
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | CHARLES VIEIRA NEVES | -- | 3726 | 2023-01-30 13:30:27 | | | |
| 2 | Camila Xu | -1 word(s) | 3725 | 2023-01-31 03:00:16 | | |
Video Upload Options
Microplastic pollution in aquatic ecosystems has drawn attention not only because microplastics are likely to accumulate anywhere but also because they cause negative impacts both to aquatic biota and, indirectly, to public health, as a result of their presence. Between diffuse and point sources, there are a variety of paths for microplastics to migrate through riverine environments, traverse transition environments between fresh and salty water sites and finally enter sea waters then accumulate along marine environments. On the other hand, plastic particles can also be transferred to other environments through percolation through the soil until they reach subterranean reservoirs and, finally, emerge in the most different ecosystems.
1. Microplastic Sources
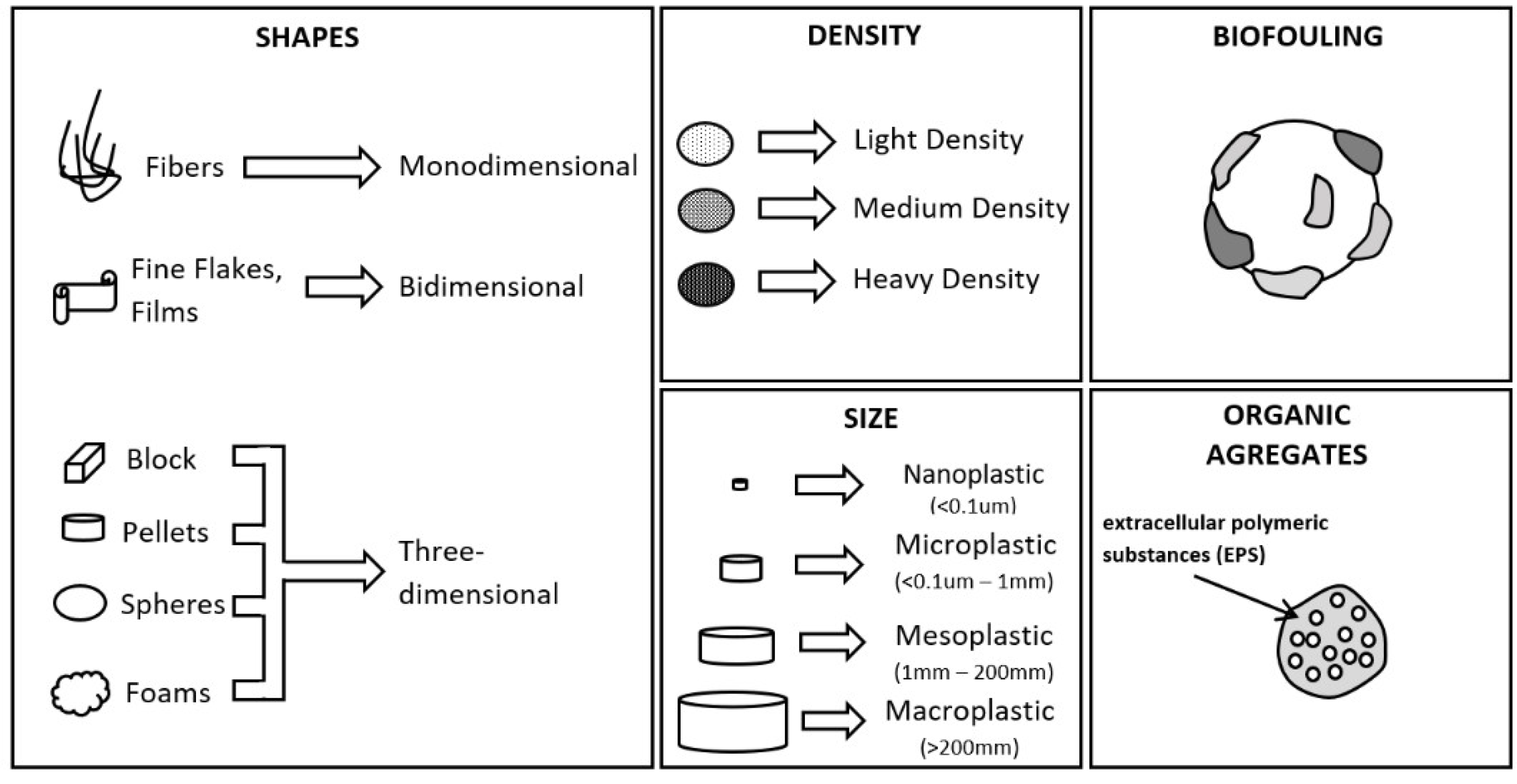
2. Characteristics of Microplastics That Influence Their Transport Dynamics
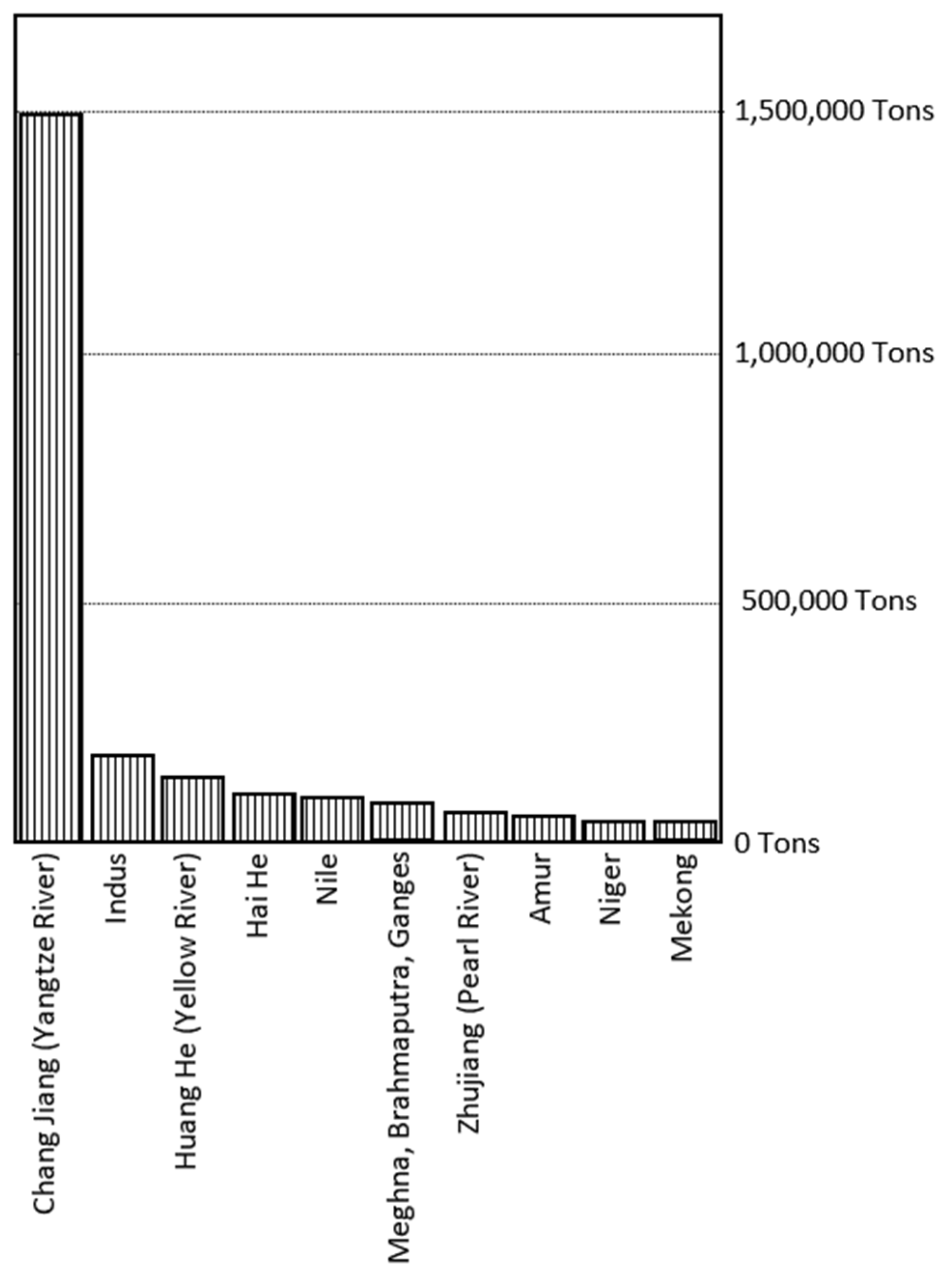
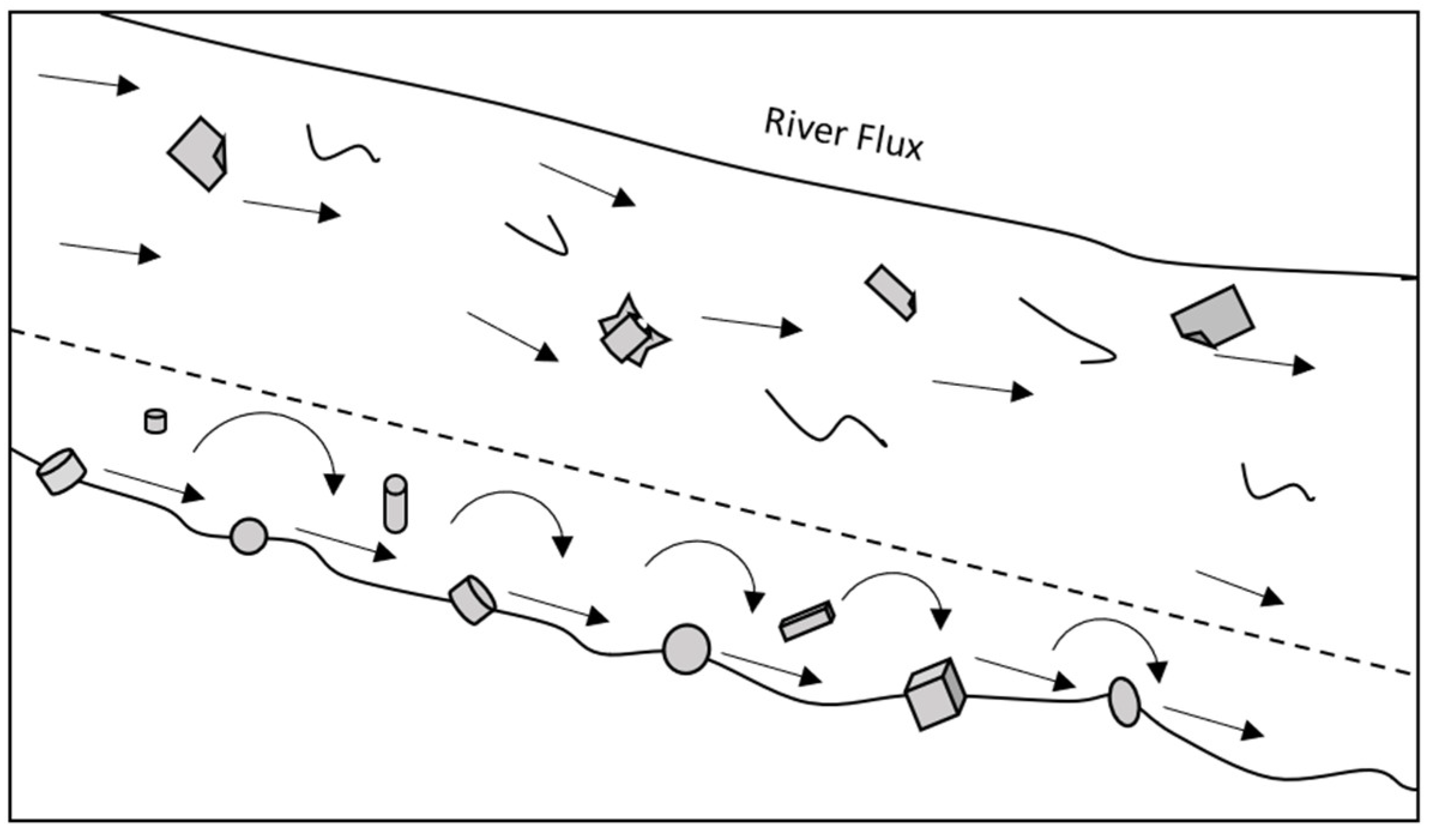
3. Riverine Transport
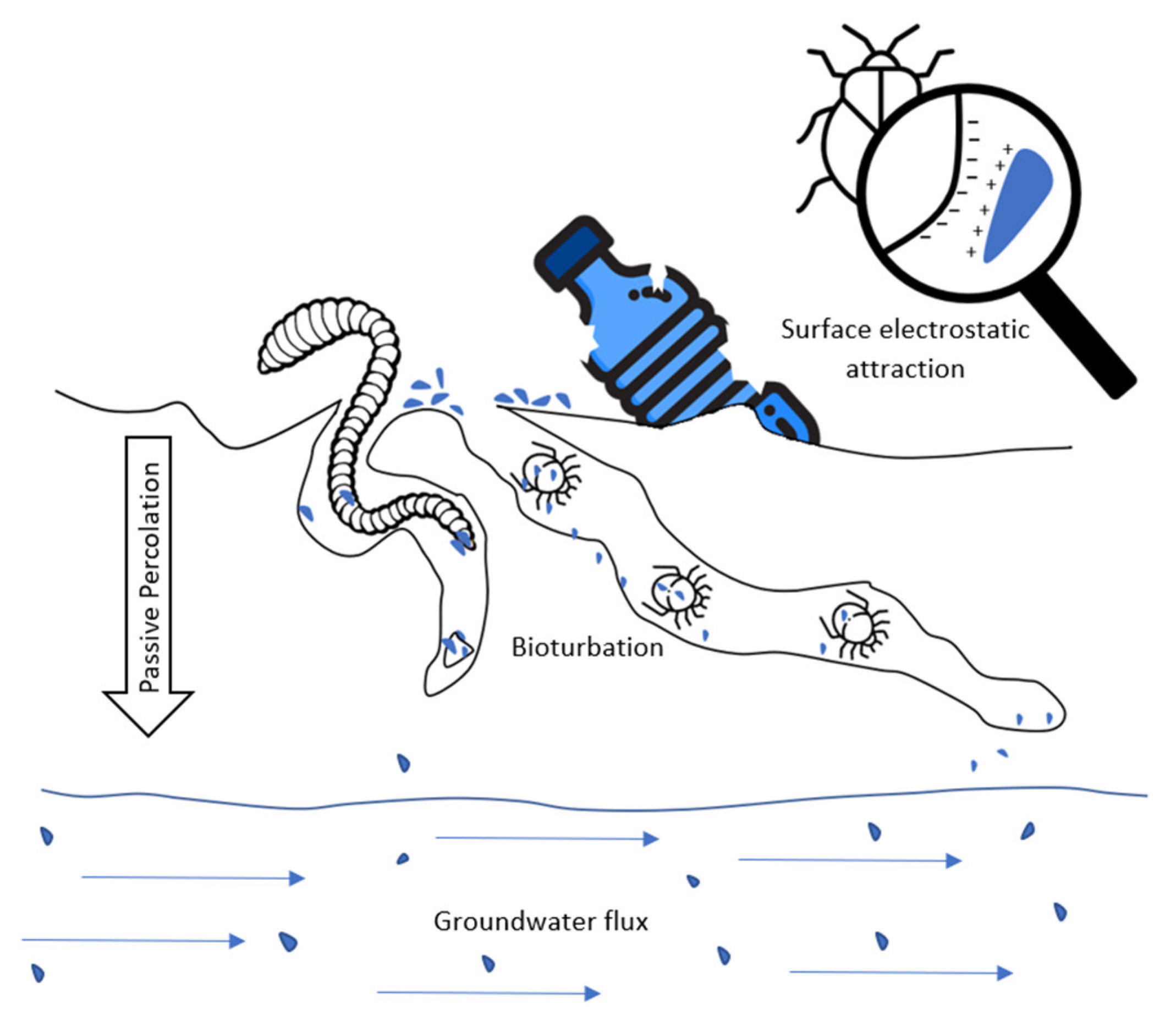
4. Underground Water Transport
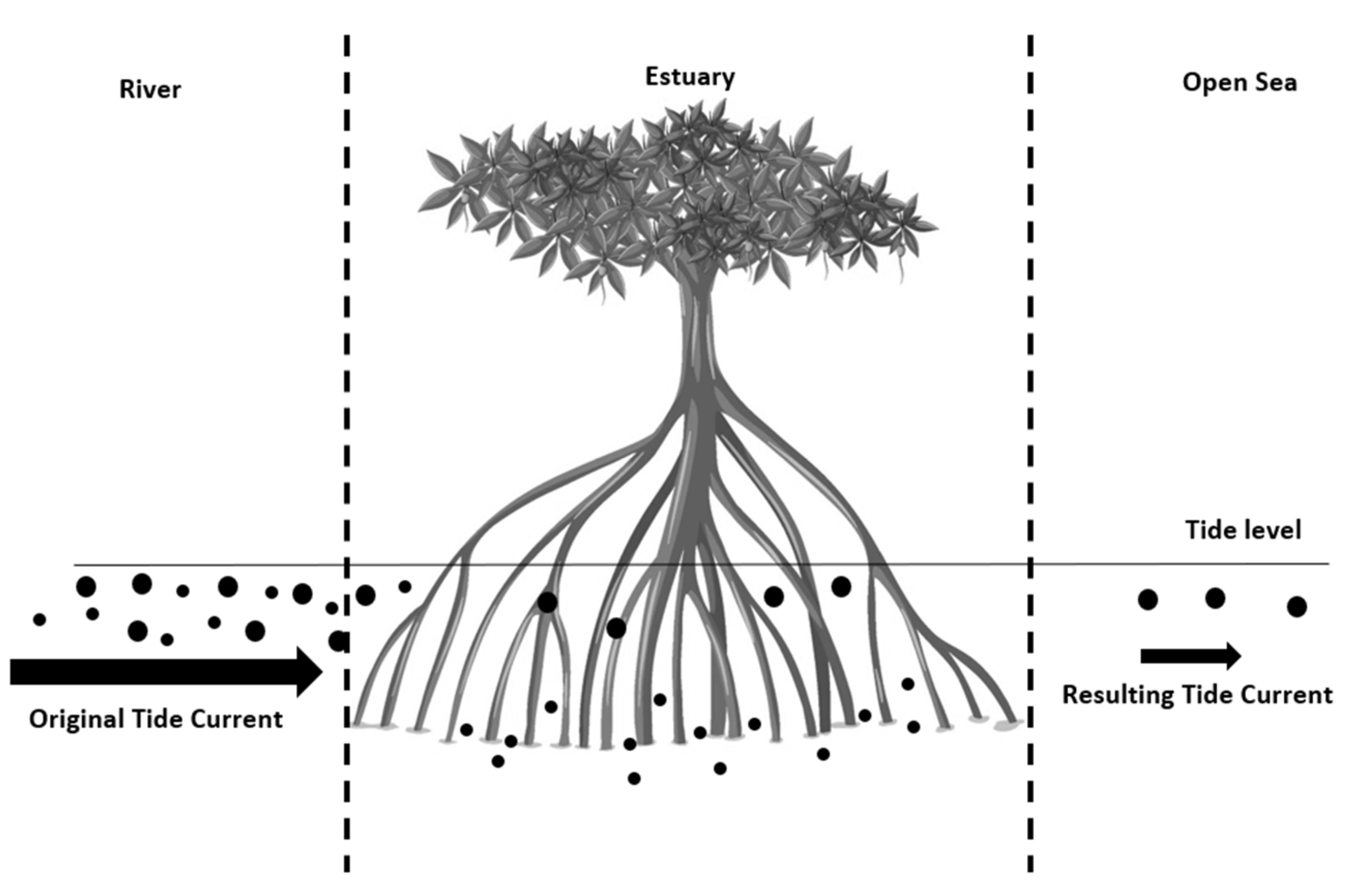
5. Estuarine Transport
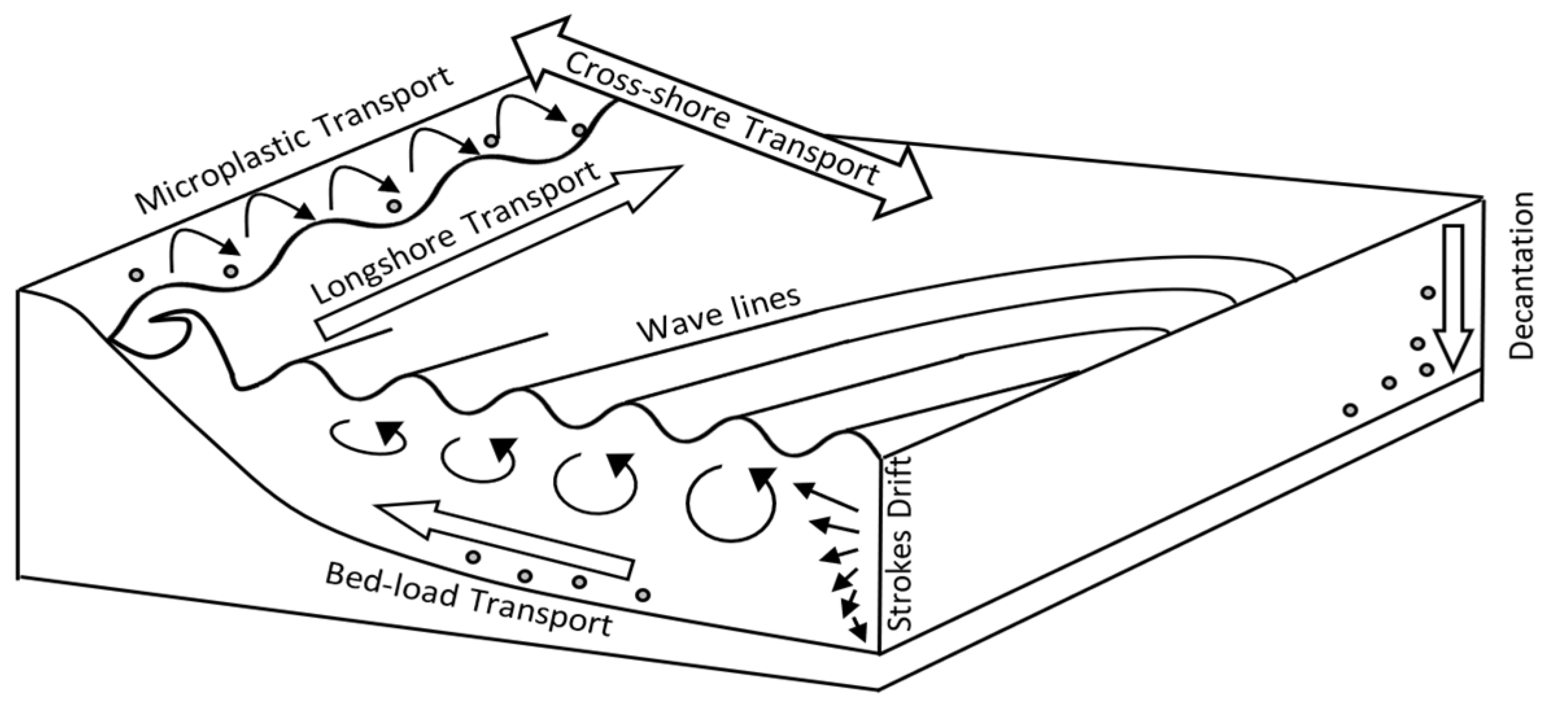
6. Sea Coast Transport
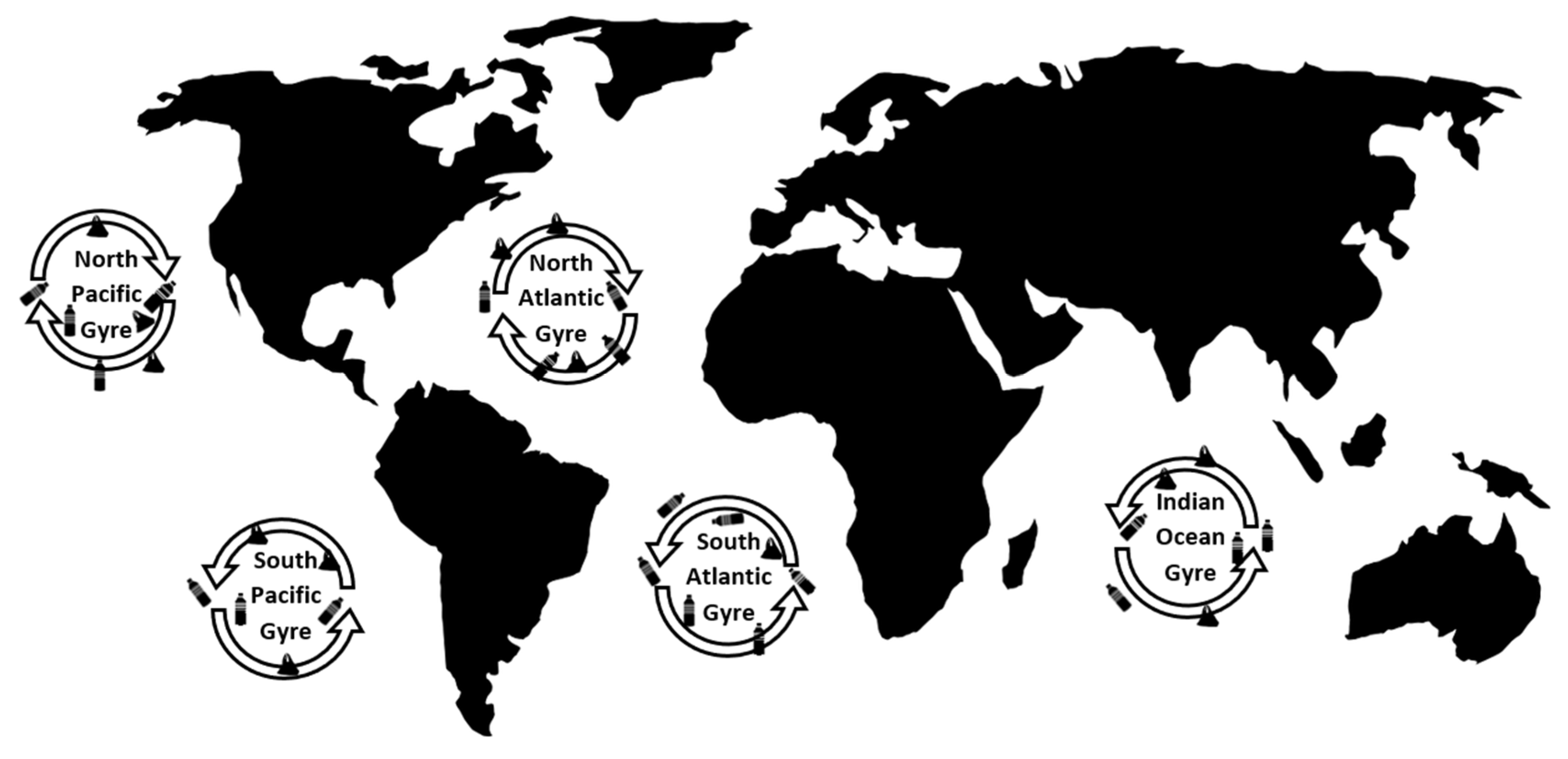
7. Oceanic Transport
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771.
- Sundt, P.; Schulze, P.-E.; Syversen, F. Sources of Microplastics-Pollution to the Marine Environment; Report M-321, 2015, Mepex; Norwegian Environment Agency: Trondheim, Norway, 2014.
- Lassen, C.; Hansen, S.F.; Magnusson, K.; Noren, F.; Bloch Hartmann, N.I.; Jensen, P.R.; Nielsen, T.G.; Brinch, A. Microplastics—Occurrence, Effects and Sources of Releases to the Environment in Denmark; The Danish Environmental Protection Agency: Copenhagen, Denmark, 2015.
- Gaylard, C.C.; Baptista Neto, J.A.; Fonseca, E.M. Paint fragments as polluting microplastics: A brief review. Mar. Pollut. Bull. 2020, 162, 9.
- Gaylard, C.; Baptista Neto, J.A.; Fonseca, E.M. Plastic microfibre pollution: How important is clothes’ laundering? Heliyon 2021, 1, 1.
- Ivar Do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364.
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in four estuarine rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 2014, 48, 14195–14202.
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605.
- EPA. Methods to Manage and Control Plastic Wastes; Report to the Congress/530-SW-89-051; Environmental Protection Agency: Washington, DC, USA, 1990.
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment 90; Report Stud. GESAMP No. 90, 96; GESAMP: London, UK, 2015.
- Allen, S.; Allen, D.; Phoenix, V.; Le Roux, G.; Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344.
- Napper, I.E.; Baroth, A.; Barrett, A.C.; Bhola, S.; Chowdhury, G.W.; Davies, B.F.; Duncan, E.M.; Kumar, S.; Nelms, S.E.; Niloy, M.N.H. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 2021, 274, 116348.
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499.
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838.
- Ryan, P.G. Does size and buoyancy affect the long-distance transport of floating debris? Environ. Res. Lett. 2015, 10, 84019.
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic debris in Great Lakes tributaries: Relations to watershed attributes and hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385.
- Cózar, A.; Martí, E.; Duarte, C.M.; García-de-Lomas, J.; Van Sebille, E.; Ballatore, T.J.; Eguíluz, V.M.; González-Gordillo, J.I.; Pedrotti, M.L.; Echevarría, F.; et al. The Arctic Ocean as a dead end for floating plastics in the North Atlantic branch of the Thermohaline Circulation. Sci. Adv. 2017, 3, 1600582.
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; Van Harmelen, T. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Mar. Pollut. Bull. 2019, 143, 92–100.
- Zhang, H. Transport of microplastics in coastal seas. Estuar. Coast. Shelf Sci. 2017, 199, 74–86.
- Van Sebille, E.; Aliani, S.; Law, K.L.; Maximenko, N.; Alsina, J.M.; Bagaev, A.; Bergmann, M.; Chapron, B.; Chubarenko, I.; Cózar, A.; et al. The physical oceanography of the transport of floating marine debris. Environ. Res. Lett. 2020, 15, 23003.
- Shamskhany, A.; Li, Z.; Patel, P.; Karimpour, S. Evidence of Microplastic Size Impact on Mobility and Transport in the Marine Environment: A Review and Synthesis of Recent Research. Front. Mar. Sci. 2021, 8, 760649.
- Ballent, A.; Pando, S.; Purser, A.; Juliano, M.; Thomsen, L. Modelled transport of benthic marine microplastic pollution in the Nazaré Canyon. Biogeoscience 2013, 10, 7957–7970.
- Ballent, A.; Purser, A.; de Jesus Mendes, P.; Pando, S.; Thomsen, L. Physical transport properties of marine microplastic pollution. Biogeosci. Discuss. 2012, 9, 18755–18798.
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319.
- Zbyszewski, M.; Corcoran, P.L.; Hockin, A. Comparison of the distribution and degradation of plastic debris along shorelines of the Great Lakes, North America. J. Great Lakes Res. 2014, 40, 288–299.
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On some physical and dynamical properties of microplastic particles in marine environment. Mar. Pollut. Bull. 2016, 108, 105–112.
- Kukulka, T.; Proskurowski, G.; Morét-Ferguson, S.; Meyer, D.; Law, K. The effect of wind mixing on the vertical distribution of buoyant plastic debris. Geophys. Res. Lett. 2012, 39, 1–6.
- Isobe, A.; Kubo, K.; Tamura, Y.; Nakashima, E.; Fujii, N. Selective transport of microplastics and mesoplastics by drifting in coastal waters. Mar. Pollut. Bull. 2014, 89, 324–330.
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Aliani, S. The mediterranean plastic soup: Synthetic polymers in mediterranean surface waters. Sci. Rep. 2016, 6, 1–10.
- Di, M.; Wang, J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616, 1620–1627.
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; a meta-analysis. J. Hazard. Mater. 2019, 369, 691–698.
- Knutsen, H.; Cyvin, J.B.; Lilleeng, Ø.; Totland, C.; Wade, E.J.; Castro, V.; Pettersen, A.; Laugesen, J.; Møskeland, T.; Arp, H.P.H. Microplastic accumulation by tube-dwelling, suspension feeding polychaetes from the sediment surface: A case study from the Norwegian Continental Shelf. Mar. Environ. Res. 2020, 161, 105073.
- Rodríguez-Seijo, A.; Pereira, R. Morphological and physical characterization of microplastics. In Comprehensive Analytical Chemistry Series; Rocha-Santos, T.A.P., Duarte, A.C., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2017; pp. 49–66.
- Jung, J.W.; Park, J.W.; Eo, S.; Young, J.C.; Song, K.; Sang, Y.C.; Hong, H.; Shim, W.J. Ecological risk assessment of microplastics in coastal, shelf, and deep-sea waters with a consideration of environmentally relevant size and shape. Environ. Pollut. 2021, 270, 116217.
- Reisser, J.; Slat, B.; Noble, K.; Du Plessis, K.; Epp, M.; Proietti, M.; Sonneville, J.; Becker, T.; Pattiaratchi, C. The vertical distribution of buoyant plastics at sea: An observational study in the North Atlantic Gyre. Biogeosciences 2015, 12, 1249–1256.
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gunnar, G. High quantities of microplastic in Arctic deep-sea sediments from the Hausgarten observatory. Environ. Sci. Technol. 2017, 51, 11000–11010.
- Heitmuller, F.T.; Hudson, P.F. Downstream trends in sediment size and composition of channel-bed, bar, and bank deposits related to hydrologic and lithologic controls in the Llano River watershed, central Texas, USA. Geomorphology 2009, 112, 246–260.
- Yang, H.; Shi, C. Sediment grain-size characteristics and its sources of ten wind-water coupled erosion tributaries (the Ten Kongduis) in the Upper Yellow River. Water 2019, 11, 115.
- Filella, M. Questions of size and numbers in environmental research on microplastics: Methodological and conceptual aspects. Environ. Chem. 2015, 12, 527–538.
- Sagawa, N.; Kawaai, K.; Hinat, H. Abundance and size of microplastics in a coastal sea: Comparison among bottom sediment, beach sediment, and surface water. Mar. Pollut. Bull. 2018, 133, 532–542.
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ Pollut. 2020, 261, 114089.
- Torikai, N.; Matsushita, Y.; Langridge, S.; Bucknall, D.; Penfold, J.; Takeda, M. Interfacial structures of block and graft copolymers with lamellar microphase-separated structures. Phys. B 2000, 283, 12–16.
- Neves, C.V.; Gaylarde, C.C.; Baptista Neto, J.A.; Vieira, K.S.; Pierri, B.; Waite, C.C.; Scott, D.C.; Fonseca, E.M. The transfer and resulting negative effects of nano- and micro-plastics through the aquatic trophic web—A discreet threat to human health. Water Biol. Secur. 2022, 1, 2772–7351.
- Bour, A.; Avio, C.G.; Gorbi, S.; Regoli, F.; Hylland, K. Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ. Pollut. 2018, 243, 1217–1225.
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116.
- Lobelle, D.; Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 2011, 62, 197–200.
- Morét-Ferguson, S.; Law, K.L.; Proskurowski, G.; Murphy, E.K.; Peacock, E.E.; Reddy, C.M. The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar. Pollut. Bull. 2010, 60, 1873–1878.
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655.
- Cózar, A.; Echevarría, F.; González-Gordillo, J.; Irigoien, X.; Úbeda, B.; Hernandez-Leon, S.; Palma, A.; Navarro, S.; Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244.
- Long, M.; Moriceau, B.; Gallinari, M.; Lambert, C.; Huvet, A.; Raffray, J.; Soudant, P. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar. Chem. 2015, 175, 39–46.
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic 748 debris. Environ. Res. Lett. 2015, 10, 124006.
- Lebreton, L.C.M.; Van der Zwet, J.; Damsteeg, J.W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611.
- John, J.; Nandhini, A.R.; Velayudhaperumal, C.P.; Sillanpääl, M. Microplastics in mangroves and coral reef ecosystems: A review. Environ. Chem. Lett. 2022, 20, 397–416.
- Waldschlager, K.; Schüttrumpf, H. Erosion Behavior of Different Microplastic Particles in Comparison to Natural Sediments. Environ. Sci. Technol. 2019, 53, 13219–13227.
- Li, Y.; Liu, S.; Liu, M.; Huang, W.; Chen, K.; Ding, Y.; Wu, F.; Ke, H.; Lou, L.; Lin, Y.; et al. Mid-Level Riverine Outflow Matters: A Case of Microplastic Transport in the Jiulong River, China. Front. Mar. Sci. 2021, 8, 712727.
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in Soil: A Review on Methods, Occurrence, Sources, and Potential Risk. Sci. Total Environ. 2021, 780, 146546.
- Balestra, V.; Bellopede, R. Microplastic pollution in show cave sediments: First evidence and detection technique. Environ. Pollut. 2022, 292, 118261.
- Chia, Z.L.; Ptaszynski, M.; Masui, F.; Leliwa, G.; Wroczynskib, M. Machine learning and feature engineering-based study into sarcasm and irony classification with application to cyberbullying detection. Inf. Process. Manag. 2021, 58, 102600.
- Wanner, P. Plastic in agricultural soils—A global risk for groundwater systems and drinking water supplies? A review. Chemosphere 2021, 264 Pt 1, 128453.
- Fahrenfeld, N.L.; Arbuckle-Keil, G.; Beni, N.N.; Bartelt-Hunt, S.L. Source tracking microplastics in the freshwater environment. TrAC Trends Anal. Chem. 2019, 112, 248–254.
- McGechan, M.B. SW—Soil and Water: Transport of Particulate and Colloid-sorbed Contaminants through Soil, Part 2: Trapping Processes and Soil Pore. Geom. Biosyst. Eng. 2002, 83, 387–395.
- Yu, M.; van der Ploeg, M.; Lwanga, E.H.; Yang, X.; Zhang, S.; Ma, X.; Ritsema, C.J.; Geissen, V. Leaching of microplastics by preferential flow in earthworm (Lumbricus terrestris) burrows. Environ. Chem. 2019, 16, 31–40.
- Krueger, M.C.; Harms, H.; Schlosser, D. Prospects for microbiological solutions to environmental pollution with plastics. Appl. Microbiol. Biotechnol. 2015, 99, 8857–8874.
- Lwanga, H.E.; Gertsen, H.; Gooren, H.; Peters, P.; Salanki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531.
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 2020, 239, 124792.
- Kennish, M.J. Ecology of Estuaries: Anthropogenic Effects; CRC Press: Boca Raton, FL, USA, 1991.
- Kennish, M.J. Pollution Impacts on Marine Biotic Communities; CRC Press: Boca Raton, FL, USA, 1998.
- Prandle, D. Estuaries: Dynamics, Mixing, Sedimentation and Morphology; Cambridge University Press: Cambridge, UK, 2009.
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179.
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597.
- Dalrymple, R.W.; Zaitlin, B.A.; Boyd, R. Estuarine Facies Models: Conceptual Basis and Stratigraphic Implications: PERSPECTIVE. J. Sed. Petrol. 1992, 62, 1130–1146.
- Costa, L.A.; Pessoa, D.M.M.; da Silva Carreira, R. Chemical and biological indicators of sewage river input to an urban tropical estuary (Guanabara Bay, Brazil). Ecol. Indic. 2018, 90, 513–518.
- Le Roux, J.P. Grains in motion: A review. Sediment. Geol. 2005, 178, 285–313.
- Li, Y.; Lu, Z.; Zheng, H.; Wang, J.; Chen, C. Microplastics in surface water and sediments of Chongming Island in the Yangtze Estuary, China. Environ. Sci. Eur. 2020, 32, 15.
- Hitchcock, J.N.; Mitrovic, S.M. Microplastic pollution in estuaries across a gradient of human impact. Environ. Pollut. 2019, 247, 457–466.
- Rodrigues, S.M.; Almeida, C.M.R.; Silva, D.; Cunha, J.; Antunes, C.; Freitas, V.; Ramos, S. Microplastic contamination in an urban estuary: Abundance and distribution of microplastics and fish larvae in the Douro estuary. Sci. Total Environ. 2019, 659, 1071–1081.
- Rodrigues, D.; Antunes, J.; Otero, V.; Sobral, P.; Costa, M.H. Distribution patterns of microplastics in seawater surface at a Portuguese estuary and marine park. Front. Environ. Sci. 2020, 8, 582217.
- Cheng, M.L.H.; Lippmann, T.C.; Dijkstra, J.A.; Bradt, G.; Cook, S.; Choi, J.-G.; Brown, B.L. A baseline for microplastic particle occurrence and distribution in Great Bay Estuary. Mar. Pollut. Bull. 2021, 170, 112653.
- Stead, J.L.; Cundy, A.B.; Hudson, M.D.; Thompson, C.E.L.; Williams, I.D.; Russell, A.E.; Pabortsava, K. Identification of tidal trapping of microplastics in a temperate salt marsh system using sea surface microlayer sampling. Sci. Rep. 2020, 10, 14147.
- Duan, J.; Han, J.; Cheung, S.G.; Chong, R.K.Y.; Lo, C.-M.; Lee, F.W.-F.; Xu, S.J.-L.; Yang, Y.; Tam, N.F.-Y.; Zhou, H.-C. How mangrove plants affect microplastic distribution in sediments of coastal wetlands: Case study in Shenzhen Bay, South China. Sci. Total Environ. 2021, 767, 144695.
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998.
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics ≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Poll. Bull. 2015, 100, 70–81.
- Moore, C.J.; Moore, S.L.; Leecaster, M.K.; Weisberg, S.B. A comparison of plastic and plankton in the North Pacific Central Gyre. Mar. Pollut. Bull. 2001, 42, 1297–1300.
- Costa, M.F.; Silva-Cavalcanti, J.S.; Barbosa, C.C.; Barletta, M. Plastic buried in the inter-tidal plain of a tropical estuarine ecosystem. J. Coast. Res. 2011, 64, 339–343.
- Browne, M.A.; Dissanayake, A.; Galloway, T.S.; Lowe, D.M.; Thompson, R.C. Ingested microplastic translocate to the circulatory system of the mussel, Mytilus edulis (L.). Environ. Sci. Technol. 2008, 42, 5026–5031.
- Graham, E.G.; Thompson, J.T. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J. Exp. Mar. Biol. Ecol. 2009, 368, 22–29.
- Iribarne, O.; Botto, F.; Martinetto, P.; Gutierrez, J.L. The role of burrows of the SW Atlantic intertidal crab Chasmagnathus granulata in trapping debris. Mar. Pollut. Bull. 2000, 40, 1057–1062.
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139.
- McLachlan, A.; Defeo, O. The Ecology of Sandy Shores; Academic Press: Cambridge, MA, USA, 2017.
- Brown, A.C.; Mclachlan, A. Sandy shore ecosystems and the threats facing them: Some predictions for the year 2025. Environ. Conserv. 2002, 29, 62–77.
- Li, J.; Zhang, H.; Zhang, K.; Yang, R.; Li, R.; Li, Y. Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China. Mar. Pollut. Bull. 2018, 136, 401–406.
- Tiwari, M.; Rathod, T.D.; Ajmal, P.Y.; Bhangare, R.C.; Sahu, S.K. Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. Pollut. Bull. 2019, 140, 262–273.
- De-la-Torre, G.E.; Dioses-Salinas, D.C.; Castro, J.M.; Antay, R.; Fernández, N.Y.; Espinoza-Morriberón, D.; Saldaña-Serrano, M. Abundance and distribution of microplastics on sandy beaches of Lima, Peru. Mar. Pollut. Bull. 2020, 51, 110877.
- Mazariegos-Ortíz, C.; de los Ángeles Rosales, M.; Carrillo-Ovalle, L.; Cardoso, R.P.; Muniz, M.C.; Dos Anjos, R.M. First evidence of microplastic pollution in the El Quetzalito sand beach of the Guatemalan Caribbean. Mar. Pollut. Bull. 2020, 156, 111220.
- Patchaiyappan, A.; ZakiAhmed, S.; Dowarah, K.; Khadanga, S.S.; Singh, T.; Jayakumar, S.; Thirunavukkarasuc, C.; Devipriyaad, S.P. Prevalence of microplastics in the sediments of Odisha beaches, southeastern coast of India. Mar. Pollut. Bull. 2021, 167, 112265.
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204.
- Leslie, H.; Brandsma, S.; Van Velzen, M.; Vethaak, A. Microplastics in route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142.
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61.
- DiBenedetto, M.H.; Ouellette, N.T.; Kose, J.R. Transport of anisotropic particles under waves. J. Fluid Mech. 2018, 837, 320–340.
- Thiel, M.; Hinojosa, I.; Miranda, L.; Pantoja, J.; Rivadeneira, M.; Vasquez, N. Anthropogenic marine debris in the coastal environment: A multi-year comparison between coastal waters and local shores. Mar. Pollut. Bull. 2013, 71, 307–316.
- Sebille, E.; England, M.H.; Froyland, G. Origin, dynamics, and evolution of ocean garbage patches from observed surface drifters. Environ. Res. Lett. 2012, 4, 7.
- Lebreton, L.C.M.; Borrero, J.C. Modeling the transport and accumulation floating debris generated by the 11 March 2011 tohoku tsunami. Mar. Pollut. Bull. 2013, 66, 53–58.
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075.
- Chubarenko, I.; Esiukova, E.E.; Bagaev, A.V.; Bagaeva, M.A.; Grave, A.N. Three-dimensional distribution of anthropogenic microparticles in the body of sandy beaches. Sci. Total Environ. 2018, 628–629, 1340–1351.
- Onink, V.; Wichmann, D.; Delandmeter, P.; Van Sebille, E. The role of ekman currents, geostrophy, and stokes drift in the accumulation of floating microplastic. J. Geophys. Res. Ocean 2019, 124, 1474–1490.
- Soulsby, R. Dynamics of Marine Sands: A Manual for Practical Applications; Thomas Telford: London, UK, 1997.
- Nielsen, P. Coastal and Estuarine Processes; World Scientific Publishing Company: Singapore, 2009.
- Forsberg, P.L.; Sous, D.; Stocchino, A.; Chemin, R. Behaviour of plastic litter in nearshore waters: First insights from wind and wave laboratory experiments. Mar. Pollut. Bull. 2020, 153, 111023.
- Fredsøe, J.; Deigaard, R. Mechanics of Coastal Sediment Transport; World Scientific: Singapore; New Jersey, NJ, USA, 1992.
- Dean, R.G.; Dalrymple, R.A. Water Wave Mechanics for Engineers and Scientists; Prentice-Hall, Inc.: New Jersey, NJ, USA, 1984; 353p.
- Horikawa, K. Nearshore Dynamics and Coastal Processes: Theory, Measurement, and Predictive Models; University of Tokyo Press: Tokyo, Japan, 1988.
- Critchell, K.; Lambrechts, J. Modeling accumulation of marine plastics in the coastal zone; what are the dominant physical processes? Estuar. Coast. Shelf Sci. 2016, 171, 111–122.
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913.
- Kanhai, L.D.; Gårdfeldt, K.; Lyashevska, O.; Hassellöv, M.; Thompson, R.; O’Connor, I. Microplastics in sub-surface waters of the Arctic Central Basin. Mar. Pollut. Bull. 2018, 130, 8–18.
- Howell, E.A.; Bograd, S.J.; Morishige, C.; Seki, M.P.; Polovina, J.J. On North Pacific circulation and associated marine debris concentration. Mar. Pollut. Bull. 2012, 65, 16–22.
- Maximenko, N.; Corradi, P.; Law, K.L.; Van Sebille, E.; Garaba, S.P.; Lampitt, R.S.; Galgani, F.; Martinez-Vicente, V.; Goddijn-Murphy, L.; Veiga, J.M.; et al. Toward the Integrated Marine Debris Observing. Front. Mar. Sci. 2012, 10, 2296–7745.
- Ballent, A.; Corcoran, P.L.; Madden, O.; Helm, P.A.; Longstaffe, F.J. Sources and Sinks of Microplastics in Canadian Lake Ontario Nearshore, Tributary and beach Sediments. Mar. Pollut. Bull. 2016, 110, 383–395.
- Galgani, F.; Hanke, G.; Maes, T. Global distribution, composition and abundance of marine litter. In Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015; pp. 29–56.
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.J.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317.
- Thompson, R.; Moore, C.; Andrady, A.; Gregory, M.; Takada, H.; Weisberg, S. New Directions in Plastic Debris. Science 2005, 310, 1117.
- Luo, H.; Zeng, Y.; Zhao, Y.; Xiang, Y.; Li, Y.; Pan, X. Effects of advanced oxidation processes on leachates and properties of microplastics. J. Hazard. Mater. 2021, 413, 125342.




