Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fevzi Bardakci | -- | 2631 | 2023-01-30 08:53:48 | | | |
| 2 | Jessie Wu | + 6 word(s) | 2637 | 2023-01-30 09:13:31 | | | | |
| 3 | Jessie Wu | + 8 word(s) | 2645 | 2023-01-30 09:17:40 | | | | |
| 4 | Jessie Wu | Meta information modification | 2645 | 2023-01-30 09:19:40 | | | | |
| 5 | Hui Jing Hong | + 6 word(s) | 2651 | 2023-03-01 08:48:54 | | | | |
| 6 | Jessie Wu | Meta information modification | 2651 | 2023-03-01 10:23:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jing, H.H.; Bardakci, F.; Akgöl, S.; Kusat, K.; Adnan, M.; Alam, M.J.; Gupta, R.; Sahreen, S.; Chen, Y.; Gopinath, S.C.B.; et al. Biomedical Application of Carbon Dots. Encyclopedia. Available online: https://encyclopedia.pub/entry/40555 (accessed on 04 March 2026).
Jing HH, Bardakci F, Akgöl S, Kusat K, Adnan M, Alam MJ, et al. Biomedical Application of Carbon Dots. Encyclopedia. Available at: https://encyclopedia.pub/entry/40555. Accessed March 04, 2026.
Jing, Hong Hui, Fevzi Bardakci, Sinan Akgöl, Kevser Kusat, Mohd Adnan, Mohammad Jahoor Alam, Reena Gupta, Sumaira Sahreen, Yeng Chen, Subash C. B. Gopinath, et al. "Biomedical Application of Carbon Dots" Encyclopedia, https://encyclopedia.pub/entry/40555 (accessed March 04, 2026).
Jing, H.H., Bardakci, F., Akgöl, S., Kusat, K., Adnan, M., Alam, M.J., Gupta, R., Sahreen, S., Chen, Y., Gopinath, S.C.B., & Sasidharan, S. (2023, January 30). Biomedical Application of Carbon Dots. In Encyclopedia. https://encyclopedia.pub/entry/40555
Jing, Hong Hui, et al. "Biomedical Application of Carbon Dots." Encyclopedia. Web. 30 January, 2023.
Copy Citation
Carbon dots (CDs), which are a new category of carbon nanoparticles that consist of quasi-spherical, discrete fluorescent carbon nanomaterials with a diameter of less than 10 nm, have multiple advantages over semiconductor quantum dots (QDs), including high water solubility, low cost, excellent biocompatibility, chemically inertness, highly tunable photoluminescence and electrochemical luminescence. Because of their unique properties, carbon quantum dots (CQDs) have acquired significance in nano-chemistry, which has resulted in the discovery of CDD applications, especially in biomedical applications.
carbon dots
biomedical applications
bioimaging
sensing
catalysis
therapy
drug delivery
1. Carbon Dots in Bioimaging
Bioimaging technology requires the assistance of technology, including X-ray, ultrasound and magnetic resonance imaging, to process images of living organisms [1]. Properties such as low toxicity, biocompatibility and fluorescent properties make carbon dots (CDs) more favourable in the visualization of the biological system both in vitro and in vivo [1]. For instance, Ray’s team successfully synthesized water-soluble fluorescent CDs with a diameter between 2 to 6 nm by nitric acid oxidation of carbon soot. The synthesized CDs with green fluorescence under UV exposure showed promising applications in cell imaging, as they can penetrate HepG2 cells beyond any further functionalization [2]. Carbon is generally non-toxic and green; thus, carbon cores of CDs are also non-toxic. It has been demonstrated that the cytotoxicity effect mainly originates from the passivating agent on the surface of CDs. Therefore, surface passivating chemicals with low toxicity can be utilized securely for in vivo imaging at high concentrations [1][3]. In a typical in vivo experiment, the cytotoxicity and in vivo toxicity of the oligomeric polyethylene glycol (PEG1500N)-dopped carbon quantum dots (CQDs) were investigated by Yang and colleagues. This 28-day in vivo experiment involved intravenous injection of PEGylated CQDs into rats (8–40 mg/kg), proving that these PEGylated CQDs did not show any noticeable toxic effect in rats for toxicity assessment [4]. Physiological indicators were at the same level when the rats were exposed to different CQD concentrations and the control (0.9% NaCl only). The dissected liver and spleen of mice also showed no abnormalities, even though the dosage of CQDs presented in the harvested organs was greater than those presented in other organs [4]. Accordingly, this research proved the non-cytotoxicity and non-toxicity effect of CQDs at various concentrations and times that can be applied for in vivo imaging performance. In addition, in vitro research carried out by Ding et al. [5] found that the DNA-CQDs isolated from bacteria DNA were internalized by human embryonic kidney cell lines (HEK 293). The fluorescent DNA-CDs, which can be easily imaged by using a confocal microscope, suggest the promising application of DNA-CDs as a luminescent vehicle for cell imaging study [5]. Another study conducted by Zheng’s group showed the CQDs possess excellent biocompatibility and highly tunable photoluminescence and can easily pass through the blood–brain barrier and target the C6 glioma cells accurately without the aid of any other targeting molecules [6]. This indicated the potential application of CQDs in fluorescent imaging, with integration in the construction of CQDs as intelligent nanomedicine.
2. Carbon Dots in Biosensing and Chemical Sensing
CDs also have a wide application in biosensing and chemical sensing (Figure 1) due to characteristics such as excitation-dependent emission, biocompatibility, low cytotoxicity, water solubility as well as higher photostability [7]. The biosensors established from CDs might be utilized to examine numerous parameters or materials such as cellular ions, antibodies, proteins and nucleic acids [8]. For example, Yu’s team suggested that CQDs can form a fluorescence resonance energy transfer (FRET) system with organic dyes, which can serve as a highly sensitive radiometric sensor that can penetrate the cells easily to detect intracellular H2S levels. In this case, the CQD–organic dye conjugates, which emit blue fluorescence, will convert to green in the sight of H2S [9]. Yu’s team also expressed that the alteration of H2S physiological levels inside the HeLa and murine aneuploid fibrosarcoma cell line (L929) can be detected via CQD–organic dye conjugates under a fluorescence microscope. This is because a change in emission colour from blue to green was detected as a consequence of the exposure of cells to H2S [9]. This research provided new insight into the potential application of CQDs as detecting analytes in human health diagnosis [9]. CQDs can be utilized as fluorescent labels in immunoassays. For example, Posthuma-Trumpie et al. [10] reported that CQDs can act as signalling labels for the detection of biomolecules, including antibodies, nucleic acid and proteins. CQDs can also be employed as an impressive fluorescence sensor in the detection of nucleic acid. The cationically modified CQD probes that were synthesized from Han’s group proved that these CQD probes were able to emit spectrally detectable fluorescence when bound with double-stranded DNA as well as single-stranded RNA in living cells [11]. Interestingly, these CQD probes can pass through various biological barriers in vitro and in vivo [11]. This research also provides a new platform to study the CQD application by exploring the localization and motion of DNA and RNA in living cells.
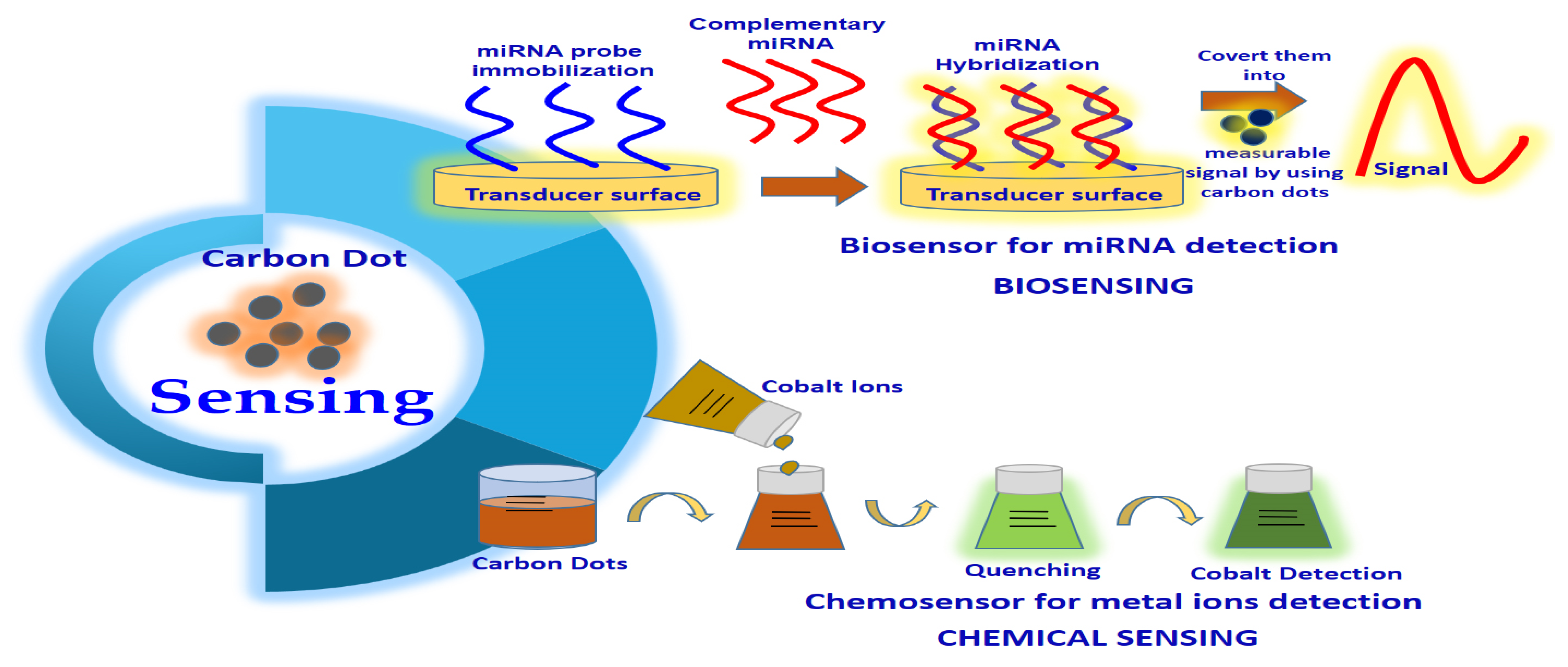
Figure 1. Carbon dot application in sensing.
CDs are promising candidates for chemical sensing or fluorescence sensing. The interaction of metal ions and the surface functional groups of CQDs will result in the production of new electron-hole recombination and lead to a change in the fluorescent nature of CDs with the aid of the energy transfer route [7]. In this case, CDs can be utilized as a fluorescence sensor for heavy metal ion detection in aqueous solutions or living cells. In a typical experiment, Zhou and colleagues demonstrated the use of unmodified CQDs as fluorescence-sensing agents to detect highly toxic metal ions (Hg2+ ions) and biothiols (e.g., cysteine, homocysteine, glutathione) [12]. They found that Hg2+ was able to quench the fluorescence of the CQDs, whereas biothiols can efficiently prevent fluorescence quenching because of their ability to remove Hg2+ from the surface of CQDs [12]. Other applications of CDs in chemical sensing included Fe3+, Cu2+, Cr(VI), Ag+ and Pb2+ detection due to the formation of fluorescent quenching of CQDs with these heavy metal ions. For instance, Liu and colleagues designed a highly sensitive lysine (Lys)-enhancing CD-BSA fluorescent probe to detect Cu2+ in tap water and hair [13]. They found that Cu2+ was able to react with the –COOH and –NH2 functional group on the surface of the Lys-enhancing CD-BSA fluorescent probe, causing fluorescent quenching [13]. CDs possessing excellent electro-chemiluminescence and chemiluminescence also led to the development of the use of CDs in electro-chemiluminescent assay and chemiluminescent assay for the detection of various ions and compounds, such as Co+2, NO2− and pentachlorophenol [3].
3. Carbon Dots in Photocatalysis
One of the most predominant and exciting topics in nanoscience and nanochemistry is nano-photocatalysis [8][14]. Recently, various studies were conducted in the discovery of new and more potent nanocatalysts with excellent specificity, high selectivity and strong and tunable chemical activity. In this case, CQDs that exhibit the potentiality of harnessing long-wavelength light and vitality conversion provide the possibility for application as photocatalysts in organic synthesis [3][8]. In fact, Ma’s group indicated that the water-soluble fluorescent CQDs synthesized via simple ultrasonic reaction showed incredible catalytic activity to decompose H2O2 and also NIR light-determined electron transfer activity [15]. In this experiment, CQDs with sizes of 1–4 nm acted as an effective NIR light-determined photocatalyst that could selectively oxidize alcohols to benzaldehyde; the photocatalytic action of CQDs could be efficiently regulated by the doping of CQDs as well as modifying the CQDs surface [1][3][15]. TiO2 is one of the most well-known photocatalysts and is commonly used to eliminate organic pollutants as well as generate H2 by splitting of water [16]. Nonetheless, a noteworthy disadvantage of TiO2 photocatalytic efficiency is the impotent use of visible light as the illumination source. Hence, bandgap construction by possible adjustment of TiO2-based media can be done to enhance the execution of TiO2 catalysts [1][3][16]. In this case, a nanocomposite of CQDs and TiO2 is recommended to understand the effective usage of the full sunlight spectrum [1][3]. For example, Li’s team designed a TiO2/CQDs complex system to exploit the employment of sunlight with a full spectrum [17]. They found that the TiO2/CQDs complex was able to deteriorate methylene blue (MB) under visible light illumination, whereas the control groups, which used only pure TiO2 without CQDs, showed no or little reduction of MB [17]. The result proved that CQDs are essential for effective photodegradation under visible light [17]. The above studies provide a new approach to the utilization of CQD-designed photocatalysts in bioscience and energy technology.
4. Carbon Dots in Nanomedicine (Photodynamic, Photothermal, Drug Delivery Applications)
One of the most interesting applications of CDs is nanomedicine. CDs, which are a type of fluorescent nanoparticles, show no toxicity under in vitro and in vivo experiments. In a typical experiment, Singh’s team performed in vitro and in vivo toxicity studies of nitrogen-doped CQDs (NCQDs). In a typical in vitro experiment, lactate dehydrogenase (LDH) profile, cell apoptosis analysis, DNA fragmentation and growth cycle assessment was analysed by treating the HeLa cell line with NCQDs [18]. The results showed no apparent toxicity of NCQDs against these cancer cell lines. Singh et al. [18], also in an in vivo toxicity study, treated mice with two different concentrations of NCQDs for 30 days. The antioxidant, serum biochemical, haematological and histopathological analysis indicated that CQDs did not show any noticeable toxicity towards mice at both NCQD concentrations [18]. Hence, CQDs are safe enough to be used in nanomedicine.
Photothermal therapy (PTT), which offers numerous advantages in conventional cancer therapy methods such as chemotherapy, radiotherapy and surgery, has attracted attention from researchers in studying its application in the cancer field [7]. PTT usually employs photothermal agents (PAs), including carbon nanomaterials, silver nanomaterials, gold nanomaterials and germanium nanocrystals, which can absorb light (preferable to the NIR region) strongly and convert light energy into hyperthermia, which leads to the generation of local heat and the destruction of cancer cells with little adverse effect on normal cells [7][19][20]. Various studies have proven that CQDs can be used in PTT for cancer treatment. For instance, Ge et al. [21] investigated the use of CDs prepared from polythiophene phenylpropionic acid in PTT. They found that the as-prepared CQDs showed significant cytotoxicity towards HeLa cells when exposed to an NIR laser. Moreover, the high in vivo PTT efficacy of CQDs towards tumour-bearing mice without extensive toxicity proposed the possible application of CQDs in the field of cancer diagnosis and treatment as well as bioimaging in living mice [21]. Das’s group studied the photothermal ablation effect of the designed mesoporous hollow NCQDs to capture carbon spheres (HCS) in human oral cancer cells (FaDu). In this in vitro experiment, they found that NCQDs-HCS can internalize with the FaDu cells and activate a significant thermal ablation effect in the cells when exposed to a 980 nm NIR laser [19]. The excellent fluorescent property of the designed NCQDs-HCS could be used to monitor the remedial reaction during the treatment [19].
Photodynamic therapy (PDT) is a clinical therapy that is commonly applied for superficial tumour treatment. The photosensitizer that is used in PDT must be highly sensitive so that it can localize and accumulate at the tumour tissue specifically. The photosensitizer that is used in PDT will then absorb visible light and can give rise to the production of an excited single state followed by the transition of a long-lived triplet state that can react with oxygen molecules to produce ROS, destroying cancer cells effectively [7][22]. CDS are exclusively used as a photosensitizer in PDT because of their unique and beneficial properties, such as excellent biocompatibility, low-cost synthesis and ability to conduct light energy to heat. For example, Beack et al. [23] suggested the use of transdermal CDs-chlorine e6-hyaluronate (CDs-Ce6-HA) conjugate in PDT of melanoma skin cancer. In this experiment, the CDs-Ce6-HA synthesized from the coupling response of diamino-hexane modified HA and the carboxylic group of Ce6 generated singlet oxygen compared to free Ce6. These synthesized CDs-Ce6-HA conjugates were able to target the B16F10 melanoma cells in a tumour model in mice when observed under a confocal microscope and two-photon microscope [23]. Complete suppression of melanoma skin cancers was also observed in this experiment after transdermal treatment using CDs-Ce6-HA conjugate [23]. Another study conducted by Guo’s team proved that the as-prepared Cu and N co-doped CDs can inhibit the growth of B16 melanoma cells significantly by both PTT and PDT [24]. In addition to PTT and PDT, CQDs can also be applied in radiotherapy. Kleinauskas and colleagues described that the coating of the silver shell (C-Ag-PEG CQDs) on PEGylated CQDs can be utilized as a radiosensitizer in prostate adenocarcinoma cell lines (Du145 cells). C-Ag-PEG CQD that accumulates at Du145 cells will eject electrons when exposed to low-energy X-ray and generate free radicals that can damage the cancer cells [25].
Recently, drug delivery systems (DDSs) depending on nanotechnology have been widely investigated. Unfortunately, various nanomaterials that can act as drug delivery vehicles, such as graphene oxides, mesoporous silica (MS), polymeric nanoparticles and gold nanoparticles (AuNPs), have toxicity and biocompatibility issues that limit their application in clinical therapy [3][8]. For example, the fluorophore quenching ability of AuNPs makes them hard to monitor in in vivo systems [3][8]. The requirement of thiol groups for drug loading through the Au-Thiol interaction further limits their application in drug delivery [26]. This has resulted in considerable attention of researchers in discovering more promising nanomaterials to replace AuNPs. As an emerging class of luminescent nanomaterials, CDs have shown tremendous potential as drug delivery agents, as they have demonstrated excellent biocompatibility properties, high water solubility and flexibility in surface modification with other chemical molecules [3][8]. For example, Zheng and colleagues conjugated oxidized oxaliplatin (Oxa(IV)-COOH), which is a type of platinum-based anticancer pro-drug that is applied in the pharmacotherapy of metastatic colorectal cancer on the surface of CQDs by condensation response between the carboxyl group of Oxa(IV)-COOH and amino group on the CQDs’ surface through chemical coupling. Zheng et al. [27] expressed that the CQD-conjugated drug absorbed by the cancer cells by endocytosis will then release upon the reduction of Oxa(IV)-COOH to oxaliplatin(II). This further suggests the application of pro-conjugated CQDs in diagnosis, estimating the proper dosage of medicine during cancer treatment [27]. CDs can also act as nanocarriers, as they can efficiently track and deliver genes or drugs; branched polyethyleneimine CQDs can be used as a potential gene delivery agent [1]. CDs also play a crucial role in controlling the release of the drug; the loading of CDs with doxorubicin can regulate the release of the drug in HeLa cells [1][3][28][29]. In a typical example, D’souzza and colleagues suggested the as-synthesized CQDs by utilizing carrot roots as a carbon precursor that can act as nano-vehicles for the delivery of mitomycin. In this experiment, the fluorescent CQDs produced were able to interact with mitomycin effectively by hydrogen bonding; this hydrogen bonding further breaks in the extracellular microenvironment of pH 6.8 and the drug to be diffused [30]. D’souzza’s group expressed that mitomycin drug-loaded CQDs with ultra-small diameter and high biocompatibility properties exhibits] high affinity towards cancer cell membranes and facilitated high degree internalization of mitomycin-CQDs by Bacillus subtilis. In addition, the in vitro research results from this experiment further suggest the mitomycin-loaded CQDs nanocarrier can effectively enter the tumour cells, exhibit pH-dependent release behaviour and be biocompatible with MCF-7 cancer cells [30]. In summary, the unique optical and physiochemical properties of CQDs allow them to be utilized as an effective vehicle to deliver drugs, which further suggests their potential application in diagnosis, estimating proper dosage of medicine, customizing the drug injection time and monitoring the response during cancer treatment by tracking the fluorescence signal of CQDs. The development of a highly biocompatible and fluorescent delivery system based on fluorescent CQDs further suggests the potential application of CQDs in personal medicine, with such CQDs holding great promise for specific drug delivery with minimal toxicity and adverse effects in cancer patients. Nonetheless, CDs’ specificity to target certain states of diseases remains unclear, which limits their therapeutic application. Hence, further study is required for understanding whether CQDs can specifically target diseases as well as their possible therapeutic applications.
References
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon quantum dots: Synthesis, characterization and biomedical applications. Turk. J. Pharm. Sci. 2018, 15, 219–230.
- Ray, S.C.; Saha, A.; Jana, N.R.; Sarkar, R. Fluorescent carbon nanoparticles: Synthesis, characterization, and bioimaging application. J. Phys. Chem. C 2009, 113, 18546–18551.
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381.
- Yang, S.-T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.-H.; Liu, Y.; Chen, M.; et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114.
- Ding, H.; Du, F.; Liu, P.; Chen, Z.; Shen, J. DNA–Carbon dots function as fluorescent vehicles for drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 6889–6897.
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-Targeting Fluorescent Carbon Dots for Diagnosis of Brain Cancer Cells. ACS Nano 2015, 9, 11455–11461.
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92.
- Farshbaf, M.; Davaran, S.; Rahimi, F.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: Recent progresses on synthesis, surface modification and applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1331–1348.
- Yu, C.; Li, X.; Zeng, F.; Zheng, F.; Wu, S. Carbon-dot-based ratiometric fluorescent sensor for detecting hydrogen sulfide in aqueous media and inside live cells. Chem. Commun. 2013, 49, 403–405.
- Posthuma-Trumpie, G.A.; Wichers, J.H.; Koets, M.; Berendsen, L.B.J.M.; van Amerongen, A. Amorphous carbon nanoparticles: A versatile label for rapid diagnostic (immuno) assays. Anal. Bioanal. Chem. 2012, 402, 593–600.
- Han, G.; Zhao, J.; Zhang, R.; Tian, X.; Liu, Z.; Wang, A.; Liu, R.; Liu, B.; Han, M.; Gao, X.; et al. Membrane-penetrating carbon quantum dots for imaging nucleic acid structures in live organisms. Angew. Chem. 2019, 131, 7161–7165.
- Zhou, L.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem. Commun. 2012, 48, 1147–1149.
- Liu, J.-M.; Lin, L.-P.; Wang, X.-X.; Lin, S.-Q.; Cai, W.-L.; Zhang, L.-H.; Zheng, Z.-Y. Highly selective and sensitive detection of Cu2+ with lysine enhancing bovine serum albumin modified-carbon dots fluorescent probe. Anal. 2012, 137, 2637–2642.
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253.
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J. Chem. 2012, 36, 861–864.
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959.
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, A.C.H.; Yang, X.; Lee, S.-T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434.
- Singh, V.; Kashyap, S.; Yadav, U.; Srivastava, A.; Singh, A.V.; Singh, R.K.; Singh, S.K.; Saxena, P.S. Nitrogen doped carbon quantum dots demonstrate no toxicity under in vitro conditions in a cervical cell line and in vivo in Swiss albino mice. Toxicol. Res. 2019, 8, 395–406.
- Das, R.K.; Panda, S.; Bhol, C.S.; Bhutia, S.K.; Mohapatra, S. N-Doped Carbon Quantum Dot (NCQD)-Deposited Carbon Capsules for Synergistic Fluorescence Imaging and Photothermal Therapy of Oral Cancer. Langmuir 2019, 35, 15320–15329.
- Zhi, D.; Yang, T.; O’Hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control. Release 2020, 325, 52–71.
- Ge, J.; Jia, Q.; Liu, W.; Guo, L.; Liu, Q.; Lan, M.; Zhang, H.; Meng, X.; Wang, P. Red-Emissive Carbon Dots for Fluorescent, Photoacoustic, and Thermal Theranostics in Living Mice. Adv. Mater. 2015, 27, 4169–4177.
- Hassan, M.; Gomes, V.G.; Dehghani, A.; Ardekani, S.M. Engineering carbon quantum dots for photomediated theranostics. Nano Res. 2018, 11, 1–41.
- Beack, S.; Kong, W.H.; Jung, H.S.; Do, I.H.; Han, S.; Kim, H.; Kim, K.S.; Yun, S.H.; Hahn, S.K. Photodynamic therapy of melanoma skin cancer using carbon dot-chlorin e6-hyaluronate conjugate. Acta Biomater. 2015, 26, 295–305.
- Guo, X.-L.; Ding, Z.-Y.; Deng, S.-M.; Wen, C.-C.; Shen, X.-C.; Jiang, B.-P.; Liang, H. A novel strategy of transition-metal doping to engineer absorption of carbon dots for near-infrared photothermal/photodynamic therapies. Carbon 2018, 134, 519–530.
- Kleinauskas, A.; Rocha, S.; Sahu, S.; Sun, Y.-P.; Juzenas, P. Carbon-core silver-shell nanodots as sensitizers for phototherapy and radiotherapy. Nanotechnology 2013, 24, 325103.
- Kumar, V.; Toffoli, G.; Rizzolio, F. Fluorescent Carbon Nanoparticles in Medicine for Cancer Therapy. ACS Med. Chem. Lett. 2013, 4, 1012–1013.
- Zheng, M.; Liu, S.; Li, J.; Qu, D.; Zhao, H.; Guan, X.; Hu, X.; Xie, Z.; Jing, X.; Sun, Z. Integrating oxaliplatin with highly luminescent carbon dots: An unprecedented theranostic agent for personalized medicine. Adv. Mater. 2014, 26, 3554–3560.
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939.
- Liu, Z.; Chen, X.; Zhang, X.; Gooding, J.J.; Zhou, Y. Carbon-Quantum-Dots-Loaded Mesoporous Silica Nanocarriers with pH-Switchable Zwitterionic Surface and Enzyme-Responsive Pore-Cap for Targeted Imaging and Drug Delivery to Tumor. Adv. Heal. Mater. 2016, 5, 1401–1407.
- D’Souza, S.L.; Chettiar, S.S.; Koduru, J.R.; Kailasa, S.K. Synthesis of fluorescent carbon dots using Daucus carota subsp. sativus roots for mitomycin drug delivery. Optik 2018, 158, 893–900.
More
Information
Subjects:
Biochemical Research Methods
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
6 times
(View History)
Update Date:
01 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





