Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Subathra Devi C. | -- | 2395 | 2023-01-31 12:13:59 | | | |
| 2 | Rita Xu | Meta information modification | 2395 | 2023-02-01 02:32:13 | | | | |
| 3 | Rita Xu | -4 word(s) | 2391 | 2023-02-01 04:04:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nair, S.R.; C, S.D. Serratiopeptidase. Encyclopedia. Available online: https://encyclopedia.pub/entry/40663 (accessed on 14 January 2026).
Nair SR, C SD. Serratiopeptidase. Encyclopedia. Available at: https://encyclopedia.pub/entry/40663. Accessed January 14, 2026.
Nair, Sreelakshmi R., Subathra Devi. C. "Serratiopeptidase" Encyclopedia, https://encyclopedia.pub/entry/40663 (accessed January 14, 2026).
Nair, S.R., & C, S.D. (2023, January 31). Serratiopeptidase. In Encyclopedia. https://encyclopedia.pub/entry/40663
Nair, Sreelakshmi R. and Subathra Devi. C. "Serratiopeptidase." Encyclopedia. Web. 31 January, 2023.
Copy Citation
Microbial products have been used for the treatment of different diseases for many centuries. The serratiopeptidase enzyme provides a new hope for COVID-19-infected patients. Anti-inflammatory drugs are easy to obtain at minimal expenditure from microbial sources. Serratia sp. is identified as one of the most efficient bacteria produced from serratiopeptidase. Screening for new and efficient bacterial strains from different sources has been of interest. Serratiopeptidase remains the most well-known anti-inflammatory drug of choice. Serratiopeptidase is a cheaper and safer anti-inflammatory drug alternative to NSAIDs.
Serratia sp.
serratiopeptidase
anti-inflammatory
COVID-19
1. Introduction
Nowadays, enzymes are used as an alternative drug of choice to treat many ailments. De Duve [1] was the first to suggest that enzymes can be an alternative treatment for hereditary diseases. Trypsin, α-chymotrypsin, prozyme, and bromelain are the most commonly administered oral anti-inflammatory enzymes [2]. Serratiopeptidase is one of the most dominant anti-inflammatory drugs, with numerous therapeutic applications. The enzyme has anti-inflammatory, anti-biofilm, mucolytic, fibrinolytic, and wound-healing properties. The enzyme has a molecular size of 52kDa, and has the ability to bind with alpha-2-macroglobulin in blood at a ratio of 1:1 [3]. It is widely used in treating carpal tunnel syndrome, arthritis, fibrocystic breast disease, bronchitis, and sinusitis [4]. Serratiopeptidase has a strong affinity for cyclooxygenase (COX) I and II, which are crucially linked with interleukin (IL), prostaglandin (PGs), and thromboxane (TXs) production [5]. Drugs such as NSAIDs (nonsteroidal anti-inflammatory drugs), either alone or in combination with other medicines, are the most often prescribed treatment for acute inflammation [5]. They act on bonds between Arg and Gly, CysSO3H and Gly, Asn and Gln, Tyr and Tyr, His and Leu, Gly and Ala, Ala and Leu, Tyr and Leu, Gly and Gly, Phen and Tyr, and Tyr and Thr. This helps with reducing inflammation and controls the release of interleukins, thromboxanes, and prostaglandins. Serratiopeptidase has a long history of use as a therapeutic enzyme, and its demand in industries has been satisfied by wild, recombinant, and mutated strains. Serratiopeptidase has also been used in the treatment of Alzheimer’s disease. The enzyme has the ability to degrade amyloid plaques. In vivo studies on rat models have shown that the enzyme is capable of fighting against Alzheimer’s disease, as it helped in amyloid fibrin degradation [6].
2. The Enzyme and Its Properties
Japanese researchers were the first to report and introduce the anti-inflammatory drug serratiopeptidase to the world. Enzyme formulations were created, and were widely used as medicines. After 1970, these enzyme formulations were eventually successfully marketed worldwide. The clinical studies carried out by researchers in Europe and Japan suggested serratiopeptidase as a potent anti-inflammatory drug [7][8]. Hence, the demand for enzyme began increasing worldwide. Serratiopeptidase is a metalloprotease enzyme with a molecular weight of 45–60 kDa. The enzyme contains zinc at the active site. Serratiopeptidase belongs to the group serralysin and has an EC number of 3.4.24.40. [7]. The enzyme consists of 470 amino acids which are important for its proteolytic activity. The enzyme is devoid of sulfur-containing amino acids such as cysteine and methionine. Serratiopeptidase showed maximum activity at pH 9 and 40 °C, and can be inactivated at 55 °C for 15 min [8][9].
2.1. Anti-Inflammatory Action of Serratiopeptidase
Inflammation is an innate immune response that causes redness, swelling, and pain in the human body. It is regarded as a response of the human body against any irritant, and can be caused by many reasons, such as pathogens, injuries, and damage of cells [3]. Hence, inflammation can be regarded as a healing mechanism of our bodies to maintain homeostasis [10]. It has been observed that NSAIDs are the most commonly used drugs for inflammation [7][10]. Anti-inflammatory drugs can interact with (cyclooxygenase) COX-I and COX-II molecules. Among these enzymes, COX-I is responsible for the breakdown of arachidonic acid, which is responsible for the production of interleukins and prostaglandins [9][11]. Serine proteases are known to have a great affinity for these molecules, and can act as anti-inflammatory agents [3]. The enzymes regulated inflammatory cytokines, modified cell adhesion molecules, and acted at the site of inflammation [4][9]. In the absence of this enzyme, pain and swelling occurred at the area of injury and initiated the release of prostaglandins. (Figure 1a). This led to the onset of cascade reactions. Serratiopeptidase has the ability to bind with cyclooxygenase and suppress the release of interleukins and prostaglandins. (Figure 1b). The oral administration of serratiopeptidase tablets reduce pain and inflammation. The enzyme has its mode of action on arachidonic acid pathway (COX I and COX II), and acts on the cyclooxygenase pathway, but not on the lipooxygenase pathway (LOX). The lipooxygenase pathway (LOX) is involved in the regulation of inflammation by mediating the catalysis of SPM (specialized pro-resolving mediators) biosynthesis, and non-specific NSAID inhibition [11].
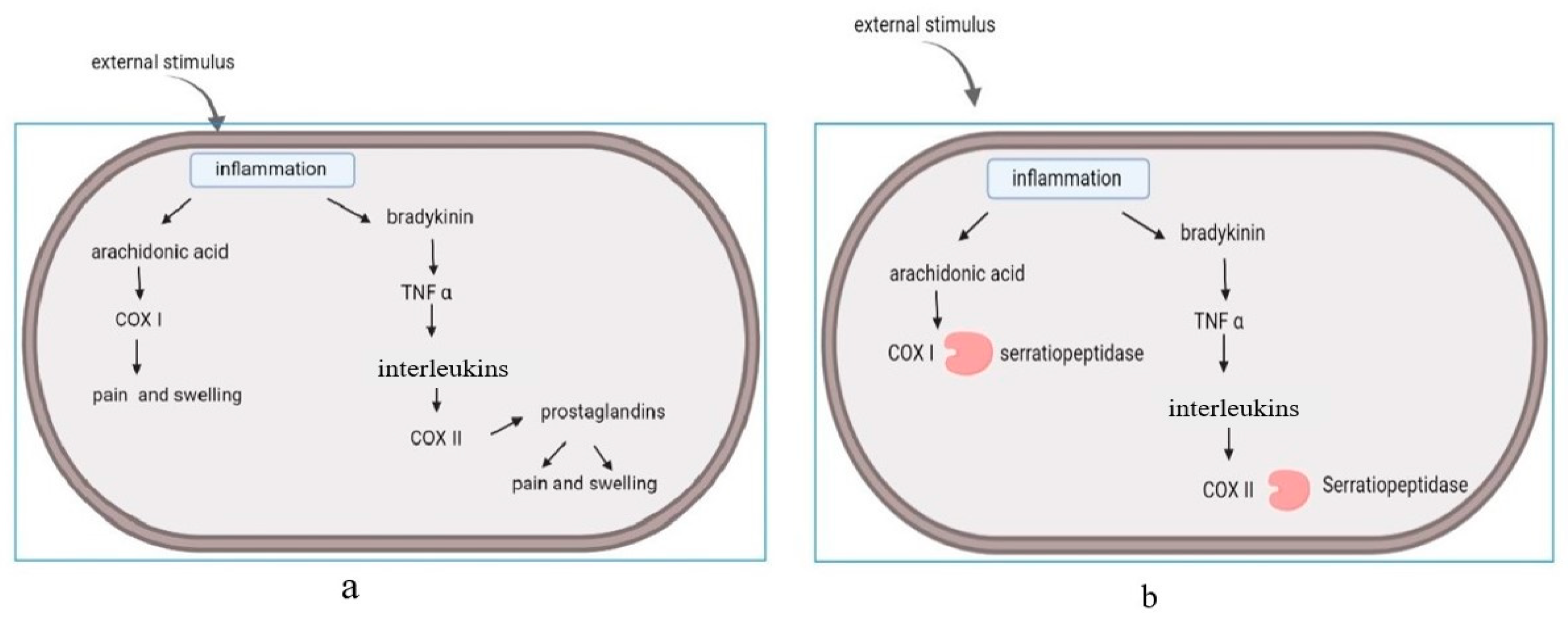
Figure 1. Arachidonic acid pathway: (a) release of interleukins and prostaglandins induce the pain and swelling. (b) Mode of action: serratiopeptidase acts on the cyclooxygenase enzyme (COX I and COX II) and suppresses the release of interleukins and prostaglandins.
2.2. Wound-Healing Activity of Serratiopeptidase
In addition to the anti-inflammatory property, the enzyme also helps in wound healing. The enzyme acts by dissolving the dead tissue around the wound and hydrolyses bradykinin, serotonin, and histamine. This improves the microcirculation at the site of injury and results in wound healing [12]. There are four phases in a typical wound healing mechanism. These include the hemostasis phase, the inflammatory phase, the proliferative phase, and the maturation phase [12][13]. This enzyme can enhance microcirculation and help to maintain hemostasis [14]. Serratiopeptidase is known to reduce the capillary permeability induced by histamine, bradykinin, and serotonin, and has the ability to break the abnormal exudates and proteins as well as to improve the absorption of decomposed products through blood and lymphatics [13]. Serratiopeptidase, along with metronidazole, was found to be effective in improving wound healing in rabbits [15]. Another finding regarding serratiopeptidase was related to the tissue repair mechanism. At the site of an inflamed wound, the enzyme assisted in reducing the amount of fluids drained to the wound and facilitated microcirculation, hence improving tissue repair [13]. In a recent comparative study, the effectiveness of an enteric-coated tablet comprising fixed-dose combination (FDC) of trypsin 48 mg, bromelain 90 mg, and rutoside trihydrate 100 mg with serratiopeptidase 10 mg was observed. The results showed that serratiopeptidase was less effective than trypsin, bromelain, and rutoside trihydrate [16]. One reason for the lower efficiency may be a low dosage. A higher concentration of the drug may be more stable at gastric pH, and can facilitate the healing process.
2.3. Antibiofilm Activity of Serratiopeptidase
In biofilms, serratiopeptidase can alter the pathogenic phenotype of a bacterium. The use of dispersion agents may improve the effectiveness of current therapeutics. The enzymatic agents dispersin B, lysostaphin, alpha amylase, V8 protease, and serratiopeptidase were tested against methicillin-resistant and susceptible strains of S. aureus biofilms, both individually and in combination with vancomycin and rifampicin. When coupled with any of the dispersal agents, the effectiveness of the antibiotics was increased. Lysostaphin and serratiopeptidase were found to be the most effective dispersion agents against all of the tested strains [14]. Serratiopeptidase, a proteolytic enzyme, was originally suggested by Selan et al. [17] for the treatment of biofilm-related illnesses nearly twenty years ago. Most recently, an S. epidermidis (a high-slime-producing strain) infected rat model was treated with an intramuscular injection of serratiopeptidase. It was noted that 94.4% of the infected mice were recovered when compared to 62.5% in the group treated with antibiotics [18]. In the in vivo animal models, serratiopeptidase effectively acted against bacteria that produced biofilms. The antibiofilm function of enzyme may enhance the effectiveness of antibiotics in reducing Staphylococcal infections [18].
Another observation regarding the serratiopeptidase enzyme based on its anti-biofilm activity was against a fully matured Staphylococcus aureus biofilm [19]. The researchers constructed an Spep mutant by replacing the glutamic acid in the catalytic site with another amino acid (alanine), and evaluated the anti-biofilm activity of the Spep mutant. The research reports revealed that there was no proteolytic activity for the mutant strain; nevertheless, it was able to retain its anti-biofilm activity [19]. Serratiopeptidase is known to exhibit the property of modifying the adhesion molecules and thereby reducing the cell surface proteins [19]. Selan et al. [20] reported that the enzyme could alter the biofilm association of virulent strains, and that it showed activity against a completely developed biofilm. Biofilms are normally difficult to destroy. Serratiopeptidase, in combination with other antibiotics, exhibited potent anti-bioflim activity. The serratiopeptidase enzyme has reduced the expression of Listeria monocytogens cell surface proteins such as Ami4b, internalin B, Act A, and autolysin. The enzyme significantly precluded the adhesion of Listeria monocytogens in the human digestive tract [21]. According to previous reports, interestingly, it was found that the enzyme has the ability to interact only with the cell adhesion molecules that formed the biofilm. No cytotoxic activity was recorded [19][20]. The enzyme showed its effect on discrete surface proteins such as At1. It can act on these surface proteins by altering adhesins and autolysins. In a study reported by Artini et al. [22], it was stated that serratiopeptidase and carboxypeptidase showed activity against biofilm formation of different strains of Staphylococcus aureus and Staphylococcus epidermidis. The test results of the previous studies showed that only serratiopeptidase inhibited the activity of all strains. The enzyme has the ability to modify the phenotype of virulent bacteria and enhance anti-bacterial properties [22]. Another interesting fact was reported regarding the enzyme: it regulates the recruitment of immune cells to the site of inflammation [23]. The efficacy of serratiopeptidase against biofilm-forming bacteria was proven in experimental animal models. The enzyme serratiopeptidase increased the effectiveness of antibiotics in the treatment of Staphylococcal infections [18]. The enzyme can be supplemented with antibiotics for more effective medication.
2.4. Mucolytic Activity of Serratiopeptidase
Sputum production, nasal congestion, and cough are observed as some of the prevalent symptoms in COVID-19 patients. Mucolytics can increase bronchial mucus output or decrease mucus viscosity and make it easier to cough up the mucus. Serratiopeptidase may be helpful due to its caseinolytic and mucolytic effects on sputum. In patients with respiratory disorders, serratiopeptidase has improved mucociliary transportability and mucociliary clearance by lowering neutrophils and modifying the viscoelasticity of sputum [24]. Research has revealed a new combination therapy for COVID-19. A combination of vitamin D and serratiopeptidase acts as a strong mucolytic agent, and has the ability to fight against the severe effects of COVID-19 syndrome [25]. Kim et al. [26] has detailed the occurrence of other symptoms such as rhinorrhoea, hypogeusia, and nasal congestion in a large number of patients. Treatment methods such as administration of bronchodilators and mucolytic agents, along with tracheal suction, were the remedial measures for such patients [26]. Several proteolytic enzymes are known to act in a synchronized manner in the control and coordination mechanism of viral entry, viral propagation, and establishment in host cells [27]. The serratiopeptidase enzyme plays a vital role in the treatment of COVID-19 infection. Sharma et al. [28] has conferred the possibility of serratiopeptidase being used as a mucolytic drug in COVID-19 patients. It was found that serratiopeptidase can inhibit the cytokine storm in COVID-19 patients. The elevated expression of transforming growth factor (TGF-α), IL-6, and other chemokines may lead to cytokine storms in COVID-19 patients. Increased levels of IL-6 may cause acute lung disorders. This condition can be treated with medicines. Serratiopeptidase has been suggested as an effective medicine to treat the severe complications of COVID-19 [28].
3. Therapeutic Aspects of Serratiopeptidase
The anti-inflammatory effects of serratiopeptidase, aspirin, trypsin, and chymotrypsin in Albino rats against carrageenan-induced paw edoema were compared by Viswanatha, Swamy, and Patil [29]. In both acute and subacute types of inflammation in rats, serratiopeptidase had superior anti-inflammatory action both on its own and in combination with aspirin. Along with a histological analysis, several inflammatory indicators, such as C-reactive protein, glutathione, myeloperoxidase, and nitric oxide, were found. When compared to the control group, serratiopeptidase decreased the disease activity index and stopped the formation of nitric oxide, as well as colonic shortening, glutathione depletion, spleen enlargement, and lipid peroxidation. Serratiopeptidase-treated mice had significantly lower C-reactive protein levels than the control mice. Moreover, the use of serratiopeptidase decreased myeloperoxidase, a significant enzyme marker of inflammation. These findings support serratiopeptidase’s ability to reduce inflammation, and thus it has been recognized as a multi-channel enzyme in terms of its wide application in treatments [30]. The enzyme has been successfully applied in atherosclerosis, in which plaques in arteries were dissolved by the proteolytic action of the enzyme. When compared with other enzymes, serratiopeptidase has been successfully used in ortholaryngiology [31]. Researchers have reported the fibrinolytic activity of serratiopeptidase and successfully used it in fibrinolytic therapy [29]. Another known application of serratiopeptidase is in dental implantation, where soft and hard gums developed inflammation upon peri implants, and anti-inflammatory enzymes were used as a treatment [31]. Serine proteases, along with other drugs, are commonly used in orthopedic medicines to treat chronic inflammation, pain, and swelling. The enzyme has great affinity with COX I and COX II, which are pain mediators [1]. An appropriate study on dosage of the enzyme must be conducted in order to control levels of the enzyme concentration in plasma, as it was found that the amount of enzymes in blood varies with body mass [32]. In 2022, it was reported that the enzyme was not able to bind with LOX or to block lipoxygenase-catalyzed biosynthesis of specialized pro-resolving mediators [33]. A pre-clinical study reported by Jadav et al. [34] indicated that serratiopeptidase was orally effective, and had anti-inflammatory activity which was equivalent to diclofenac sodium in both chronic and acute phases of inflammation. Serratiopeptidase can be used to treat osteoarthritis in combination with metformin. Ateia et al. [35] reported the impact of metformin and serratiopeptidase on knee osteoarthritis in obese patients. Metformin and serratiopeptidase combination tablets were efficient in the treatment of knee osteoarthritis. Ai-Khateeb and Nusair’s [32]. clinical study reports revealed the effect of serratiopeptidase in pain reduction, trismus, and post-operative swelling after molar surgery. Small studies in the field of dentistry, otorhinolaryngology, and orthopaedics have revealed reductions in pain and inflammation for ailments such as carpal tunnel syndrome, arthritis, and tooth extraction. Serratiopeptidase tablets have also been used in the treatment of pneumonitis, joint pain, and dermatitis. According to clinical case reports, serratiopeptidase did not show many adverse effects in treated patients [8]. Very few studies have been reported on the anti-cancer activity of serratiopeptidase. The in vitro cytotoxic activity of serratiopeptidase against colon cancer cell lines (Caco-2) was reported by Araghi et al. [36]. The findings of previous reports suggested that the enzyme has anti-cancer potential, but further in vitro and in vivo mechanistic pathway studies are needed in order to confirm the biological activity of the enzyme.
References
- De Duve, C. The significance of lysosomes in pathology and medicine. Proc. Inst. Med. Chic. 1966, 26, 73–76.
- Tasaka, K.; Meshi, T.; Akagi, M.; Kakimoto, M.; Saito, R.; Okada, I.; Maki, K. Anti-Inflammatory Activity of a Proteolytic Enzyme, Prozime-10. Pharmacology 1980, 21, 43–52.
- Devi, C.S.; Elizabeth, J.R.; Saravanan, H.; Naine, S.J.; Srinivansan, V.M. Screening and molecular characterization of Serratia mar-cescens VITSD2: A strain producing optimum serratiopeptidase. Front. Biol. 2013, 8, 632–639.
- Mohankumar, A.; Raj, R.K. Production and Characterization of Serratiopeptidase Enzyme from Serratia Marcescens. Int. J. Biol. 2011, 3, 39.
- Steiger, S.; Harper, J.L. Mechanisms of Spontaneous Resolution of Acute Gouty Inflammation. Curr. Rheumatol. Rep. 2013, 16, 392.
- Metkar, S.K.; Girigoswami, A.; Vijayashree, R.; Girigoswami, K. Attenuation of subcutaneous insulin induced amyloid mass in vivo using Lumbrokinase and Serratiopeptidase. Int. J. Biol. Macromol. 2020, 163, 128–134.
- Maeda, H.; Morihara, K. Serralysin and related bacterial proteinases. Methods Enzymol. 1995, 248, 395–413.
- Gupte, V.; Luthra, U. Analytical techniques for serratiopeptidase: A review. J. Pharm. Anal. 2017, 7, 203–207.
- Bhagat, S.; Agarwal, M.; Roy, V. Serratiopeptidase: A systematic review of the existing evidence. Int. J. Surg. 2013, 11, 209–217.
- Suma, K.C.; Manasa, H.; Likhitha, A.; Nagamani, T.S. Isolation, Purification, and Characterization of Serratiopeptidase Enzyme from Serratia marcescens. Int. J. Innov. Sci. Res. Technol. 2020, 5, 156–161.
- Srivastava, S.; Singh, D.; Patel, S.; Singh, M.R. Treatment of rheumatoid arthritis by targeting macrophages through folic acid tailored superoxide dismutase and serratiopeptidase. J. Drug Deliv. Sci. Technol. 2017, 41, 431–435.
- Teller, P.; White, T.K. The Physiology of Wound Healing: Injury Through Maturation. Perioper. Nurs. Clin. 2011, 6, 159–170.
- Strodtbeck, F. Physiology of wound healing. Newborn Infant Nurs. Rev. 2001, 1, 43–52.
- Hogan, S.; Zapotoczna, M.; Stevens, N.; Humphreys, H.; O’Gara, J.; O’Neill, E. Potential use of targeted enzymatic agents in the treatment of Staphylococcus aureus biofilm-related infections. J. Hosp. Infect. 2017, 96, 177–182.
- Rath, G.; Johal, E.S.; Goyal, A.K. Development of Serratiopeptidase and Metronidazole Based Alginate Microspheres for Wound Healing. Artif. Cells Blood Substit. Biotechnol. 2010, 39, 44–50.
- Mungantiwar, A.; Bhatt, N.; Shrivastava, P.; More, J.; Shaikh, R. A Randomized, Open-Label, Phase IV Clinical Study to Compare the Safety and Efficacy of the Fixed-Dose Combination of Trypsin, Bromelain, and Rutoside versus Serratiopeptidase in Minor Sur-gical Wound. IJRAMT. 2021, 2, 308–313.
- Selan, L.; Berlutti, F.; Passariello, C.; Comodi-Ballanti, M.R.; Thaller, M.C. Proteolytic enzymes: A new treatment strategy for prosthetic infections? Antimicrob. Agents Chemother. 1993, 37, 2618–2621.
- Mecikoglu, M.; Saygi, B.; Yildirim, Y.; Karadag-Saygi, E.; Ramadan, S.S.; Esemenli, T. The effect of proteolytic enzyme serratiopep-tidase in the treatment of experimental implant-related infection. JBJS. 2006, 88, 1208–1214.
- Selan, L.; Papa, R.; Tilotta, M.; Vrenna, G.; Carpentieri, A.; Amoresano, A.; Pucci, P.; Artini, M. Serratiopeptidase: A well-known metallo-protease with a new non-proteolytic activity against S. aureus biofilm. BMC Microbiol. 2015, 15, 207.
- Selan, L.; Artini, M.; Papa, R. Compounds from natural sources for new diagnostics and drugs against biofilm infections. In Microbial Biofilms—Importance and Applications; Dhanasekaran, D., Ed.; 2016; pp. 487–509. Available online: https://www.intechopen.com/chapters/50474 (accessed on 13 July 2016).
- Longhi, C.; Scoarughi, G.L.; Poggiali, F.; Cellini, A.; Carpentieri, A.; Seganti, L.; Pucci, P.; Amoresano, A.; Cocconcelli, P.S.; Artini, M.; et al. Protease treatment affects both invasion ability and biofilm formation in Listeria monocytogenes. Microb. Pathog. 2008, 45, 45–52.
- Artini, M.; Papa, R.; Scoarughi, G.L.; Galano, E.; Barbato, G.; Pucci, P.; Selan, L. Comparison of the action of different proteases on virulence properties related to the staphylococcal surface. J. Appl. Microbiol. 2012, 114, 266–277.
- Tiwari, M. The role of serratiopeptidase in the resolution of inflammation. Asian J. Pharm. Sci. 2017, 12, 209–215.
- Gioia, M.; Ciaccio, C.; Calligari, P.; De Simone, G.; Sbardella, D.; Tundo, G.; Fasciglione, G.F.; Di Masi, A.; Di Pierro, D.; Bocedi, A.; et al. Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches. Biochem. Pharmacol. 2020, 182, 114225.
- Gupta, K.K.; Rahman, A.; Kumar, A.; Gavel, P.; Asia, P. Adjuvant therapy with Serratiopeptidase and Vitamin D for COVID-19 patients: A new perspective. Int. J. Med. Sci. 2021, 4, 282–287.
- Kim, G.-U.; Kim, M.-J.; Ra, S.; Lee, J.; Bae, S.; Jung, J.; Kim, S.-H. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin. Microbiol. Infect. 2020, 26, 948.e1–948.e3.
- Farooqi, F.I.; Morgan, R.C.; Dhawan, N.; Dinh, J.; Yatzkan, G.; Michel, G. Airway Hygiene in COVID-19 Pneumonia: Treatment Responses of 3 Critically Ill Cruise Ship Employees. Am. J. Case Rep. 2020, 21, e926596–e926596-8.
- Sharma, C.; Jha, N.K.; Meeran, M.N.; Patil, C.R.; Goyal, S.N.; Ojha, S. Serratiopeptidase, a serine protease anti-inflammatory, fibrinolytic, and mucolytic drug can be a useful adjuvant for management in COVID-19. Front. Pharmacol. 2021, 12, 1188.
- Swamy, A.V.; Patil, P. Effect of some clinically used proteolytic enzymes on inflammation in rats. Indian J. Pharm. Sci. 2008, 70, 114–117.
- Rajinikanth, B.; Venkatachalam, V.V.; Manavalan, R. Investigations on the potential of serratiopeptidase—A proteolytic enzyme, on acetic acid induced ulcerative colitis in mice. Int. J. Pharm. Pharm. Sci. 2014, 6, 525–531.
- Jadhav, S.B.; Shah, N.; Rathi, A.; Rathi, V.; Rathi, A. Serratiopeptidase: Insights into the therapeutic applications. Biotechnol. Rep. 2020, 28, e00544.
- Al-Khateeb, T.; Nusair, Y. Effect of the proteolytic enzyme serrapeptase on swelling, pain and trismus after surgical extraction of mandibular third molars. Int. J. Oral Maxillofac. Surg. 2008, 37, 264–268.
- Luthra, U.; Babu, P.; Patel, Y.; Ramesh, J.V.; Sharma, M.; Majeed, I.; Subbiah, S.K.; Pandiyan, R. Serratiopeptidase: A statistical approach towards enhancement of fermentation and biomass product recovery. Biomass-Convers. Biorefinery 2022, 1–8.
- Jadav, S.P.; Patel, N.H.; Shah, T.G.; Gajera, M.V.; Trivedi, H.R.; Shah, B.K. Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J. Pharmacol. Pharmacother. 2010, 1, 116–117.
- Ateia, Y.A.; Al-Edanni, M.S.; Al-Qurtas, M.I. Impact of metformin and serratiopeptidase in obese patients with knee osteoarthritis. Int. J. Pharm. Pharm. Sci. 2018, 10, 37.
- Araghi, A.; Hashemi, S.; Sepahi, A.A.; Faramarzi, M.A.; Amin, M. Purification and study of anti-cancer effects of Serratia mar-cescens ser-ralysin. Iran J. Microbiol. 2019, 11, 320.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.0K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
3 times
(View History)
Update Date:
01 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




