through the central veins thereafter (3) (see Figure 1[6] (Biorender)). Furthermore, Iliff et al. showed that aquaporin-4 (AQP4) knockout mice displayed a 65% reduction in the CSF flux through the parenchyma, suggesting that the influx of water into the brain interstitium is controlled via the bidirectional water channel, predominantly expressed and located in the astrocytic endfeet (polarized expression) [5]. Based on these observations, the Nedergaard group proposed the term “glymphatic system“ in recognition of the glial-mediated water influx into the parenchyma that contributes to the compensatory lymphatic system within the brain [5]. While the proposed concept of an astrocyte-mediated clearance system initially produced some controversies, now, numerous studies have corroborated the presence of a perivascular waste-removal network [] Intriguingly, recent studies have revealed that glymphatic activity is significantly enhanced during sleep, rendering sleep crucial in clearing neurotoxic waste and supporting the correlation between proteinopathies and sleep deprivation [7][8]. The increase in glymphatic flux can be linked to a decrease in the neurotransmitter norepinephrine, which has been shown to expand the extracellular space and thus result in a lowered hydraulic resistance [9]. In addition, a drastic age-dependent reduction in the glymphatic activity of 80-90% was observed in mice, providing a possible explanation for the increased vulnerability to developing neurodegenerative diseases with advancing age [10]. This decrease might be explained by the loss of polarization or depolarization of AQP4 (see Figure 2[6] (Biorender)), which has been reported in astrocytes of aging mice and was linked to reduced cognitive performance in elder individuals (over 85 years old) [11][12]. Furthermore, both age-associated alterations in circadian rhythms and a decrease in CSF turnover rate might contribute to an abated clearance efficiency in the aging cerebrum [10][13].
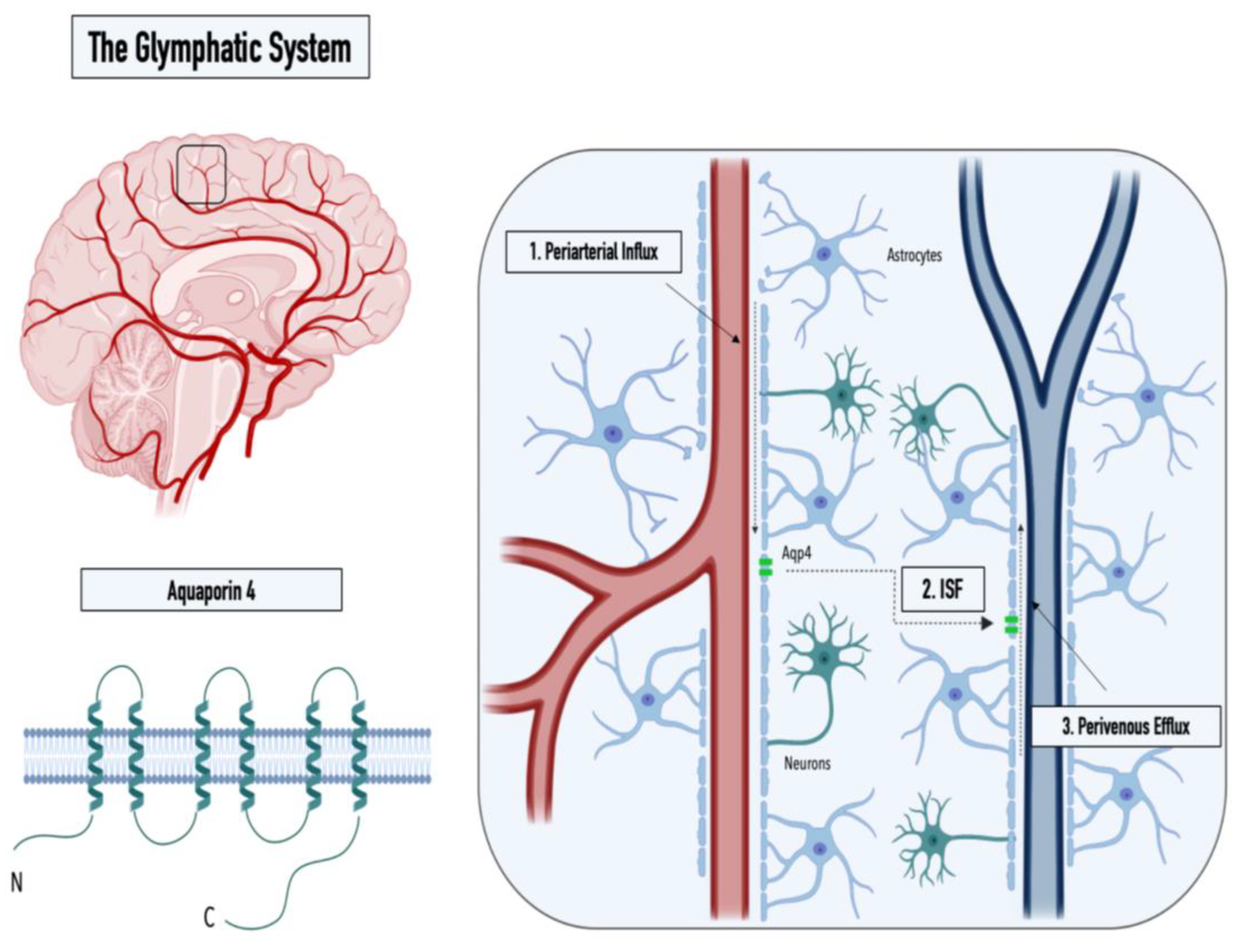
Figure 1. Graphical illustration of the structure of the water channel aquaporin-4 (left panel) and the three steps of the glymphatic pathway: (1) CSF influx into the Virchow–Robin spaces along arterioles, (2) influx of CSF into the parenchyma, and (3) perivenous efflux of the CSF/ISF mixture (right panel).
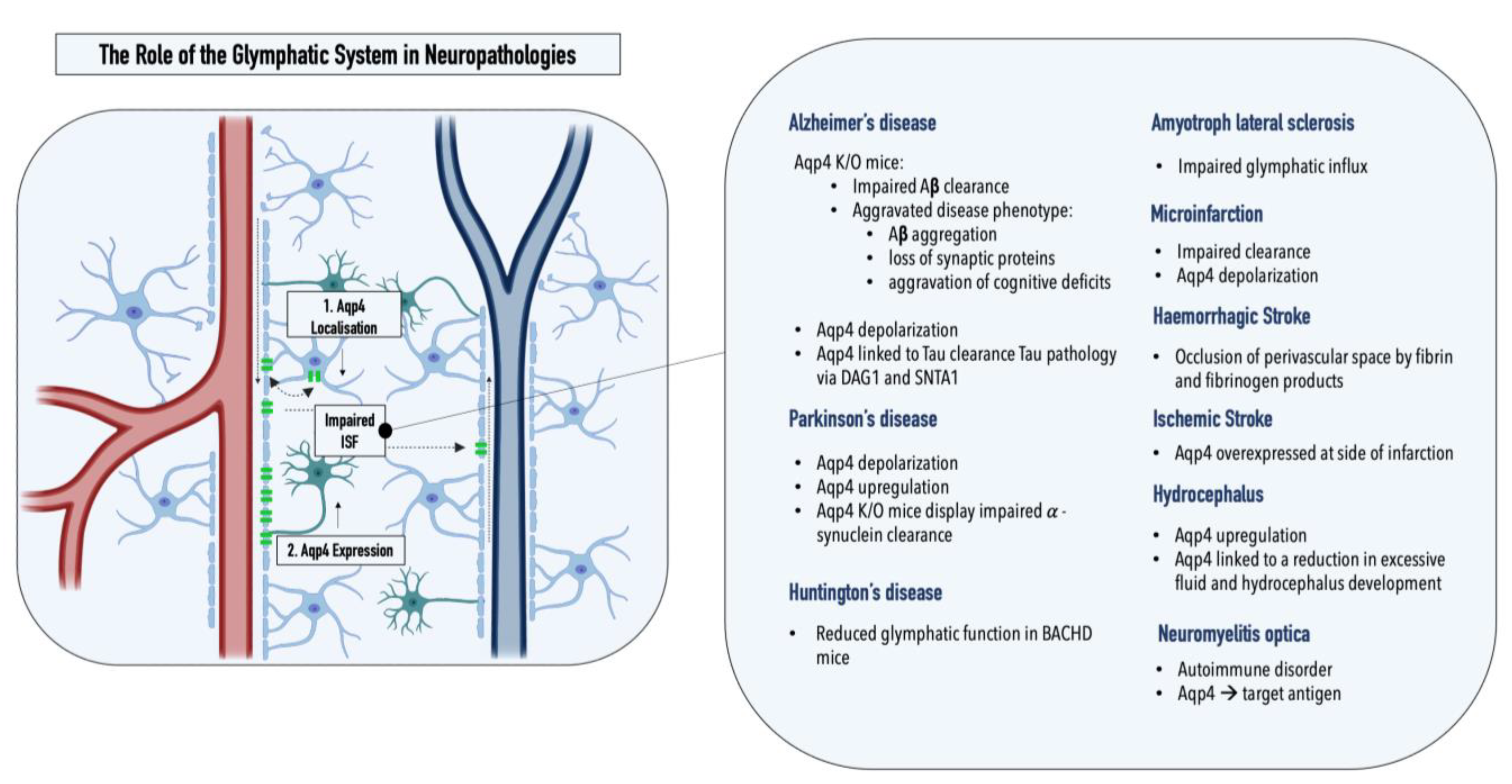
Figure 2. Graphical illustration of the two key pathological alterations in the glymphatic system observed in neuropathologies: (1) AQP4 depolarization and (2) altered AQP4 expression. Overview of pathological changes in the glymphatic system observed in various neuropathologies.
Considering the critical role of the glymphatic system in maintaining cerebral homeostasis, fluctuations in this glial-mediated balance can consequently have detrimental effects. For this reason, over recent years, several studies have started to look into the potential role of the glymphatic system in neuropathologies, specifically within neurodegenerative proteinopathies, which comprise disorders characterized by abnormal aggregation of proteins as well as insufficient waste clearance.
2. The Glymphatic System and Neuropathologies
A pathological inter-relationship between neurodegenerative proteinopathies and the glymphatic system was first reported by Iliff et al., who connected a marked decrease (70% for [3H]mannitol) in interstitial solute clearance with increased levels of amyloid β, the main constituent of plaques in Alzheimer’s disease, in an AQP4-/- mouse model. Specifically, the authors reported a ~55% decrease in the rate of 125I-amyloid β 1–40 clearance in AQP4-/- mice compared with that in wild-type controls, indicating that a significant fraction of soluble amyloid β is cleared via perivascular routes as opposed to BBB-mediated local removal [5]. This hypothesis was recently supported by a study by Nauen and Troncoso, who demonstrated the presence of amyloid β for the first time in human lymph nodes. Compared with lymph nodes of the inguine, a greater abundance of amyloid β was reported in cervical lymph nodes, pointing toward the cervical lymph nodes as the primary entry point of amyloid β into the systemic lymphatics [14]. Furthermore, Peng et al. showed that chimeric human amyloid precursor protein (APP)/presenilin-1 (PS1) double-transgenic mice displayed reduced glymphatic clearance that preceded marked amyloid β deposition, rendering glymphatic influx a potential target for therapeutic intervention. Interestingly, the treatment of wild-type mice with amyloid β40 resulted in a significant reduction of CSF influx, pointing toward a negative AQP4-dependent reinforcement loop within Alzheimer’s disease [15]. The reported impaired glymphatic influx might be linked to depolarization of AQP4, which is observed in the post-mortem tissues of Alzheimer’s patients. Interestingly, a comparative analysis of AQP4 in the CSF of Alzheimer’s disease patients and healthy controls revealed a 1.7× increase in AQP4, which positively correlated with tau levels [16]. Both SNTA1 and DAG1, which mediate the anchoring of AQP4 to the astrocytic endfeet, as well as MLC1, which encodes an astroglial membrane transporter linked to AQP4, positively correlated with tau levels, further relating the water channel AQP4 to tau pathology [17]. A study by Ishida et al. employing transgenic mice expressing P301S mutant tau showed that a deletion of AQP4 also markedly elevated tau levels within the CSF and significantly exacerbated p-tau deposition, aggravating the ensuing neuronal degeneration [18]. As hypothesized by Mogensen et al., these observations suggest an uncoupling of AQP4 expression and glymphatic influx, which might be explained by a concomitant depolarization of the water channel under pathological conditions [19]. Furthermore, as amyloid β oligomers and fibrils stimulate the release of proinflammatory cytokines in the microglia, inflammation constitutes an essential element in Alzheimer’s disease pathology [19][20][21]. Consequently, morphological changes in astrocytes mediated by astrogliosis might further enhance alterations within the glymphatic system [22]. Lastly, recent genetic studies have linked single-nucleotide polymorphisms (SNPs) within the AQP4 gene with protein depolarization, Aβ accumulation, disease stage progression, and cognitive decline, making SNPs potential predictors for the disease burden in Alzheimer’s patients [23][24].
Alterations within the glymphatic system were also linked to Parkinson’s disease, the second most common neurodegenerative proteinopathy [25]. While α-synuclein, the protein affected within Parkinson’s disease, is traditionally assigned to the intracellular space of neurons, studies showed that α-synuclein can be excreted into the extracellular space and thereby might contribute to the interneuron transfer of protein aggregates [26]. The quantification of α-synuclein within post-mortem brain tissue samples from Parkinson’s patients, displaying sleep disturbances, a common nonmotor symptom of the disease, revealed an increased synuclein burden within cerebral tissues compared with that of patients without impaired sleep patterns [27]. The potential inter-relationship between the glymphatic system and REM sleep, circadian rhythms, and clock gene dysfunction in Parkinson’s disease was recently summarized in a comprehensive review by Sundaram et al., and can be found elsewhere [28]. A potential involvement of the glymphatic system within Parkinson’s disease can also be derived from the multifaceted role of the affected neurotransmitter, dopamine, as the cholinergic monoamine transmitter was shown to both mediate ISF influx and directly modulate glial differentiation and AQP4 expression [29][30]. AQP4 deficiency, for example, was shown to enhance dopaminergic neurodegeneration and increase the susceptibility of TH-positive cells to the prodrug MPTP, a chemical inducer of PD in mice [31]. In addition, a potential interplay between the glymphatic system and Parkinson’s disease was supported by a study assessing α-synuclein levels in the CSF of patients and controls, revealing a 13% reduction in the unaggregated form of α-synuclein in Parkinson’s patients [32]. The hypothesis was further supported by a recent study conducted by Zou et al., who demonstrated perivascular α-synuclein aggregation and AQP4 depolarization in a mouse model of Parkinson’s disease (A53T). In addition to glymphatic dysfunction, pathology-related phenotypes such as neurodegeneration and exacerbated α-synuclein aggregation were further enhanced upon the ligation of the cervical lymph nodes [33]. Furthermore, two recent studies have reported changes in the glymphatic system in two neurodegenerative disorders, Huntington’s and amyotrophic lateral sclerosis. Zamani et al. reported significantly disrupted glymphatic function in a transgenic mouse model emulating key pathological events implicated in amyotrophic lateral sclerosis; Wu et al. demonstrated reduced CSF–ISF exchange in a huntingtin (HTT)-expressing mouse model (BACHD) [34][35].
In to neurodegenerative diseases, alterations within the glymphatic system have also been reported for hydrocephalus, ischemic and hemorrhagic stroke, multiple microinfarctions, traumatic brain injury, cerebral amyloid angiopathy, as well as diabetes mellitus [36]. Hydrocephalus, for example, which is a pathological accumulation of CSF within the brain, has been associated with increased cerebrovascular pulsatility, decreased CSF influx, and impaired glymphatic clearance [37]. As a result, compounds modulating AQP4 function are currently under investigation in clinical trials [38]. Traumatic brain injury, a risk factor for neurodegenerative diseases, was shown to promote tau pathology in an AQP4-deficient mouse model [39]. In addition, reduced glymphatic influx was reported for both ischemic and hemorrhagic stroke, as well as multiple microinfarctions [36]. AQP4 was overexpressed at the site of infarction in ischemic stroke, and fibrin and fibrinogen deposits were shown to occlude perivascular spaces in hemorrhagic stroke, entrapment of CSF solutes was reported within small, dispersed ischemic lesions characteristic of multiple microinfarctions [36][39][40]. Impaired glymphatic transport was also reported for cerebral amyloid angiopathy, which, next to increased arterial stiffness, has been associated with a decreased arterial pulse and reduced perivascular spaces [41]. Lastly, an impaired glymphatic influx has also been observed in type II diabetes mellitus, a common metabolic disorder associated with cognitive impairment. Herein, the pathological phenotype is characterized by an increase in CSF influx, while interstitial solute clearance is reduced [42][43].
To summarize, over the last few years, substantial evidence has highlighted the critical role of the glymphatic system in maintaining cerebral homeostasis, and the detrimental effects of imbalances within this perivascular clearance system have been demonstrated. However, that most of our understanding of how the glymphatic system operates has been extracted from nonhuman in vivo models. While the development of new imaging techniques and post mortem studies corroborated initial hypotheses surrounding the glymphatic system, the limited access to the human cerebrovascular system necessitates alternative investigation strategies, preferably in the form of personalized in vitro models.
References
- Stewart, R.H. A Modern View of the Interstitial Space in Health and Disease. . Front. Vet. Sci. 2020, 7, 609583.
- Abbott, N.J. Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. . Neurochem. Int. 2004, 45, 545–552.
- Absinta, M.; Ha, S.K.; Nair, G.; Sati, P.; Luciano, N.J.; Palisoc, M.; Louveau, A.; Zaghloul, K.A.; Pittaluga, S.; Kipnis, J.; et al.et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife 2017, 6, e29738.
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back.. Physiol. Rev. 2019, 99, 21–78.
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, Y.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, s.A.; et al.et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid beta.. Sci. Transl. Med. 2012, 4, 147ra111.
- Spitz, S.; Ko, E.; Ertl, P.; Kamm, R.D. How Organ-on-a-Chip Technology Can Assist in Studying the Role of the Glymphatic System in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 2171.
- Mendelsohn, A.R.; Larrick, J.W. Sleep Facilitates Clearance of Metabolites from the Brain: Glymphatic Function in Aging and Neurodegenerative Diseases. Rejuvenation Res. 2013, 16, 518–523.
- Reddy, O.C.; van der Werf, Y.D. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020, 10, 868.
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al.et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861.
- Valenza, M.; Facchinetti, R.; Steardo, L.; Scuderi, C. Altered waste disposal system in aging and Alzheimer’s disease: Focus on astrocytic aquaporin-4. Front. Pharmacol. 2020, 10, 1656.
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. . Neurol Res. Pract. 2021, 3, 5.
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1434.
- Nilsson, C.; Stahlberg, F.; Thomsen, C.; Henriksen, O.; Herning, M.; and Owman, C. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992, 262 Pt 2, R20–R24.
- Nauen, D.W.; Troncoso, J.C. Amyloid-beta is present in human lymph nodes and greatly enriched in those of the cervical region. Alzheimer’s Dement. 2022, 18, 205–210.
- Peng, W.; Achariyar, T.M.; Li, B.; Liao, Y.; Mestre, H.; Hitomi, E.; Regan, S.; Kasper, T.; Peng, S.; Ding, F.; et al.et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016, 93, 215–225.
- Arighi, A.; Arcaro, M.; Fumagalli, G.G.; Carandini, T.; Pietroboni, A.M.; Sacchi, L.; Fenoglio, C.; Serpente, M.; Sorrentino, F.; Isgrò, G.; et al.et al. Aquaporin-4 cerebrospinal fluid levels are higher in neurodegenerative dementia: Looking at glymphatic system dysregulation. Alzheimers Res. Ther. 2022, 14, 135.
- Simon, M.J.; Wang, M.X.; Murchison, C.F.; Roese, N.E.; Boespflug, E.L.; Woltjer, R.L.; Iliff, J.J. Transcriptional network analysis of human astrocytic endfoot genes reveals region-specific associations with dementia status and tau pathology. Sci. Rep. 2018, 8, 12389.
- Ishida, K.; Yamada, K.; Nishiyama, R.; Hashimoto, T.; Nishida, I.; Abe, Y.; Yasui, M.; Iwatsubo, T. Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J. Exp. Med. 2022, 219, e20211275.
- Mogensen, F.L.H.; Delle, C.; Nedergaard, M. The glymphatic system (En)during inflammation. Int. J. Mol. Sci. 2021, 22, 7491.
- da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.G.; Wall, M.; Jiang, H.; Kodira, C.D.; de Lima, K.A.; Herz, J.; et al.et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature 2021, 593, 255–260.
- Buccellato, F.R.; D’Anca, M.; Serpente, M.; Arighi, A.; Galimberti, D. The Role of Glymphatic System in Alzheimer’s and Parkinson’s Disease Pathogenesis. Biomedicines 2022, 10, 2261.
- Zhou, B.; Zuo, Y.X.; Jiang, R.T. Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neuroscience and Therapeutics 2019, 25, 665–673.
- Chandra, A.; Farrell, C.; Wilson, H.; Dervenoulas, G.; de Natale, E.R.; Politis, M. Aquaporin-4 polymorphisms predict amyloid burden and clinical outcome in the Alzheimer’s disease spectrum. Neurobiol. Aging 2021, 97, 1–9.
- Burfeind, K.G.; Murchison, C.F.; Westaway, S.K.; Simon, M.J.; Erten-Lyons, D.; Kaye, J.A.; Quinn, J.F.; Iliff, J.J. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 348–359.
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al.et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy.. Mov. Disord. 2017, 32, 1264–1310.
- Emmanouilidou, E.; Elenis, D.; Papasilekas, T.; Stranjalis, G.; Gerozissis, K.; Ioannou, P.C.; Vekrellis, K. Assessment of α-synuclein secretion in mouse and human brain parenchyma. PLoS ONE 2011, 6, e22225.
- Kalaitzakis, M.E.; Gentleman, S.M.; Pearce, R.K.B. Disturbed sleep in Parkinson’s disease: Anatomical and pathological correlates. Neuropathol. Appl. Neurobiol. 2013, 39, 644–653.
- Sundaram, S.; Hughes, R.L.; Peterson, E.; Müller-Oehring, E.M.; Brontë-Stewart, H.M.; Poston, K.L.; Faerman, A.; Bhowmick, C.; Schulte, T. Establishing a framework for neuropathological correlates and glymphatic system functioning in Parkinson’s disease. Neurosci. Biobehav. Rev. 2019, 103, 305–315.
- Fuxe, K.; Agnati, L.F.; Marcoli, M.; Borroto-Escuela, D.O. Volume Transmission in Central Dopamine and Noradrenaline Neurons and Its Astroglial Targets. Neurochem. Res. 2015, 40, 2600–2614.
- Küppers, E.; Gleiser, C.; Brito, V.; Wachter, B.; Pauly, T.; Hirt, B.; Grissmer, S. AQP4 expression in striatal primary cultures is regulated by dopamine—Implications for proliferation of astrocytes. Eur. J. Neurosci. 2008, 28, 2173–2182.
- Fan, Y.; Fan, Y.; Kong, H.; Shi, X.; Sun, X.; Ding, J.; Wu, J.; Hu, G. Hypersensitivity of aquaporin 4-deficient mice to 1-methyl-4- phenyl-1,2,3,6-tetrahydropyrindine and astrocytic modulation. Neurobiol. Aging 2008, 29, 1226–1236.
- van Dijk, K.D.; Bidinosti, M.; Weiss, A.; Raijmakers, P.; Berendse, H.W.; van de Berg, W.D.J. Reduced α-synuclein levels in cerebrospinal fluid in Parkinson’s disease are unrelated to clinical and imaging measures of disease severity. Eur. J. Neurol. 2014, 21, 388–394.
- Zou, W.; Pu, T.; Feng, W.; Lu, M.; Zheng, Y.; Du, R.; Xiao, M.; Hu, G. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl. Neurodegener. 2019, 8, 1–17.
- Zamani, A.; Walker, A.K.; Rollo, B.; Ayers, K.L.; Farah, R.; O’Brien, T.J.; Wright, D.K. Impaired glymphatic function in the early stages of disease in a TDP-43 mouse model of amyotrophic lateral sclerosis. Transl. Neurodegener. 2022, 11, 17.
- Wu, T.; Su, F.; Feng, Y.; Liu, B.; Li, M.; Liang, F.; Li, G.; Li, X.; Zhang, Y.; Cai, Z.; et al.et al. Mesenchymal stem cells alleviate AQP-4-dependent glymphatic dysfunction and improve brain distribution of antisense oligonucleotides in BACHD mice.. Stem. Cells 2020, 38, 218–230.
- Natale, G.; Limanaqi, F.; Busceti, C.L.; Mastroiacovo, F.; Nicoletti, F.; Puglisi-Allegra, S.; Fornai, F. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. Front. Neurosci. 2021, 15, 1662-453X.
- Reeves, B.C.; Karimy, J.K.; Kundishora, A.J.; Mestre, H.; Cerci, H.M.; Matouk, C.; Alper, S.L.; Lundgaard, I.; Nedergaard, M.; Kahle, K.T.; et al. Glymphatic System Impairment in Alzheimer’s Disease and Idiopathic Normal Pressure Hydrocephalus. Trends Mol. Med. 2020, 26, 285–295.
- Desai, B.; Hsu, Y.; Schneller, B.; Hobbs, J.G.; Mehta, A.I.; Linninger, A. Hydrocephalus: The role of cerebral aquaporin-4 channels and computational modeling considerations of cerebrospinal fluid. Neurosurg. Focus 2016, 41, e8.
- Wang, M.; Ding, F.; Deng, S.Y.; Guo, X.; Wang, W.; Iliff, J.J.; Nedergaard, M. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J. Neurosci. 2017, 37, 2870–2877.
- Gaberel, T.; Gakuba, C.; Goulay, R.; Martinez De Lizarrondo, S.; Hanouz, J.L.; Emery, E.; Touze, E.; Vivien, D.; Gauberti, M. Impaired Glymphatic Perfusion After Strokes Revealed by Contrast-Enhanced MRI A New Target for Fibrinolysis? . Stroke 2014, 45, 3092–3096.
- Chen, X.; Liu, X.; Koundal, S.; Elkin, R.; Zhu, X.; Monte, B.; Xu, F.; Dai, F.; Pedram, M.; Lee, H.; et al.et al. Cerebral amyloid angiopathy is associated with glymphatic transport reduction and time-delayed solute drainage along the neck arteries. Nat. Aging 2022, 2, 214–223.
- Zhang, L.; Chopp, M.; Jiang, Q.; Zhang, Z. Role of the glymphatic system in ageing and diabetes mellitus impaired cognitive function. Stroke Vasc. Neurol. 2019, 4, 90–92.
- Kim, Y.K.; Nam, K.; il Song, J. The Glymphatic System in Diabetes-Induced Dementia. . Front. Neurol. 2018, 9, 867.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
02 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




