You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Awad A Shehata | -- | 2124 | 2023-02-01 10:18:51 | | | |
| 2 | Sirius Huang | Meta information modification | 2124 | 2023-02-02 02:11:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Basiouni, S.; Tellez-Isaias, G.; Latorre, J.D.; Graham, B.D.; Petrone-Garcia, V.M.; El-Seedi, H.R.; Yalçın, S.; El-Wahab, A.A.; Visscher, C.; May-Simera, H.L.; et al. Factors for Oxidative Stress and Inflammation in Poultry. Encyclopedia. Available online: https://encyclopedia.pub/entry/40712 (accessed on 05 January 2026).
Basiouni S, Tellez-Isaias G, Latorre JD, Graham BD, Petrone-Garcia VM, El-Seedi HR, et al. Factors for Oxidative Stress and Inflammation in Poultry. Encyclopedia. Available at: https://encyclopedia.pub/entry/40712. Accessed January 05, 2026.
Basiouni, Shereen, Guillermo Tellez-Isaias, Juan D. Latorre, Brittany D. Graham, Victor M. Petrone-Garcia, Hesham R. El-Seedi, Sakine Yalçın, Amr Abd El-Wahab, Christian Visscher, Helen L. May-Simera, et al. "Factors for Oxidative Stress and Inflammation in Poultry" Encyclopedia, https://encyclopedia.pub/entry/40712 (accessed January 05, 2026).
Basiouni, S., Tellez-Isaias, G., Latorre, J.D., Graham, B.D., Petrone-Garcia, V.M., El-Seedi, H.R., Yalçın, S., El-Wahab, A.A., Visscher, C., May-Simera, H.L., Huber, C., Eisenreich, W., & Shehata, A.A. (2023, February 01). Factors for Oxidative Stress and Inflammation in Poultry. In Encyclopedia. https://encyclopedia.pub/entry/40712
Basiouni, Shereen, et al. "Factors for Oxidative Stress and Inflammation in Poultry." Encyclopedia. Web. 01 February, 2023.
Copy Citation
Chronic stress is recognized as a secret killer in poultry. It is associated with systemic inflammation due to cytokine release, dysbiosis, and the so-called leaky gut syndrome, which mainly results from oxidative stress reactions that damage the barrier function of the cells lining the gut wall. Poultry, especially the genetically selected broiler breeds, frequently suffer from these chronic stress symptoms when exposed to multiple stressors in their growing environments.
poultry
inflammation
oxidative stress
stressors
phytogenic substances
1. Introduction
Mitochondria, commonly referred as the “powerhouse of eukaryotic cells”, are responsible for the production of cellular energy [1]. However, mitochondria are also involved in numerous additional metabolic processes, such as signaling through mitochondrial reactive oxygen species (ROS), hormonal signaling, heme synthesis reactions, steroid synthesis, regulation of membrane permeability, apoptosis-induced cell death, calcium trafficking, and control of cellular metabolism [2][3]. As a result, mitochondrial damage and subsequent malfunction are significant contributing factors to a variety of animal diseases, owing to their influence on cellular metabolism [4][5]. Additionally, ROS can be generated in the cytosol and other cellular compartments, including the plasma membrane, but also the nucleus, peroxisome, endoplasmic reticulum (ER), and Golgi apparatus [6][7][8]. Due to the high contents of polyunsaturated fatty acids (PUFAs) in these membranes [9], lipid peroxidation can occur and, as a result, phospholipids become directly damaged and may also act as a signal for death [10].
Stress, regardless of its source or type (biological, environmental, nutritional, physical, chemical, or psychological), can lead to inflammation and further malicious downstream reactions [11][12][13]. Several synthetic compounds have been developed to significantly lower inflammation, but most of these drugs are accompanied by unwanted side effects, especially when used at higher doses and during long-term therapies. Natural compounds appear to be less compromised by these side effects [14] and, especially in poultry farming, phytogenic feed additives (PFAs) have attracted considerable interest [15]. Generally, the utilization of natural feed additives that contain anti-inflammatory phytochemicals has become very common for the enhancement of productivity, digestive enzymes, nutrient utilization and as an alternative to antibiotics in livestock species and poultry in particular. The phytochemical compounds of interest are diverse in their structures and include polyphenols, flavonoids, terpenoids, alkaloids and plant sterols [16]. In addition to their anti-inflammatory and antioxidant properties, they may also have a number of other effects, including anticancer, antimicrobials, anti-diarrheal, and analgesic actions [17], which in turn enhance the profitability of poultry.
2. Factors for Oxidative Stress and Inflammation in Poultry: Secret Killers
In animal farming, a variety of environmental, nutritional, microbiological, and management factors contribute to oxidative stress. These stressors can be termed as “secret killers”, since they multiply in malignant states in animals [18]. This section focus on the most important factors that are relevant to poultry farming, such as heat stress, dysbiosis and mycotoxins.
During chronic inflammation, an increase in the generation of ROS causes the peroxidation of lipids in cell membranes, as well as mitochondrial and other endomembranes, finally leading to cell death [19]. When these membranes are damaged over time, it is not surprising that multiple cells and organs of an organism are affected [20]. Animal studies [21][22] have established that the complex interactions among diet ingredients, the gut microbiome, the nervous system, the immune system, and the endocrine system are crucial for metabolic and gastrointestinal health. Any disturbances in this delicate equilibrium, such as chronic oxidative stress, result in mitochondrial dysfunction, with its severe impacts upon the immune system and microbiota (see below).
Ninety percent of pathological problems are linked to intestinal chronic inflammation [23]. Disbalance of the gut microbiota has negative effects on the health and biology of metazoans because the gut integrity, biology, metabolism, nutrition, immunity, and neuroendocrine system are all dependent on a healthy microbiota [24][25][26][27][28][29], which is in constant interaction with the microbiota–brain–gut axis. In conclusion, it is justified to qualify oxidative stress and intestinal inflammation as the “secret killers” in animal farming, especially in poultry farming [18][24][30].
2.1. Heat Stress
High temperature is one of the most challenging stressors associated with poultry production [31][32]. It is a serious problem for poultry reared in tropical and subtropical regions, as well as in temperate climate zones, including central and eastern Europe [33]. Heat stress occurs when the ambient temperature exceeds the animal’s thermoneutral zone, and the animal’s physiological capacity to disperse heat through sweating, breathing, or panting fails to prevent a rise in body temperature [34]. Chickens are susceptible to high ambient temperatures due to their feathers, lack of skin sweat glands, and high production of heat, unlike mammals. Chickens lose excess heat by panting to prevent the increase in their body temperature [35]. Heat stress causes several adverse effects on the intestinal mucus layer, tight junctions, enteric immune system, and the antioxidant system [36], which are as follows: (i) a decrease in the size of mucin layers. Heat stress reduces the amount of goblet cells, as well as the expression and secretion of mucins, leading to the thinning of mucin protective layers [37]. As a result, their resistance to opportunistic bacteria decreases and these come in more contact with the intestinal epithelial cells. The following effects are also caused by heat stress: (ii) disruption of tight junctions, as heat stress alters the expression of tight junction protein constituents, such as occludin (OCLN), various claudins (CLDN) and zonula occludens (ZO)-1, -2 and -3 [37][38]; (iii) intestinal barrier dysfunction, as the intestinal hyperpermeability is increased [39][40][41][42]; (iv) endotoxemia and systemic inflammation, which results from the translocation of opportunistic bacteria, endotoxins and lipopolysaccharides (LPS), leading to an increase in pro-inflammatory mediators, such as interleukins (IL-1β, IL-6) and tumor necrosis factor-α (TNF-α) [43]; v) hepatic and hypothalamic inflammation, which mainly results from the translocation of microbial-associated molecular patterns, such as LPS [44]; (vi) redox imbalance between the pro- and antioxidants in favor of pro-oxidants. Heat stress is a key contributor to systemic oxidative stress by increasing the levels of pro-oxidants (e.g., ROS). Several studies have revealed that heat stress leads to higher cellular energy demand, promoting the generation of ROS in the mitochondria [45][46]. Consequently, oxidative stress occurs in multiple tissues, leading to cell apoptosis or necrosis [47].
In summary, heat-induced oxidative stress disrupts the intestinal barrier and alters many cellular processes. Thus, the presence of ROS increases the intestinal permeability, which facilitates the translocation of bacteria and their molecular patterns (e.g., LPS) from the gut (leaky gut syndrome) [48] (see also Figure 1).
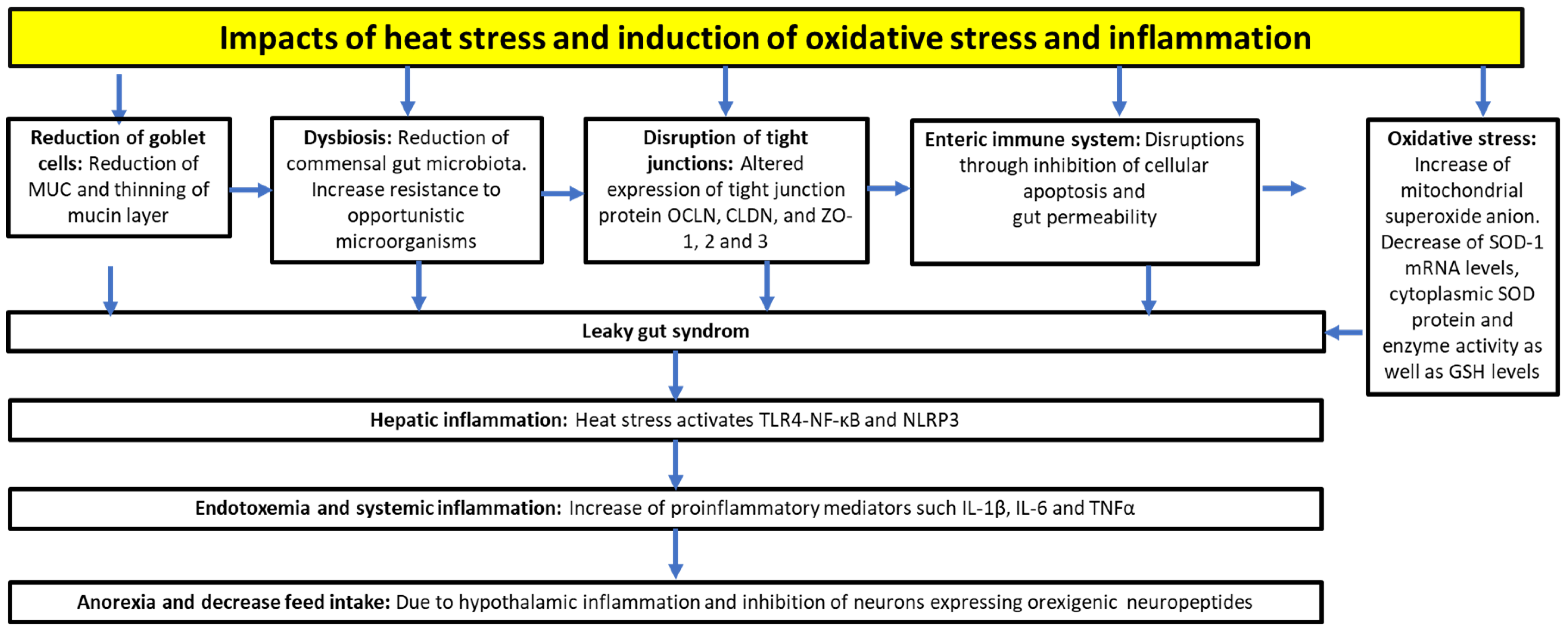
Figure 1. Impacts of heat stress on physiological functions, and induction of inflammation and oxidative stress. OCLN, occludin; CLDN, claudins; ZO, zonula occludens; TLR4, toll-like receptor 4; NF-κB, nuclear factor-kappa B; IL, interleukin; TNFα, tumor necrosis factor α; SOD, superoxide dismutase 1; GSH, glutathione.
2.2. Dysbiosis
Poultry production relies heavily on the animals’ intestinal health and intestinal function to maximize nutrient uptake and growth, which in turn are associated with animal performance. Their gut microbiota mainly consists of bacteria, fungi, and protozoa. As a result of commensal bacteria, intestinal epithelial cells create ROS, which serve as second messengers in cellular signaling. Tight junctions between intestinal epithelial cells form a barrier and prevent the invasion of microorganisms into the host organism [49]. Dysbiosis refers to the alteration in the composition of the gut microbiota with an imbalanced host–microbe relationship [50][51]. As a result, this can lead to increasing amounts of microbial metabolites (see below) that mediate oxidative stress and inflammation (Figure 2).
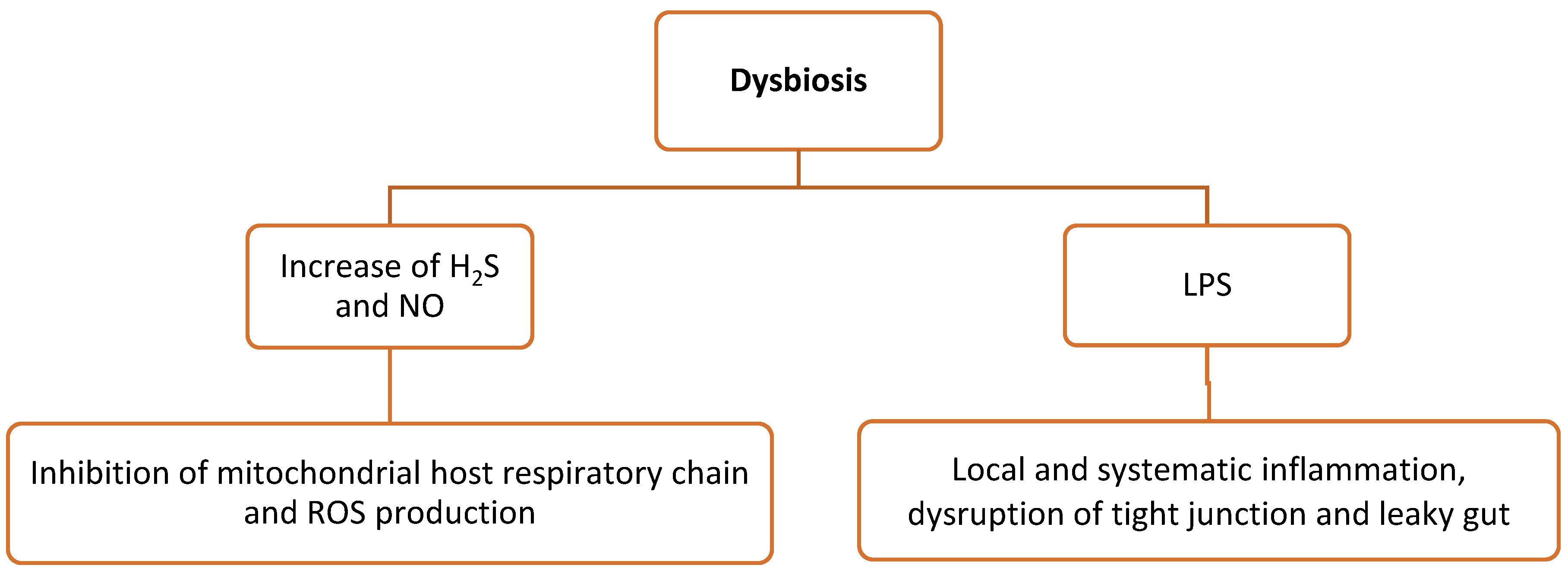
Figure 2. Microbial metabolites during dysbiosis-mediated oxidative stress and inflammation. H2S, hydrogen sulfide; ROS, reactive oxygen species; IL, interleukins; LPS, lipopolysaccharides.
More specifically, ROS are generated in the gut epithelial cells by several ROS stressors that disrupt the redox balance and cause inflammation, which are as follows [21]: (i) NO is produced by the gut microbiota in the intestinal tissues via the conversion of nitrite and nitrate [52]. Excessive production of NO due to dysbiosis generates ROS associated with cellular damages, e.g., due to the inhibition of the host mitochondrial respiratory chain [53]. (ii) Some intestinal bacteria such as E. coli produce hydrogen sulfide (H2S) in high amounts by the degradation of sulfur-containing peptides and amino acids in the gut. In the case of dysbiosis, the elevated H2S concentration inhibits cytochrome oxidase, which in turn inhibits the host mitochondrial respiratory chain and leads to the overexpression of pro-inflammatory factors [54]. However, H2S can also be detoxified by the cecal mucosa by converting it into thiosulfate, which is subsequently converted by ROS into tetrathionate, serving as an electron acceptor for salmonellae, as an example. As a result, a new nutrient niche in the gut is shaped by supporting the growth of more pathogenic bacteria and, thus, increasing dysbiosis and gut inflammation [55][56]. (iii) The TCA cycle can be stimulated by short-chain fatty acids (SCFAs), particularly butyrate. In addition, SCFAs can promote the production of the signaling hormone GLP-1 and the anti-inflammatory IL-10 cytokines to decrease energy intake [54]. (iv) During dysbiosis, LPS production by Gram-negative bacteria is increased and induces local and systematic inflammation by the stimulation of the intestinal epithelial cells and macrophages. As a result, tight junctions are damaged, leading to leaky gut syndrome [57][58][59][60][61][62].
2.3. Mycotoxins
Foods, grains, and animal diets are suitable substrates for a wide array of fungi and molds. In particular, molds such as Aspergillus, Fusarium, and Penicillium species produce their own strain-specific mycotoxins as secondary metabolites and the mycotoxin-contaminated diets have to be discarded [63]. Due to significant economic losses, mycotoxins are a global issue. Aflatoxin B1 (AFB1), deoxynivalenol (DON), nivalenol (NIV), fumonisin B1 (FB1), ochratoxin A (OTA), and zearalenone (ZEN) are the main mycotoxins [64][65][66] (Figure 3).
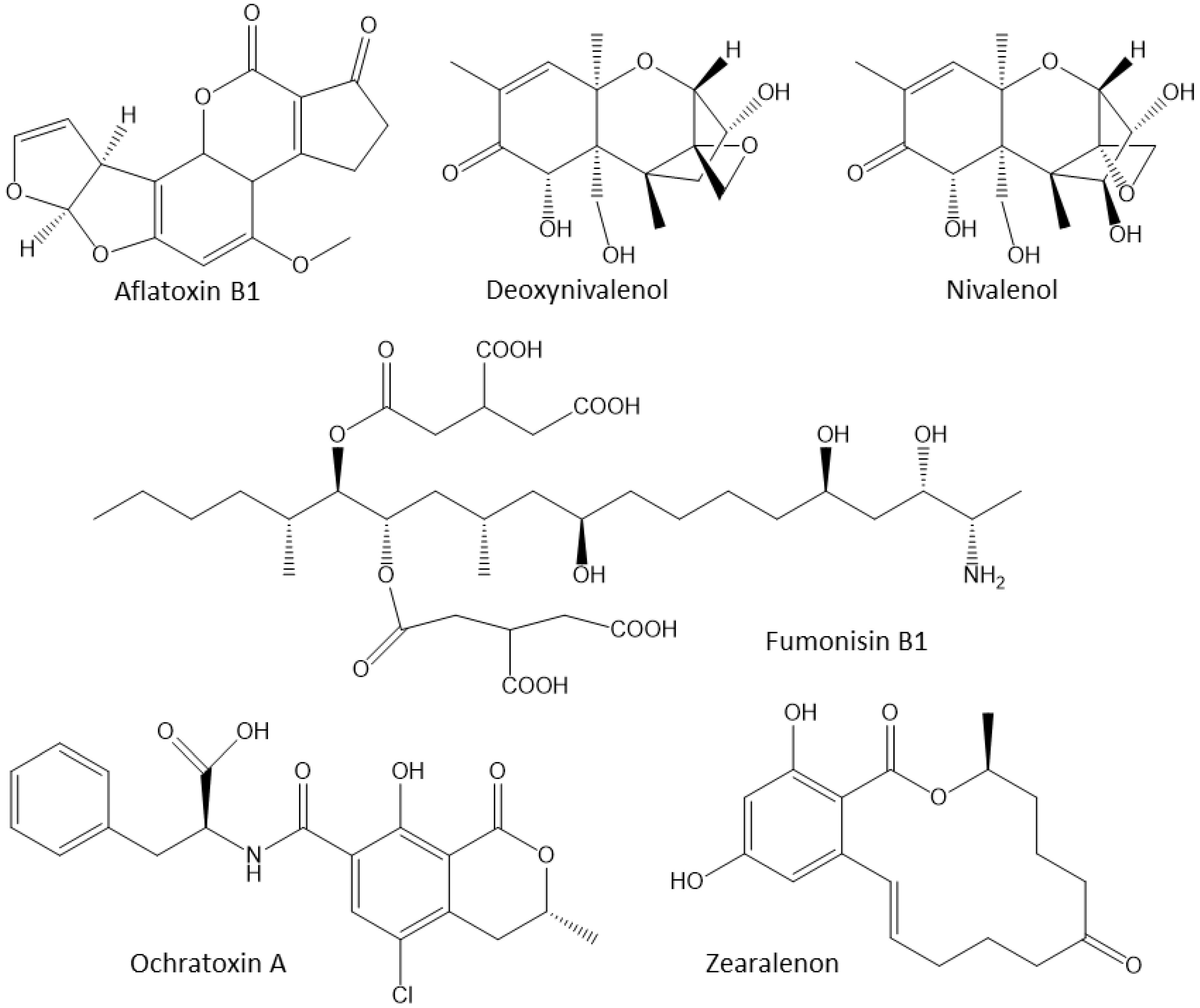
Figure 3. The most common mycotoxins that affect poultry. Aflatoxin B1 (AFB1) and fumonisin B1 (FB1) are polar mycotoxins that are more easily adsorbed by adsorbents than non-polar mycotoxins. Ochratoxin A, T-2 toxin, deoxynivalenol (DON) and zearalenone (ZEN) are non-polar.
In poultry farming, mycotoxins reduce feed intake, feed efficiency, growth performance, immunity, and hatchability [67][68]. The toxins increase mortality, organ damage, carcinogenicity, teratogenicity, and decrease egg production. On a molecular level, mycotoxins induce the generation of ROS, and thereby contribute to lipid peroxidation [69]. They also alter cellular redox signaling, antioxidant status, and membrane integrity [70]. Mycotoxins, particularly aflatoxin, suppress the intracellular levels of antioxidants Nrf2, SOD, GPx and CAT [71][72], and, thus, increase lipid peroxidation and reduce GSH levels [73][74]. The main intracellular endogenous antioxidants and pro-inflammatory cytokines that are associated with oxidative stress mediated by the different mycotoxins (adapted from [75]) are summarized in Table 1.
Table 1. Modulatory effect of mycotoxins on intracellular antioxidants and pro-inflammatory cytokines.
| Mycotoxin | Downregulation of Intracellular Antioxidants | Upregulation of Pro-Inflammatory Cytokines |
|---|---|---|
| AFB1 | Nrf2, CAT, GPx; SOD | Cytokines, NO; NO2 |
| DON | CAT, GPx; SOD | AP-1; ERK-MAPK |
| OTA | Nrf2, CAT, GPx; SOD | Fenton reaction |
| ZEN | CAT, GPx; SOD | CoX-2, cytokines; iNOS |
| T-2 | Nrf2, CAT, GPx, GPx; SOD | Cytokines, iNOS; NO |
AFB1, aflatoxin B1; DON, deoxynivalenol; NIV, nivalenol; FB1, fumonisin B1; OTA, ochratoxin A; ZEN, zearalenon. Nrf2, erythroid 2-related factor 2; CAT, catalase; GPx, glutathione peroxidase; SOD, superoxide dismutase; NO, nitric oxide; NO2, nitrogen dioxide; AP-1, activator protein 1; ERK-MAPK, extracellular signal-regulated kinase-mitogen-activated protein kinase; CoX-2, cyclooygenase-2; iNOS, inducible nitric oxide synthetase.
2.4. Diet-Mediated Oxidative Stress
The supplementation of poultry diets with oils that are high in PUFAs is common as an efficient source of energy and as a means to increase palatability, to improve pellet quality, and to enhance the absorption of fat-soluble vitamins [76][77]. As mentioned earlier, PUFAs have a faster oxidation rate than saturated fats, meaning that they will become rancid more quickly. This is due to the oxidation of the reactive double bonds, which allows molecular oxygen to react with these moieties [78]. A number of additional factors, such as light exposure, the presence of catalytic transition metal ions, and high temperature during feed pelleting and storage, can lead to the production of free radicals, which in turn lead to lipid autoxidation [79][80]. The oxidation of lipids results in the production of more reactive substances, which exhibit potentially biological harmful effects and give the product an undesirable odor [81][82][83][84]. Notably, even mild oxidation can produce biologically reactive and toxic oxidation products. Lipid peroxidation results in a variety of degradation products, such as peroxides, aldehydes, and polar compounds that are differentially absorbed and metabolized. Peroxidation varies depending on the temperature, the duration of the thermal processing steps, and the composition of the oil. In this regard, feeding poultry with peroxidized oils that contain inadequate supplies of endogenous antioxidants may lead to in vivo metabolic oxidative stress [85][86][87][88]. As a result of this oxidative stress, ROS and free radical products cannot be converted into less reactive species by antioxidants and antioxidant enzymes, resulting in tissue-damaging free radicals that bind to lipids, proteins, and DNA [89] (see above). Indeed, it was demonstrated that, during the consumption of oxidized oils, reactive aldehydes accumulate in the stomach, which are adsorbed into the small intestine, where they are concentrated and metabolized in the liver [90]. Broilers that received oxidized oils had a slower growth rate, and the animals’ plasma and tissues had higher thiobarbituric acid reactive substances (TBARS) as a marker of lipid damage and a low quantity of antioxidants [91].
References
- Quintana-Cabrera, R.; Mehrotra, A.; Rigoni, G.; Soriano, M.E. Who and how in the regulation of mitochondrial cristae shape and function. Biochem. Biophys. Res. Commun. 2018, 500, 94–101.
- Mannella, C.A. The relevance of mitochondrial membrane topology to mitochondrial function. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2006, 1762, 140–147.
- Papadopoulos, V.; Miller, W.L. Role of mitochondria in steroidogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 771–790.
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723.
- Maechler, P. Mitochondrial signal transduction in pancreatic β-cells. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 739–752.
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-Imetabolism: Implications for human disease. Biochim. Biophys. Acta 2012, 1822, 1363–1373.
- Li, J.-M.; Shah, A.M. Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1014–R1030.
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950.
- Nowak, J.Z. Oxidative Stress, Polyunsaturated Fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: Focus on age-related macular degeneration. Pharmacol. Rep. 2013, 65, 288–304.
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425.
- Kiecolt-Glaser, J.K. Stress, food, and inflammation: Psychoneuroimmunology and nutrition at the cutting edge. Psychosom. Med. 2010, 72, 365–369.
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616.
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797.
- Mahesh, G.; Anil Kumar, K.; Reddanna, P. Overview on the discovery and development of anti-inflammatory drugs: Should the focus be on synthesis or degradation of PGE2? J. Inflamm. Res. 2021, 14, 253–263.
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86 (Suppl. S14), E140–E148.
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic Compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48.
- Achilonu, M.C.; Umesiobi, D.O. Bioactive phytochemicals: Bioactivity, sources, preparations, and/or modifications via silver tetrafluoroborate mediation. J. Chem. 2015, 2015, 629085.
- Tellez-Isaias, G.; Eisenreich, W.; Shehata, A.A. Nutraceuticals to mitigate the secret killers in animals. Vet. Sci. 2022, 9, 435.
- Bickler, S.W.; Prieto, J.M.; Cauvi, D.M.; De Cos, V.; Nasamran, C.; Ameh, E.; Amin, S.; Nicholson, S.; Din, H.; Mocumbi, A.O.; et al. Differential Expression of nuclear genes encoding mitochondrial proteins from urban and rural populations in Morocco. Cell Stress Chaperones 2020, 25, 847–856.
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62.
- Mishra, B.; Jha, R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60.
- Zhao, H.; He, Y.; Li, S.; Sun, X.; Wang, Y.; Shao, Y.; Hou, Z.; Xing, M. Subchronic arsenism-induced oxidative stress and inflammation contribute to apoptosis through mitochondrial and death receptor dependent pathways in chicken immune organs. Oncotarget 2017, 8, 40327–40344.
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Research 2020, 9, 69.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol Rev. 2010, 90, 859–904.
- Dimitrov, D.V. The human gutome: Nutrigenomics of the host-microbiome interactions. OMICS 2011, 15, 419–430.
- Fukui, H.; Xu, X.; Miwa, H. Role of gut microbiota-gut hormone axis in the pathophysiology of functional gastrointestinal disorders. J. Neurogastroenterol. Motil. 2018, 24, 367–386.
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The microbiota-gut-brain axis and Alzheimer’s disease: Neuroinflammation is to blame? Nutrients 2020, 13, 37.
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521.
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9.
- Stecher, B. The Roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Microbiol. Spectr. 2015, 3, 3.
- Lara, L.; Rostagno, M. Impact of heat stress on poultry production. Animals 2013, 3, 356–369.
- ICAR-National Institute of Abiotic Stress Management; Pawar, S.S.; Basavaraj, S.; Dhansing, L.V.; Pandurang, K.N.; Sahebrao, K.A.; Vitthal, N.A.; Pandit, B.M.; Kumar, B.S. Assessing and mitigating the impact of heat stress in poultry. Adv. Anim. Vet. Sci. 2016, 4, 332–341.
- Hirakawa, R.; Nurjanah, S.; Furukawa, K.; Murai, A.; Kikusato, M.; Nochi, T.; Toyomizu, M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020, 7, 46.
- Mount, L.E. Heat transfer between animal and environment. Proc. Nutr. Soc. 1978, 37, 21–27.
- Lasiewski, R. Physiological responses to heat stress in the poorwill. Am. J. Physiol.-Leg. Content 1969, 217, 1504–1509.
- Ortega, A.D.S.V.; Szabó, C. Adverse effects of heat stress on the intestinal integrity and function of pigs and the mitigation capacity of dietary antioxidants: A review. Animals 2021, 11, 1135.
- Yi, H.; Xiong, Y.; Wu, Q.; Wang, M.; Liu, S.; Jiang, Z.; Wang, L. Effects of Dietary supplementation with L-arginine on the intestinal barrier function in finishing pigs with heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1134–1143.
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701.
- Alhenaky, A.; Abdelqader, A.; Abuajamieh, M.; Al-Fataftah, A.-R. The effect of heat stress on intestinal integrity and salmonella invasion in broiler birds. J. Therm. Biol. 2017, 70, 9–14.
- Mercer, E.H.; Singh, A.P. Endosymbiosis and cellular tolerance in the hawaiian soft coral sarcothelia edmondsoni verrill. Adv. Exp. Med. Biol. 1975, 64, 69–76.
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat Stress Reduces Intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215.
- Wu, Q.J.; Liu, N.; Wu, X.H.; Wang, G.Y.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683.
- Yu, Q.; Tang, C.; Xun, S.; Yajima, T.; Takeda, K.; Yoshikai, Y. MyD88-dependent signaling for IL-15 production plays an important role in maintenance of CD8 alpha alpha TCR alpha beta and TCR gamma delta intestinal intraepithelial lymphocytes. J. Immunol. 2006, 176, 6180–6185.
- von Meyenburg, C.; Hrupka, B.H.; Arsenijevic, D.; Schwartz, G.J.; Landmann, R.; Langhans, W. Role for CD14, TLR2, and TLR4 in bacterial product-induced anorexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, 298–305.
- Altan, Ö.; Pabuçcuoğlu, A.; Altan, A.; Konyalioğlu, S.; Bayraktar, H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003, 44, 545–550.
- Kumar, B. Stress and its impact on farm animals. Front. Biosci. 2012, E4, 1759–1767.
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.J.; Tersteeg-Zijderveld, M.H.G.; Koolmees, P.A.; Fink-Gremmels, J. Quantitative histo-morphometric analysis of heat-stress-related damage in the small intestines of broiler chickens. Avian Pathol. 2015, 44, 19–22.
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914.
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776.
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. 2016, 160, 461–466.
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 2022, 10, 395.
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971.
- Tse, J.K.Y. Gut microbiota, nitric oxide, and microglia as prerequisites for neurodegenerative disorders. ACS Chem. Neurosci. 2017, 8, 1438–1447.
- Saint-Georges-Chaumet, Y.; Edeas, M. Microbiota–mitochondria inter-talk: Consequence for microbiota–host interaction. Pathog. Dis. 2016, 74, ftv096.
- Winter, S.E.; Thiennimitr, P.; Winter, M.G.; Butler, B.P.; Huseby, D.L.; Crawford, R.W.; Russell, J.M.; Bevins, C.L.; Adams, L.G.; Tsolis, R.M.; et al. Gut inflammation provides a respiratory electron acceptor for salmonella. Nature 2010, 467, 426–429.
- Tsolis, R.M.; Bäumler, A.J. Gastrointestinal host-pathogen interaction in the age of microbiome research. Curr. Opin. Microbiol. 2020, 53, 78–89.
- Faralli, A.; Shekarforoush, E.; Ajalloueian, F.; Mendes, A.C.; Chronakis, I.S. In vitro permeability enhancement of curcumin across CACO-2 cells monolayers using electrospun xanthan-chitosan nanofibers. Carbohydr. Polym. 2019, 206, 38–47.
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin Improves intestinal barrier function: Modulation of Intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, 438–445.
- Baumgard, L.H.; Rhoads, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337.
- Pearce, S.C.; Mani, V.; Weber, T.E.; Rhoads, R.P.; Patience, J.F.; Baumgard, L.H.; Gabler, N.K. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J. Anim. Sci. 2013, 91, 5183–5193.
- Gilani, S.; Chrystal, P.V.; Barekatain, R. Current experimental models, assessment and dietary modulations of intestinal permeability in broiler chickens. Anim. Nutr. 2021, 7, 801–811.
- Kvidera, S.K.; Dickson, M.J.; Abuajamieh, M.; Snider, D.B.; Fernandez, M.V.S.; Johnson, J.S.; Keating, A.F.; Gorden, P.J.; Green, H.B.; Schoenberg, K.M.; et al. Intentionally Induced intestinal barrier dysfunction causes inflammation, affects metabolism, and reduces productivity in lactating Holstein cows. J. Dairy Sci. 2017, 100, 4113–4127.
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol. Res. 2019, 35, 1–7.
- Murugesan, G.R.; Ledoux, D.R.; Naehrer, K.; Berthiller, F.; Applegate, T.J.; Grenier, B.; Phillips, T.D.; Schatzmayr, G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci. 2015, 94, 1298–1315.
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19.
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74.
- Wang, R.J.; Fui, S.X.; Miao, C.H.; Feng, D.Y. Effects of different mycotoxin adsorbents on performance, meat characteristics and blood profiles of avian broilers fed mold contaminated corn. Asian Australas. J. Anim. Sci. 2005, 19, 72–79.
- Ghareeb, K.; Awad, W.A.; Böhm, J.; Zebeli, Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: Poultry and swine: Effect of deoxynivalenol on gut health. J. Appl. Toxicol. 2015, 35, 327–337.
- Niemiec, J.; Borzemska, W.; Goliński, P.; Karpińska, E.; Szeleszczuk, P.; Celeda, T. The effect of ochratoxin A on egg quality, development of embryos and the level of toxinin eggs and tissues of hens and chicks. J. Anim. Feed Sci. 1994, 3, 309–316.
- Longobardi, C.; Andretta, E.; Romano, V.; Lauritano, C.; Avantaggiato, G.; Schiavone, A.; Jarriyawattanachaikul, W.; Florio, S.; Ciarcia, R.; Damiano, S. Effects of some new antioxidants on apoptosis and ROS production in AFB1 treated chickens. Med. Sci. Forum 2020, 2, 12.
- Liu, Y.; Wang, W. Aflatoxin B1 Impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes: AFB1 on apoptosis and Nrf2 pathway in PBHs. Anim. Sci. J. 2016, 87, 1490–1500.
- Wang, W.-J.; Xu, Z.-L.; Yu, C.; Xu, X.-H. Effects of aflatoxin B1 on mitochondrial respiration, ROS generation and apoptosis in broiler cardiomyocytes. Anim. Sci. J. 2017, 88, 1561–1568.
- Ma, Q.; Li, Y.; Fan, Y.; Zhao, L.; Wei, H.; Ji, C.; Zhang, J. Molecular mechanisms of lipoic acid protection against aflatoxin b₁-induced liver oxidative damage and inflammatory responses in broilers. Toxins 2015, 7, 5435–5447.
- Maurya, B.K.; Trigun, S.K. Fisetin modulates antioxidant enzymes and inflammatory factors to inhibit aflatoxin-B1 induced hepatocellular carcinoma in rats. Oxidative Med. Cell. Longev. 2016, 2016, 1972793.
- da Silva, E.O.; Bracarense, A.P.F.L.; Oswald, I.P. Mycotoxins and oxidative stress: Where are we? World Mycotoxin J. 2018, 11, 113–134.
- Mousa, S.A.; Abdel-Raheem, S.M.; Abdel-Raheem, H.A.; Sadeek, A.L.S. Effect of dietary fat sources and antioxidant types on growth performance and carcass quality of japanese quails. Int. J. Poult. Sci. 2017, 16, 443–450.
- Attia, Y.A.; Al-Harthi, M.A.; Abo El-Maaty, H.M. The effects of different oil sources on performance, digestive enzymes, carcass traits, biochemical, immunological, antioxidant, and morphometric responses of broiler chicks. Front. Vet. Sci. 2020, 7, 181.
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and omega-6 Fatty acids in poultry nutrition: Effect on production performance and health. Animals 2019, 9, 573.
- Labuza, L.R.; Dugan, J.R. Kinetics of lipid oxidation in foods critical reviews in food science and nutrition. Crit. Rev. Food Sci. Nutr. 1971, 2, 355–405.
- St. Angelo, A.J. (Ed.) Lipid Oxidation in Food; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; Volume 500, ISBN 978-0-8412-2461-2.
- Goicoechea, E.; Brandon, M.H.; Blokland, M.D. Guillén Fate in digestion in vitro of several food components, including some toxic compounds coming from omega-3 and omega-6 lipids. Food Chem. Toxicol. 2011, 49, 115–124.
- Goicoechea, E.; Guillén, M.D. Volatile compounds generated in corn oil stored at room temperature. presence of toxic compounds. Eur. J. Lipid Sci. Technol. 2014, 116, 395–406.
- Grigorakis, K.; Giogios, I.; Vasilaki, A.; Nengas, I. Effect of the fish oil, oxidation status and of heat treatment temperature on the volatile compounds of the produced fish feeds animal feed science and technology. Anim. Feed Sci. Technol. 2010, 158, 73–84.
- Hammouda, I.B.; Zribi, A.; Mansour, A.B.; Bouaziz, M. Effect of deep-frying on 3-MCPD esters and glycidyl esters contents and quality control of refined olive pomace blended with refined palm oil. Eur. Food Res. Technol. 2017, 243, 1219–1227.
- Takahashi, K.; Akiba, Y. Effect of oxidized fat on perfor mance and some physiological responses in broiler chickens. Jpn. Poult. Sci. 1999, 36, 304–310.
- Anjum, M.I.; Mirza, I.H.; Khan, A.G.; Azim, A. Effect of fresh versus oxidized soybean oil on growth performance, or gans weights and meat quality of broiler chicks. Pakistan Vet. J. 2004, 24, 173–178.
- Tavárez, M.A.; Boler, D.D.; Bess, K.N.; Zhao, J.; Yan, F.; Dilger, A.C.; McKeith, F.K.; Killefer, J. Effect of antioxidant inclusion and oil quality on broiler performance, meat quality, and lipid oxidation. Poult. Sci. 2011, 90, 922–930.
- Boler, D.D.; Fernández-Dueñas, D.M.; Kutzler, L.W.; Zhao, J.; Harrell, R.J.; Campion, D.R.; McKeith, F.K.; Killefer, J.; Dilger, A.C. Effects of oxidized corn oil and a synthetic antioxidant blend on performance, oxidative status of tissues, and fresh meat quality in finishing barrows. J. Anim. Sci. 2012, 90, 5159–5169.
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257.
- Kanazawa, K.; Ashida, H. Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochim. Biophys. Acta 1998, 1393, 349–361.
- Engberg, R.M.; Lauridsen, C.; Jensen, S.K.; Jakobsen, K. Inclusion of oxidized vegetable oil in broiler diets. its influence on nutrient balance and on the antioxidative status of broilers. Poult. Sci. 1996, 75, 1003–1011.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
780
Revisions:
2 times
(View History)
Update Date:
02 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




