Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luis Fontana | -- | 1472 | 2023-01-30 08:28:56 | | | |
| 2 | Jessie Wu | Meta information modification | 1472 | 2023-01-30 08:40:18 | | | | |
| 3 | Jessie Wu | Meta information modification | 1472 | 2023-01-30 08:41:24 | | | | |
| 4 | Jessie Wu | -7 word(s) | 1465 | 2023-01-30 08:43:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Álvarez-Mercado, A.I.; Cano, A.D.V.; Fernández, M.F.; Fontana, L. Microbiota on Clinical Outcomes and Chemotherapy Resistance. Encyclopedia. Available online: https://encyclopedia.pub/entry/40554 (accessed on 07 February 2026).
Álvarez-Mercado AI, Cano ADV, Fernández MF, Fontana L. Microbiota on Clinical Outcomes and Chemotherapy Resistance. Encyclopedia. Available at: https://encyclopedia.pub/entry/40554. Accessed February 07, 2026.
Álvarez-Mercado, Ana Isabel, Ana Del Valle Cano, Mariana F. Fernández, Luis Fontana. "Microbiota on Clinical Outcomes and Chemotherapy Resistance" Encyclopedia, https://encyclopedia.pub/entry/40554 (accessed February 07, 2026).
Álvarez-Mercado, A.I., Cano, A.D.V., Fernández, M.F., & Fontana, L. (2023, January 30). Microbiota on Clinical Outcomes and Chemotherapy Resistance. In Encyclopedia. https://encyclopedia.pub/entry/40554
Álvarez-Mercado, Ana Isabel, et al. "Microbiota on Clinical Outcomes and Chemotherapy Resistance." Encyclopedia. Web. 30 January, 2023.
Copy Citation
The dual role of the gut microbiota in the preservation of host health and in the development of different pathologies, cancer among them. Our gut microbiota is capable of producing metabolites that protect host homeostasis but can also produce molecules with deleterious effects, which, in turn, may trigger inflammation and carcinogenesis, and even affect immunotherapy.
microbiota
cancer
immune
1. Importance of Gut Microbiota in Cancer Therapies
The common goal of the different cancer therapies is to effectively eliminate cancer cells in order to eradicate the disease in the patient and prevent a future recurrence. Despite the great advances in cancer treatments, almost all are also toxic for non-cancerous cells, which leads to the appearance of different side effects of varying severity, some of them even affecting the survival of patients. Gut microbiota and cancer therapies are closely related [1]. Treatments, such as radiotherapy, chemotherapy, and immunotherapy, can modify the microbiota of patients and, at the same time, the composition of the microbiota can influence efficacy and development of side effects of such therapies [2].
The gut microbiota can modulate the progression of cancer pathogenesis through its ability to synthesize different antitumor compounds, as well as to regulate the immune response and host inflammatory pathways. These combined mechanisms may explain the strong influence of the microbiota with the efficacy of different therapies.
2. Intestinal Microbiota and Chemotherapy
The gut microbiota can modulate the metabolism of different drugs used in chemotherapy, thus affecting both the response of cancer cells to this treatment and the susceptibility of healthy cells.
2.1. Gemcitabine
Gemcitabine (2′-2′-difluoro-deoxycytidine) is a pyrimidine antagonist, which therefore competes with deoxycytidine (a component of deoxyribonucleic acids derived from cytosine) during DNA synthesis. The antitumor activity of gemcitabine, used in the treatment of different types of cancer, is based on its intracellular activation and subsequent degradation, through its transformation into the inactive metabolite difluoro-deoxy-uridine by cytidine deaminase (CDD) [3]. Studies in mice have concluded that gemcitabine resistance may be due to enhanced metabolic degradation of the drug into difluoro-deoxy-uridine due to the expression of a long isoform of the bacterial enzyme cytidine deaminase (CDDL), which is mainly observed in Gammaproteobacteria [4] On the other hand, the combined action of the antibiotic ciprofloxacin, together with gemcitabine, seems to increase the antitumor activity of the drug through the inhibition of bacterial growth caused by the antibiotic, demonstrating that modulation of the intestinal microbiota can influence the activity of gemcitabine in mice [5].
2.2. Cyclophosphamide
Cyclophosphamide is an alkylating agent used in different types of cancer, which acts by stimulating the immune response against cancer. Studies in mice have shown that when cyclophosphamide is administered together with gram-positive bacteria antibiotics, there is an inhibition of the immune response elicited by cyclophosphamide, and therefore of the anticancer effect of the drug, which is restored by oral administration of Gram-positive bacteria, such as Lactobacillus johonsoni and Enterobacter Hirae [6][7].
2.3. Irinotecan
Irinotecan (CPT-11) is an inhibitor of DNA replication through its anti-topoisomerase I action. This drug, used in different types of cancer, has an active form (SN-38) and an inactive form (SN-38-G) that are excreted into the intestine. When SN-38G is excreted into the intestinal lumen, it is converted back to SN-38 by the bacterial ß-glucuronidase of E. coli, a process that can cause enteric injury and, therefore, diarrhea, this being one of the main side effects of the drug. In mice, it has been shown that administration of this drug with a bacterial ß-glucuronidase inhibitor can prevent gastrointestinal toxicity [8].
2.4. Cisplatin
Cisplatin is an effective anticancer agent and is used in many advanced cancers. It has antibiotic effects on Gram-negative and Gram-positive bacteria and can cause intestinal dysbiosis [9][10]. In addition, cisplatin can also cause loss of intestinal mucosal integrity by binding to the DNA of epithelial cells, impairing their replication, which could lead to serious infections of different parasites [11]. Cisplatin also has other side effects in which the microbiota is involved, such as ototoxicity, mucositis, and weight loss. It has been determined that the administration of D-methionine, together with cisplatin treatment, protects against drug toxicity through, not only its antioxidant and anti-inflammatory properties, but also by promoting the growth of beneficial bacteria, such as Lachnospiraceae and Lactobacillus, thus regulating the imbalance of the intestinal microbiota [12]. On the other hand, the intestinal microbiota also seems to affect the efficacy of cisplatin. In mice with lung tumors, it has been shown that, when administering this drug with anti-Gram positive antibiotics, the efficacy of the treatment is reduced, as mice survive less and develop larger tumors than mice in which cisplatin is combined with probiotics, such as Lactobacillus [10].
2.5. 5-fluorouracil
5-fluorouracil (5-FU) is a thymidylate synthase inhibitor used for the treatment of gastrointestinal tumors. Its usefulness is limited due to the acquisition of resistance and the gastrointestinal toxicity effects it causes, one of the most relevant side effects of 5-FU being intestinal mucositis. 5-FU can cause intestinal dysbiosis even with a single dose; different studies have reported a drastic change in the microbiota, decreasing species such as Bifidobacterium and Lactobacillus and increasing others, such as Escherichia, Clostridium, and Enterococcus. Regarding drug efficacy, it has been shown, in mice, that combined administration with an antibiotic cocktail decreases antitumor efficacy, while probiotic supplementation seems to increase it significantly [13].
Figure 1 summarizes the impact of gut microbiota in several common drugs used in chemotherapy.
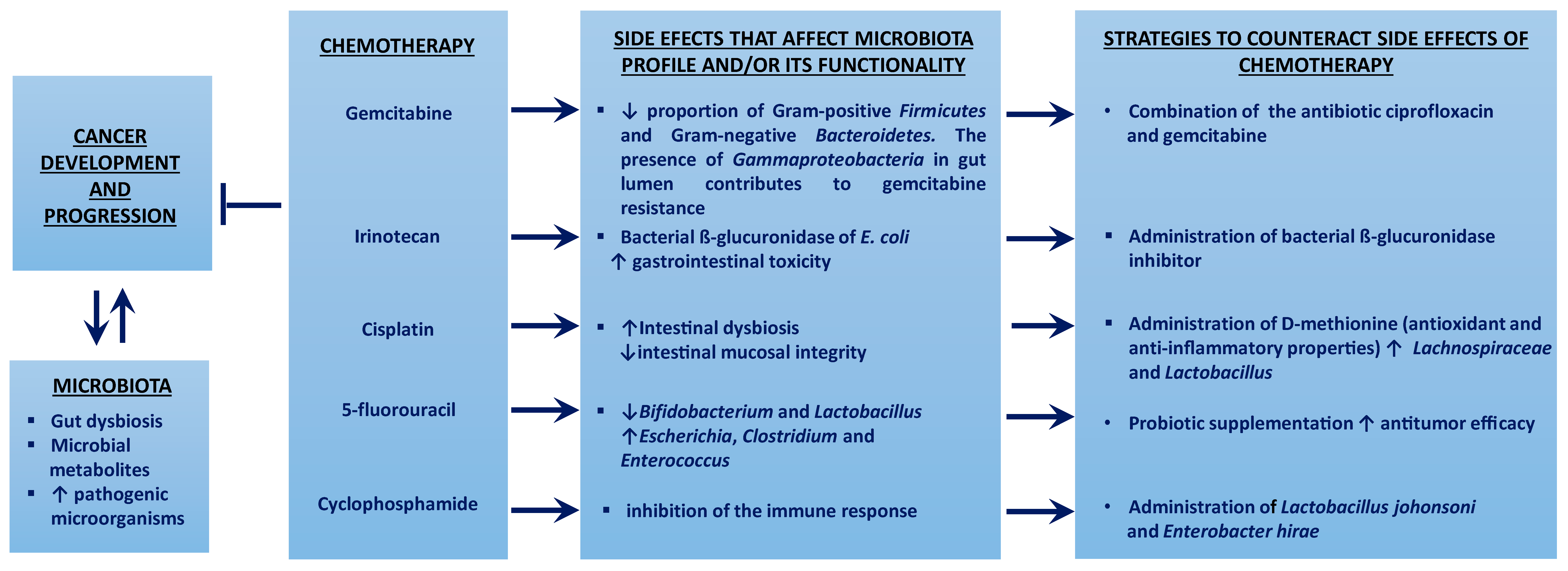
Figure 1. The gut microbiota affects cancer pathogenesis and the metabolism of chemotherapy drugs, conditioning both the response of cancer cells and the susceptibility of healthy cells. ↑ means increment; ↓ means decrease; ⱶ means inhibition.
3. Gut Microbiota and Immunotherapy
Immunotherapy is based on immune checkpoint inhibitor (ICI) molecules, which act by blocking certain immune regulatory pathways in order to enhance the antitumor immune response. ICIs are monoclonal antibodies that target receptor molecules on the surface of T lymphocytes, such as cytotoxic lymphocyte antigen 4 (CTLA-4) and programmed death receptor 1 (PD-1), or PD-1 ligands (PD-L1 or PD-L2). The mechanisms of each of these antibodies are different [14].
Because they dysregulate the immune system, ICIs cause a wide spectrum of side effects that can affect any organ. These side effects are known as immune-related adverse events (irAEs), which will differ according to the therapy used. In general, the ICI with the highest incidence and severity of irAEs are antibodies to CTLA-4, followed by those to PD1, with antibodies to PD-L1 having the least effect. In particular, intestinal side effects, such as diarrhea or colitis, are more frequently observed with anti-CTLA-4 antibodies, while dysthyroidism or pulmonary toxicity are more frequent with anti-PD-1/PD-L1 [14]. Because of this, there are a significant number of patients to whom such therapy can be applied only for a limited time due to the occurrence of strong side effects. However, oral administration of certain probiotics, such as Bacterioides fragilis and Burkholderia cepacia, has been linked to improvement of these immunotherapy-associated side effects [15].
In terms of efficacy, ICIs have demonstrated their usefulness in different solid tumors, as well as in hematologic malignancies. Although ICIs achieve a durable response and prolonged survival, a non-negligible percentage of patients do not obtain any benefit (primary resistance) or eventually progress (secondary resistance), and there is accumulated evidence that in some patients ICIs can even favor tumor growth (hyperprogression) [14]. Because of this, different studies have been carried out to identify predictive factors for the efficacy of this type of treatment, as well as strategies to avoid resistance to it, with some of these studies showing that the composition of the intestinal microbiota modulates the activity, efficacy, and toxicity of ICIs.
3.1. Anti-CTL-4
In patients treated with anti-CTLA4 antibodies, side effects are greater in those with a gut microbiota abundant in different Firmicutes species, such as Faecalibacterium, and a decreased abundance of Bacterioides [16][17]. In terms of treatment efficacy, in patients with metastatic melanoma, it was found that those whose gut microbiota was enriched in Faecalibacterium and other Firmicutes had longer progression-free survival and overall survival than those with microbiota rich in Bacteroides [16].
3.2. Anti-PD-L1
The efficacy of the antibody targeting PD-L1 in the treatment of melanoma in mice is improved in the presence of a gut microbiota enriched in Bifidobacterium species. Additionally, oral administration to patients of a cocktail of bacteria of this species combined with the anti-PD-L1 antibody specifically increases the T-cell response and blocks melanoma growth, whereas, when the treatment is combined with antibiotics, the survival rate is lower [18].
3.3. Anti-PD1
As was the case with anti-PD-L1 therapy, when combining anti-PD1 with antibiotics, the survival rate in patients is lower. In these patients, the responders to anti-PD1 treatment had a gut microbiota enriched in the Akkermansia and Alistipes genera [15]. Likewise, when analyzing the intestinal microbiota of patients with metastatic melanoma subjected to anti-PD-1 immunotherapy, a greater diversity and abundance of Faecalibacterium was observed in those with greater response to treatment and SSP, and a lower diversity and abundance of Bacteroilades in non-responders with lower SSP was observed [14].
References
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut microbiota and cancer: From pathogenesis to therapy. Cancers 2019, 11, 38.
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285.
- Gori, S.; Inno, A.; Belluomini, L.; Bocus, P.; Bisoffi, Z.; Russo, A.; Arcaro, G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol./Hematol. 2019, 143, 139–147.
- Picardo, S.L.; Coburn, B.; Hansen, A.R. The microbiome and cancer for clinicians. Crit. Rev. Oncol./Hematol. 2019, 141, 1–12.
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160.
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976.
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 2016, 45, 931–943.
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835.
- Joyce, K.; Saxena, S.; Williams, A.; Damurjian, C.; Auricchio, N.; Aluotto, S.; Tynan, H.; Demain, A.L. Antimicrobial spectrum of the antitumor agent, cisplatin. J. Antibiot. 2010, 63, 530–532.
- Gui, Q.F.; Lu, H.F.; Zhang, C.X.; Xu, Z.R.; Yang, Y.H. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 2015, 14, 5642–5651.
- Perales-Puchalt, A.; Perez-Sanz, J.; Payne, K.K.; Svoronos, N.; Allegrezza, M.J.; Chaurio, R.A.; Anadon, C.; Calmette, J.; Biswas, S.; Mine, J.A.; et al. Frontline Science: Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J. Leukoc. Biol. 2018, 103, 799–805.
- Wu, C.H.; Ko, J.L.; Liao, J.M.; Huang, S.S.; Lin, M.Y.; Lee, L.H.; Chang, L.Y.; Ou, C.C. D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther. Adv. Med. Oncol. 2019, 11, 1758835918821021.
- Yuan, L.; Zhang, S.; Li, H.; Yang, F.; Mushtaq, N.; Ullah, S.; Shi, Y.; An, C.; Xu, J. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed. Pharmacother. 2018, 108, 184–193.
- Ferrara, R.; Caramella, C.; Besse, B. Hyperprogression-immunotherapy-related phenomenon vs Intrinsic Natural History of Cancer-In Reply. JAMA Oncol. 2019, 5, 743–744.
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97.
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2019, 30, 2012.
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 2017, 19, 848–855.
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
538
Revisions:
4 times
(View History)
Update Date:
30 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




