Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pierre-Antoine Bonnet | -- | 2550 | 2023-01-30 05:14:11 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zheng, H.; Wu, P.; Bonnet, P. TLR7 Implication in Various Clinical Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/40543 (accessed on 28 February 2026).
Zheng H, Wu P, Bonnet P. TLR7 Implication in Various Clinical Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/40543. Accessed February 28, 2026.
Zheng, Haoyang, Peiyang Wu, Pierre-Antoine Bonnet. "TLR7 Implication in Various Clinical Diseases" Encyclopedia, https://encyclopedia.pub/entry/40543 (accessed February 28, 2026).
Zheng, H., Wu, P., & Bonnet, P. (2023, January 30). TLR7 Implication in Various Clinical Diseases. In Encyclopedia. https://encyclopedia.pub/entry/40543
Zheng, Haoyang, et al. "TLR7 Implication in Various Clinical Diseases." Encyclopedia. Web. 30 January, 2023.
Copy Citation
Toll-like receptor 7 (TLR7) is a class of pattern recognition receptors (PRRs) recognizing the pathogen-associated elements and damage and as such is a major player in the innate immune system. TLR7 triggers the release of pro-inflammatory cytokines or type-I interferons (IFN), which is essential for immunoregulation. Increasing reports also highlight that the abnormal activation of endosomal TLR7 is implicated in various immune-related diseases, carcinogenesis as well as the proliferation of human immunodeficiency virus (HIV).
TLR7
innate immunity
1. Introduction
Toll-like receptors (TLRs) are a major family of prototypical pattern recognition receptors (PPRs) and type I membranous glycoproteins [1]. TLRs recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). TLRs can initiate antimicrobial host defense responses to restrain pathogenic replication in the innate immune system [2][3]. The engagement of PAMP-PRR interaction allows TLRs to activate downstream signaling molecules of host defense responses [4]. To date, ten TLR subtypes (TLRs 1–10) have been identified in mammals. They are expressed in various innate immune cells, including dendritic cells (DCs), macrophages, and B cells as well as other cell types, such as epithelial cells, endothelial cells, and fibroblasts [5][6]. Notably, TLR7 and TLR9 are only expressed in plasmacytoid DCs rather than myeloid DCs [7]. Several nucleotide-sensing intracellular TLRs, including TLR3, TLR7, TLR8, and TLR9, originally synthesized in the endoplasmic reticulum (ER), are finally transferred to the endosomal compartments [8][9].
TLR7 are selectively activated by guanosine and uridine-containing single-stranded RNA (ssRNA) from viruses, bacteria, endogenous RNA, and oligoribonucleotides in the DCs’ endolysosomes [10]. TLR7 also respond to various chemical ligands, such as small heterocyclic molecules. Such recognition promotes the release of pro-inflammatory cytokines, chemokines, and type-I interferons (IFN), which are involved in the up-regulation of inflammatory reactions [11].
TLR7 is a key determinant of the protective immunity, conversely, its dysregulation is linked to the susceptibility of inflammatory diseases, such as lupus, caused by activation of host-origin nucleic-sensing pathways via TLR7 [12]. Therefore, the rational design of antagonist ligands is a primary focus for the management of autoimmune disorders, cancers, virus infection, and other potential TLR7-associated clinical disorders [13][14].
The development of small-molecule antagonists of TLR7 might start with the identification of chemical scaffolds by high-throughput screening (HTS), or chemical switches, that transform an existing agonist to an antagonist [15]. After the determination of the chemical scaffolds, structure-activity relationship (SAR) studies are then applied to optimize their antagonistic activities and might be followed by the co-crystallization of the inhibitor/TLR7 complexes [16]. Biological studies usually use HEK-Blue or HEK293 cell lines, which are engineered to overexpress TLR7 to indirectly report the nuclear factor-κB (NF-κB) translocation to the cell nucleus. Tests, such as secreted embryonic alkaline phosphatase (SEAP)-driven assay, cell proliferation assay, isothermal titration calorimetry (ITC), and immunoblotting, are also used in in vitro validations. A non-selective TLR7 agonist R848 (Resiquimod) can be commonly used as a positive control [17]. The inhibition of TLR7-induced pro-inflammatory cytokines can be measured through a real-time polymerase chain reaction (RT-PCR) [18].
2. TLR7 Main Features
2.1. Structural Studies of TLR7
TLR7 is characterized by three distinct domains (Figure 1a) [19]. Firstly, an N-terminal ectodomain (ECD) contains 26 leucine-rich repeats (LRRs). They form a large parallel β-sheet, which lies in the inner part of the ECD allowing a protein–protein interaction between the two monomers. Opposite the β-sheet, α-helices form the convex surface. The different widths between the β-sheet and the α-helices might explain why the ECD structure is curved [20]. The unstructured Z-loop region between LRR14 and LRR15 is particularly important for TLR dimerization. TLR7 requires proteolytic cleavage at the Z-loop for its activation; TLR7 with an uncleaved Z-loop is unable to form the dimer and recognize any microbial RNA [21]. After proteolytic cleavage at the Z-loop region, the N-terminal remains connected with the C-terminal of TLR7 through a disulfide bond between Cys98 (N-ter) and Cys475 (C-ter) [22].
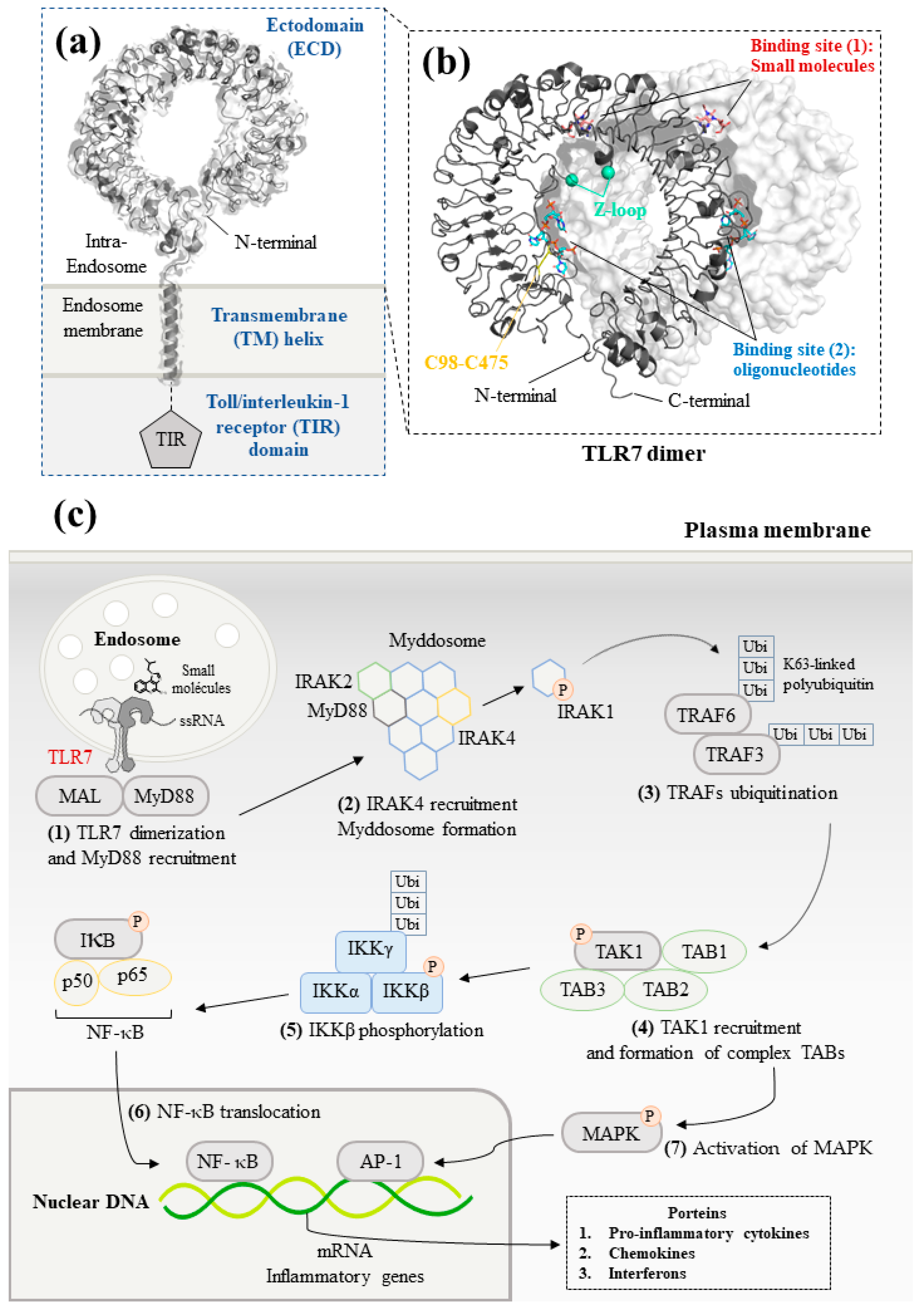
Figure 1. Structure and signaling pathways of the toll-like receptor 7 (TLR7): (a) Schematic representation of TLR7. The N-terminal ectodomain (ECD) locates in the endosome. The transmembrane helix consists of an α-helix across the endosomal membrane. The details of the structure of toll/interleukin-1 receptor (TIR) domain are not illustrated due to the lack of data for TLR7. Data are from reference [23] (PDB ID: 7CYN). (b) Front view of the ECD dimer. The cleaved Z-loop and a disulfide bridge between Cys98 and Cys475 are shown in cyan and yellow, respectively. The binding site of small molecules and synthetized oligonucleotides are illustrated in red and blue, respectively. Data are from reference [24] (PDB ID: 5GMG). (c) TLR7-mediated MyD88-dependent signaling pathways. Firstly, the recognition between small-molecule ligands or ssRNA and TLR7 allows to initiate downstream signaling. MyD88 then forms the myddosome with IRAK4, which phosphorylates and releases IRAK1. After that, TRAF6 is auto-ubiquitinated and activates TAK1. TAK1 forms a complex with TAB1/2/3. Finally, translocation of NF-κB and activation of MAPK signaling pathway generates innate immune responses that lead to the production of pro-inflammatory cytokines and IFNs.
Following ligand stimulation, two ECDs of TLR7 form an m-shape symmetrical homodimer, due to the proximity of the LRR loops. This stage is crucial to trigger a downstream signal transduction [25][26]. Moreover, TLR7 has been considered as a dual receptor. Small-molecule ligands insert into the binding site within the dimerization interface, whereas the binding of oligonucleotides is found at the concave surface, respectively (Figure 1b) [24][27].
Additionally, a transmembrane (TM) domain consisting of a single long transmembrane helix inserts into the lipid endomembrane, due to hydrophobic forces [23]. Finally, the cytoplasmic toll/interleukin-1 receptor (TIR) homology domain, a horseshoe structure, is highly conserved in the TLRs. The TIR domain is taken into consideration for interacting with the other TIR-containing systems to activate a signaling cascade through recruitment of adaptor proteins. In TLR1 and TLR10, the TIR domain has a central parallel five-stranded β-sheet flanked with five α-helices. Inside of the TIR domain, a BB-loop connects a β strand and an α helix, which play a crucial role in the formation of the dimer and activation of downstream signaling. At present, the structure of TIR domain has not been reported yet in TLR7 [28][29].
2.2. TLR7 Signaling Pathways
The homodimerization of TLR7 allows to initiate the myeloid differentiation primary response 88 (MyD88)-dependent pathway in the plasmacytoid DCs (Figure 1c) [30]. In the signaling pathway, TLR7 cooperates with protein kinases, transcription factors, and adaptor proteins [31].
Upon ligand binding, TLR7 firstly moves toward the MyD88 adaptor-like protein (MAL) and interacts with MyD88 [32]. Then, MyD88 recruits interleukin-1 receptor-associated kinase (IRAK) family members and forms a large intracellular oligomeric signaling complex: the myddosome. During the formation of the myddosome, IRAK4 activates the autophosphorylation of IRAK1, which is then released to interact with the tumor necrosis factor receptor–associated factor 6 (TRAF6) [33]. TRAF6 functions as an E3 ubiquitin ligase and promotes the non-degradative K63-linked ubiquitination of growth factor-β-activated kinase 1 (TAK1).
Subsequently, the poly-ubiquitinated TAK1 is activated after the formation of a complex with TAK1-binding proteins (TAB1, TAB2, and TAB3) [34]. TAK1 downstream cascades are then divided into two different signaling pathways: NF-κB pathway and mitogen-activated protein kinases (MAPK) pathway [35]. TAK1 phosphorylates IκB kinase β (IKKβ). IKKβ forms a complex with a catalytic subunit IKKα and a regulatory subunit NEMO termed as IKKγ. The IKK complex then phosphorylates NF-κB inhibitory protein IκBα. The inhibitory protein IKK family inactivates and keeps apart the transcription factor NF-κB dimer [36]. Subsequently, the degradation of both IκBα and IκBβ allows the nuclear translocation of the NF-κB, which stimulates the genes encoding IFNs and pro-inflammatory cytokines [37]. Additionally, TAK1 activates the AP-1 transcription factor, which leads to an increased expression of cytokines and IFNβ in the nucleus via the MAPK signaling pathway [1].
3. TLR7 Implication in a Variety of Clinical Diseases
3.1. Autoimmune Disorders
TLR7 MyD88-dependant signaling pathway drives the production of type 1 IFN in human pDCs and is implicated in the pathogenesis of autoimmune diseases [38]. The abnormal immune system turns its defenses against pathogens upon normal physiological components of the body [39].
Among them, systemic lupus erythematosus (SLE) is a polygenic autoimmune disease characterized by the elevation of two cell types; they are autoreactive age-associated B cells (ABCs) and extrafollicular helper T cells [40]. Additionally, SLE is also associated with the production of antinuclear autoantibodies in multiple organs [41]. Abnormal resistance to the degradation of self-derived RNA activates the TLR7 MyD88 signaling pathway and increases the production of pro-inflammatory cytokines [42]. In SLE pathogenesis, TLR7 can induce the transcription of IFN-stimulated genes (ISGs), which can up-regulate type 1 IFN and activate B cells [43][44]. Several data support the link between TLR7 signaling and B cell activation and production of autoantibodies [45][46]. Similarly, the TLR7Y264H variant resulted in the activation of DCs to release serum lgG in mouse and caused severe lupus in child [39][47]. Up-regulation of TLR7 also increases IFN-β production in pDCs of SLE patients [48]. A recent study also revealed that such a variant increased the affinity of TLR7 for guanosine and cGMP and caused B-cell driven autoimmunity [49]. The TLR7 copy number tightly correlates with disease severity in SLE [50]. In addition, enhanced TLR7 signaling is associated with the differentiation of inflammatory hemophagocytes (iHPCs), which are responsible for anemia and thrombocytopenia in immunity-related diseases [51].
For the management of autoimmune disorders, several studies report the use of synthesized oligonucleotides to act as immunosuppressor of TLR7 [52][53]. Among them, IMO-3100, a TLR7/9 dual antagonist of TLR7 and TLR9 can block the expression of IFN-β, TNF-α, and interleukin 17 (IL-17) and attenuate SLE, rheumatoid arthritis (RA) in a murine model [54]. A recent study also indicates TLR7 overexpression might be a relevant biomarker in preclinical RA patients [55][56]. This overexpression might be especially observed in the endosomes of CD8+ T cells [57]. IMO-3100 significantly improved the expression profile of disease-related MAD-3 genes involved in spindle-assembly checkpoint (SAC) [58]. Recently, IMO-3100 was evaluated in a 4-week phase 2 trial in psoriasis patients (NCT01622348). IMO-9200, a trinary antagonist of TLRs 7/8/9, ameliorates SLE progression in mouse model and shows safety and tolerance in healthy subjects [59]. IMO-8400 is also a dual antagonist of TLRs 7/9 that completed a phase 2 trial in psoriasis pathogenesis (NCT01899729) and dermatomyositis (NCT02612857).
Endosomal TLR7 is also implicated in the pathogenesis of organ-specific type 1 diabetes (T1D), which leads to insufficient insulin secretion and hyperglycemia [60]. T1D occurs as a consequence of the immune destruction of insulin-producing β-islet cells within the pancreas [61][62]. Further to the cytokines release, CD4+ helper T cells promote CD8+ cytotoxic T cells responses, which lead to the β-islet cells blast. Consequently, the loss of β-islet cells makes it difficult for the body to metabolize glucose [63][64]. TLR7 stimulates the up-regulation of a proinflammatory cytokine and type I/II IFNs and accelerates spontaneous onset of autoimmune diabetes [62]. Additionally, TLR7 deficiency suppresses the development of T1D by altering B-cell functions and immunoglobulin production in diabetogenic mouse. Meanwhile, TLR7 deficiency limits the number of CD4+ T cells and reduces the proliferation of antigen-specific CD8+ T cells [61].
Sjogren’s syndrome (SS) is also associated with TLR7 expression [65][66]. SS is a rheumatoid autoimmune disorder characterized by dry eyes and a dry mouth, and often accompanies lupus and rheumatoid arthritis. In this disease, the immune system destroys moisture-secreting glands, such as salivary and lacrimal glands. Patients with SS exhibit increased secretion of inflammatory cytokines in line with TLR7 and TLR9 activation. Such stimulation in peripheral blood B-cells indicates altered TLR signaling [67]. Therefore, the development of antagonists targeting TLR7 might prove beneficial for the treatment of SLE, T1D, and SS.
3.2. Immuno-Oncology
TLRs are also involved in the development of various tumors [68]. Either hyperactivation or hypoactivation of TLRs increase the survival and metastasis of a tumor. On the one hand, elevated expression of TLRs signaling induces the production of cytokines and stimulates immune cells, such as DCs, to foster tumor immunotherapy [69][70]. For example, administration of resiquimod (R848), a TLR7 agonist, upregulates TLR7 expression in dendritic cells (DCs), and enhances the activation of DCs, natural killer (NK) cells and increases the production of T helper cell associated cytokines in mice [71]. On the other hand, activation of the TLR7 Myd88 signaling pathway induces chronic inflammation, which is an important factor for further putative tumorigenesis and tumor progression [72]. Additionally, TLRs aberrant stimulation could be involved in the early initiation, carcinogenesis, and therapeutic resistance in several types of cancer, such as gastrointestinal malignancies, melanoma, and esophageal cancer [73][74][75]. Dysregulation of TLRs could also enhance immune escape and angiogenesis [76].
Furthermore, TLR7 overexpression is related to high cell proliferation in lung cancer as well as pancreatic cancer [74][76]. Up-regulation of TLR7 decreases the expression of several antitumor molecules implicated in apoptosis. TLR7 is also shown to be overexpressed in more than half primary human pancreatic ductal adenocarcinoma (PDAC), and such overexpression is associated with shorter patient survival [77]. Moreover, increased TLR7 accelerates the proliferation of human CD4+ T helper cells and induces the production of IL-10, IL-2, and IFNγ, and leads to chemoresistance in primary tumors [78][79]. Finally, TLR7 and TLR8 stimulation are associated with immune evasion in line with an increase in the nuclear factor NF-κB and cyclooxygenase-2 (COX-2) expression [80]. Finally, a recent study on experimental pancreas tumor models confirms a dual action of TLR7 on tumor growth [81].
3.3. Antiviral Immunotherapy and Infection
Endosomal TLRs might promote human immunodeficiency virus type 1 (HIV-1) replication and latency reversal via the stimulation of inflammatory responses [82]. TLR7 overexpression leads to the hypo-responsiveness of CD4+ T cells, and the production of IFN-α in HIV-1 replication [83][84]. TLR7 engagement in CD4+ T cells results in the dephosphorylation of transcription factor NFATc2 and then induces an anergic gene-expression program. In contrast, the anergy of CD4+ T cells could be eliminated by silencing TLR7 [85]. Furthermore, TLR7 and TLR9 were found to be involved in T cell CD95/Fas-mediated apoptosis by inducing Type 1 IFN upon exposure to HIV-1. This enhanced apoptosis has been shown to be inhibited by a phosphonothioate deoxyribose compound acting as a TLR7/9 specific antagonist [86]. Furthermore, an oligonucleotide TLR7/9 antagonist had the potential to abolish the production of virus-induced chemokines, such as interferon gamma-induced protein 10 (IP-10) in HIV-1 viremia [87].
By blocking related cytokines, influenza-related immunopathology could be moderated [88]. The mortality of viral respiratory diseases is often associated with the ‘cytokine storm’ along with an excessive pro-inflammatory cytokine production [89]. TLR7 antagonism demonstrated an adjustive role in the protection of an IFN-1-driven cytokine storm produced by pDCs and monocytes. TLR7 abrogation also reduced the number of lung neutrophils and attenuated inflammation and mortality in influenza in a murine model [90].
TLR7 is also implicated in other infection diseases, such as Pseudomonas aeruginosa pneumonia or Helicobacter pylori infection, and TLR7 or TLR7/8 antagonist might play a positive role in bacterial recognition and treatment [91][92][93]. TLR7 mediated antiviral protection appears to be observed in SARS-CoV-2 infection [94]. In addition, expression of TLR7/TLR9 in monocytes and B lymphocytes was shown to be increased in patients with chronic active EBV infection and such overexpression resulted in excessive inflammation [95].
3.4. Others
TLR7 is involved in the pathogenesis of knee osteoarthritis (OA) pain induced by microRNAs (miRNAs) [96]. TLR7 can detect the GU-rich motif of miRNAs; therefore, removal of this TLR recognition motif eliminates OA and blocks the analgesic effect [97]. Clinical data in humans have also suggested a possible involvement of TLR7 in atherosclerotic lesions characterized by the accumulations of lipid, cells and matrix components [55]. The knockout of TLR7 in vivo demonstrated a protective effect toward the atherosclerotic lesions by constraining inflammatory macrophage activation and cytokine production [98]. A recent study shows TLR7 activated platelets can increase the risk of thrombus formation and highlights the role of TLR7 in cardiovascular diseases and as potential therapeutic targets in such diseases [99].
References
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384.
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066.
- Pandey, S.; Kawai, T.; Akira, S. Microbial Sensing by Toll-Like Receptors and Intracellular Nucleic Acid Sensors. Cold Spring Harb. Perspect. Biol. 2015, 7, a0162462015.
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8. Science 2004, 303, 1526–1529.
- Lauterbach, M.A.; Hanke, J.E.; Serefidou, M.; Mangan, M.S.J.; Kolbe, C.-C.; Hess, T.; Rothe, M.; Kaiser, R.; Hoss, F.; Gehlen, J.; et al. Toll-like Receptor Signaling Rewires Macrophage Metabolism and Promotes Histone Acetylation via ATP-Citrate Lyase. Immunity 2019, 51, 997–1011.e7.
- Corzo, C.A.; Varfolomeev, E.; Setiadi, A.F.; Francis, R.; Klabunde, S.; Senger, K.; Sujatha-Bhaskar, S.; Drobnick, J.; Do, S.; Suto, E.; et al. The Kinase IRAK4 Promotes Endosomal TLR and Immune Complex Signaling in B Cells and Plasmacytoid Dendritic Cells. Sci. Signal. 2020, 13, eaaz1053.
- Jarrossay, D.; Napolitani, G.; Colonna, M.; Sallusto, F.; Lanzavecchia, A. Specialization and Complementarity in Microbial Molecule Recognition by Human Myeloid and Plasmacytoid Dendritic Cells. Eur. J. Immunol. 2001, 31, 3388–3393.
- Gay, N.J.; Symmons, M.F.; Gangloff, M.; Bryant, C.E. Assembly and Localization of Toll-like Receptor Signalling Complexes. Nat. Rev. Immunol. 2014, 14, 546–558.
- Pelka, K.; Bertheloot, D.; Reimer, E.; Phulphagar, K.; Schmidt, S.V.; Christ, A.; Stahl, R.; Watson, N.; Miyake, K.; Hacohen, N.; et al. The Chaperone UNC93B1 Regulates Toll-like Receptor Stability Independently of Endosomal TLR Transport. Immunity 2018, 48, 911–922.e7.
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801.
- Swiecki, M.; Colonna, M. The Multifaceted Biology of Plasmacytoid Dendritic Cells. Nat. Rev. Immunol. 2015, 15, 471–485.
- Junt, T.; Barchet, W. Translating Nucleic Acid-Sensing Pathways into Therapies. Nat. Rev. Immunol. 2015, 15, 529–544.
- O’reilly, S.; Duffy, L. Toll-like Receptors in the Pathogenesis of Autoimmune Diseases: Recent and Emerging Translational Developments. ITT 2016, 5, 69–80.
- Patinote, C.; Karroum, N.B.; Moarbess, G.; Cirnat, N.; Kassab, I.; Bonnet, P.-A.; Deleuze-Masquéfa, C. Agonist and Antagonist Ligands of Toll-like Receptors 7 and 8: Ingenious Tools for Therapeutic Purposes. Eur. J. Med. Chem. 2020, 193, 112238.
- Mukherjee, A.; Raychaudhuri, D.; Sinha, B.P.; Kundu, B.; Mitra, M.; Paul, B.; Bandopadhyay, P.; Ganguly, D.; Talukdar, A. A Chemical Switch for Transforming a Purine Agonist for Toll-like Receptor 7 to a Clinically Relevant Antagonist. J. Med. Chem. 2020, 63, 4776–4789.
- Knoepfel, T.; Nimsgern, P.; Jacquier, S.; Bourrel, M.; Vangrevelinghe, E.; Glatthar, R.; Behnke, D.; Alper, P.B.; Michellys, P.-Y.; Deane, J.; et al. Target-Based Identification and Optimization of 5-Indazol-5-Yl Pyridones as Toll-like Receptor 7 and 8 Antagonists Using a Biochemical TLR8 Antagonist Competition Assay. J. Med. Chem. 2020, 63, 8276–8295.
- Hu, Z.; Zhang, T.; Jiang, S.; Yin, H. Protocol for Evaluation and Validation of TLR8 Antagonists in HEK-Blue Cells via Secreted Embryonic Alkaline Phosphatase Assay. STAR Protoc. 2022, 3, 101061.
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845.
- Zhang, Z.; Ohto, U.; Shimizu, T. Toward a Structural Understanding of Nucleic Acid-sensing Toll-like Receptors in the Innate Immune System. FEBS Lett. 2017, 591, 3167–3181.
- Bell, J.K.; Mullen, G.E.D.; Leifer, C.A.; Mazzoni, A.; Davies, D.R.; Segal, D.M. Leucine-Rich Repeats and Pathogen Recognition in Toll-like Receptors. Trends Immunol. 2003, 24, 528–533.
- Maeda, K.; Akira, S. TLR7 Structure: Cut in Z-Loop. Immunity 2016, 45, 705–707.
- Kanno, A.; Yamamoto, C.; Onji, M.; Fukui, R.; Saitoh, S.; Motoi, Y.; Shibata, T.; Matsumoto, F.; Muta, T.; Miyake, K. Essential Role for Toll-like Receptor 7 (TLR7)-Unique Cysteines in an Intramolecular Disulfide Bond, Proteolytic Cleavage and RNA Sensing. Int. Immunol. 2013, 25, 413–422.
- Ishida, H.; Asami, J.; Zhang, Z.; Nishizawa, T.; Shigematsu, H.; Ohto, U.; Shimizu, T. Cryo-EM Structures of Toll-like Receptors in Complex with UNC93B1. Nat. Struct. Mol. Biol. 2021, 28, 173–180.
- Zhang, Z.; Ohto, U.; Shibata, T.; Krayukhina, E.; Taoka, M.; Yamauchi, Y.; Tanji, H.; Isobe, T.; Uchiyama, S.; Miyake, K.; et al. Structural Analysis Reveals That Toll-like Receptor 7 Is a Dual Receptor for Guanosine and Single-Stranded RNA. Immunity 2016, 45, 737–748.
- Shibata, T.; Ohto, U.; Nomura, S.; Kibata, K.; Motoi, Y.; Zhang, Y.; Murakami, Y.; Fukui, R.; Ishimoto, T.; Sano, S.; et al. Guanosine and Its Modified Derivatives Are Endogenous Ligands for TLR7. Int. Immunol. 2016, 28, 211–222.
- Miyake, K.; Shibata, T.; Ohto, U.; Shimizu, T.; Saitoh, S.-I.; Fukui, R.; Murakami, Y. Mechanisms Controlling Nucleic Acid-Sensing Toll-like Receptors. Int. Immunol. 2018, 30, 43–51.
- Wang, Y.; Zhang, S.; Li, H.; Wang, H.; Zhang, T.; Hutchinson, M.R.; Yin, H.; Wang, X. Small-Molecule Modulators of Toll-like Receptors. Acc. Chem. Res. 2020, 53, 1046–1055.
- Lushpa, V.A.; Goncharuk, M.V.; Lin, C.; Zalevsky, A.O.; Talyzina, I.A.; Luginina, A.P.; Vakhrameev, D.D.; Shevtsov, M.B.; Goncharuk, S.A.; Arseniev, A.S.; et al. Modulation of Toll-like Receptor 1 Intracellular Domain Structure and Activity by Zn2+ Ions. Commun. Biol. 2021, 4, 1003.
- Nyman, T.; Stenmark, P.; Flodin, S.; Johansson, I.; Hammarström, M.; Nordlund, P. The Crystal Structure of the Human Toll-like Receptor 10 Cytoplasmic Domain Reveals a Putative Signaling Dimer. J. Biol. Chem. 2008, 283, 11861–11865.
- Kim, T.H.; Oh, D.S.; Jung, H.E.; Chang, J.; Lee, H.K. Plasmacytoid Dendritic Cells Contribute to the Production of IFN-β via TLR7-MyD88-Dependent Pathway and CTL Priming during Respiratory Syncytial Virus Infection. Viruses 2019, 11, 730.
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Sig Transduct Target. Ther. 2021, 6, 291.
- AbdAllah, N.B.; Toraih, E.A.; Al Ageeli, E.; Elhagrasy, H.; Gouda, N.S.; Fawzy, M.S.; Helal, G.M. MYD88, NFKB1, and IL6 Transcripts Overexpression Are Associated with Poor Outcomes and Short Survival in Neonatal Sepsis. Sci. Rep. 2021, 11, 13374.
- Balka, K.R.; Nardo, D. Understanding Early TLR Signaling through the Myddosome. J. Leukoc. Biol. 2019, 105, 339–351.
- Chen, Z.J. Ubiquitination in Signaling to and Activation of IKK: Ubiquitin-Mediated Activation of IKK. Immunol. Rev. 2012, 246, 95–106.
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461.
- Tang, J.-R.; Michaelis, K.A.; Nozik-Grayck, E.; Seedorf, G.J.; Hartman-Filson, M.; Abman, S.H.; Wright, C.J. The NF-ΚB Inhibitory Proteins IκBα and IκBβ Mediate Disparate Responses to Inflammation in Fetal Pulmonary Endothelial Cells. J. Immunol. 2013, 190, 2913–2923.
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Sig. Transduct. Target. Ther. 2017, 2, 17023.
- Farrugia, M.; Baron, B. The Role of Toll-Like Receptors in Autoimmune Diseases through Failure of the Self-Recognition Mechanism. Int. J. Inflam. 2017, 2017, 8391230.
- Wang, T.; Marken, J.; Chen, J.; Tran, V.B.; Li, Q.-Z.; Li, M.; Cerosaletti, K.; Elkon, K.B.; Zeng, X.; Giltiay, N.V. High TLR7 Expression Drives the Expansion of CD19+CD24hiCD38hi Transitional B Cells and Autoantibody Production in SLE Patients. Front. Immunol. 2019, 10, 1243.
- Illescas-Montes, R.; Corona-Castro, C.C.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Infectious Processes and Systemic Lupus Erythematosus. Immunology 2019, 158, 153–160.
- Zhou, Y.; Yuan, J.; Pan, Y.; Fei, Y.; Qiu, X.; Hu, N.; Luo, Y.; Lei, W.; Li, Y.; Long, H.; et al. T Cell CD40LG Gene Expression and the Production of IgG by Autologous B Cells in Systemic Lupus Erythematosus. Clin. Immunol. 2009, 132, 362–370.
- Marshak-Rothstein, A.; Rifkin, I.R. Immunologically Active Autoantigens: The Role of Toll-Like Receptors in the Development of Chronic Inflammatory Disease. Annu. Rev. Immunol. 2007, 25, 419–441.
- Kiefer, K.; Oropallo, M.A.; Cancro, M.P.; Marshak-Rothstein, A. Role of Type I Interferons in the Activation of Autoreactive B Cells. Immunol. Cell Biol 2012, 90, 498–504.
- Wirth, J.R.; Molano, I.; Ruiz, P.; Coutermarsh-Ott, S.; Cunningham, M.A. TLR7 Agonism Accelerates Disease and Causes a Fatal Myeloproliferative Disorder in NZM 2410 Lupus Mice. Front. Immunol. 2020, 10, 3054.
- Hwang, S.-H.; Lee, H.; Yamamoto, M.; Jones, L.A.; Dayalan, J.; Hopkins, R.; Zhou, X.J.; Yarovinsky, F.; Connolly, J.E.; Curotto de Lafaille, M.A.; et al. B Cell TLR7 Expression Drives Anti-RNA Autoantibody Production and Exacerbates Disease in Systemic Lupus Erythematosus–Prone Mice. J. Immunol. 2012, 189, 5786–5796.
- Walsh, E.R.; Pisitkun, P.; Voynova, E.; Deane, J.A.; Scott, B.L.; Caspi, R.R.; Bolland, S. Dual Signaling by Innate and Adaptive Immune Receptors Is Required for TLR7-Induced B-Cell–Mediated Autoimmunity. Proc. Natl. Acad. Sci. USA 2012, 109, 16276–16281.
- Deane, J.A.; Pisitkun, P.; Barrett, R.S.; Feigenbaum, L.; Town, T.; Ward, J.M.; Flavell, R.A.; Bolland, S. Control of Toll-like Receptor 7 Expression Is Essential to Restrict Autoimmunity and Dendritic Cell Proliferation. Immunity 2007, 27, 801–810.
- Sakata, K.; Nakayamada, S.; Miyazaki, Y.; Kubo, S.; Ishii, A.; Nakano, K.; Tanaka, Y. Up-Regulation of TLR7-Mediated IFN-α Production by Plasmacytoid Dendritic Cells in Patients with Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 1957.
- Brown, G.J.; Cañete, P.F.; Wang, H.; Medhavy, A.; Bones, J.; Roco, J.A.; He, Y.; Qin, Y.; Cappello, J.; Ellyard, J.I.; et al. TLR7 Gain-of-Function Genetic Variation Causes Human Lupus. Nature 2022, 605, 349–356.
- Vanpouille-Box, C.; Hoffmann, J.A.; Galluzzi, L. Pharmacological Modulation of Nucleic Acid Sensors—Therapeutic Potential and Persisting Obstacles. Nat. Rev. Drug Discov. 2019, 18, 845–867.
- Akilesh, H.M.; Buechler, M.B.; Duggan, J.M.; Hahn, W.O.; Matta, B.; Sun, X.; Gessay, G.; Whalen, E.; Mason, M.; Presnell, S.R.; et al. Chronic TLR7 and TLR9 Signaling Drives Anemia via Differentiation of Specialized Hemophagocytes. Science 2019, 363, eaao5213.
- Rimbach, K.; Kaiser, S.; Helm, M.; Dalpke, A.H.; Eigenbrod, T. 2’-O-Methylation within Bacterial RNA Acts as Suppressor of TLR7/TLR8 Activation in Human Innate Immune Cells. J. Innate Immun. 2015, 7, 482–493.
- Schmitt, F.C.F.; Freund, I.; Weigand, M.A.; Helm, M.; Dalpke, A.H.; Eigenbrod, T. Identification of an Optimized 2′- O -Methylated Trinucleotide RNA Motif Inhibiting Toll-like Receptors 7 and 8. RNA 2017, 23, 1344–1351.
- Lai, C.-Y.; Su, Y.-W.; Lin, K.-I.; Hsu, L.-C.; Chuang, T.-H. Natural Modulators of Endosomal Toll-Like Receptor-Mediated Psoriatic Skin Inflammation. J. Immunol. Res. 2017, 2017, 1–15.
- Liu, C.-L.; Santos, M.M.; Fernandes, C.; Liao, M.; Iamarene, K.; Zhang, J.-Y.; Sukhova, G.K.; Shi, G.-P. Toll-like Receptor 7 Deficiency Protects Apolipoprotein E-Deficient Mice from Diet-Induced Atherosclerosis. Sci Rep 2017, 7, 847.
- Ramos-González, E.J.; Bastian, Y.; Castañeda-Delgado, J.E.; Zapata-Zúñiga, M.; Gómez-Moreno, M.; Cas-tillo-Ortiz, J.D.; Ramos-Remus, C.; Enciso-Moreno, J.A. Overexpression of TLR7 and TLR9 Occurs Before On-set Symptoms In First-Degree Relatives of Rheumatoid Arthritis Patients. Arch Med Res 2022, 53, 86–92.
- Swain, N.; Tripathy, A.; Padhan, P.; Raghav, S.K.; Gupta, B. Toll-like Receptor-7 Activation in CD8+ T Cells Modulates In-flammatory Mediators in Patients with Rheumatoid Arthritis. Rheumatol Int 2022, 42, 1235–1245.
- Suárez-Fariñas, M.; Arbeit, R.; Jiang, W.; Ortenzio, F.S.; Sullivan, T.; Krueger, J.G. Suppression of Molecular Inflammatory Pathways by Toll-Like Receptor 7, 8, and 9 Antagonists in a Model of IL-23-Induced Skin Inflammation. PLoS ONE 2013, 8, e84634.
- Zhu, F.; Wang, D.; Jiang, W.; Bhagat, L.; Agrawal, S. Sa1757 Targeting Innate Immune Receptors to Treat Inflammatory Bowel Disease: Preclinical Activity of IMO-9200, an Antagonist of TLRS 7, 8, and 9 in Mouse Models of Colitis. Gastroenterology 2015, 148, S-324.
- Pearson, J.A.; Tai, N.; Ekanayake-Alper, D.K.; Peng, J.; Hu, Y.; Hager, K.; Compton, S.; Wong, F.S.; Smith, P.C.; Wen, L. Norovirus Changes Susceptibility to Type 1 Diabetes by Altering Intestinal Microbiota and Immune Cell Functions. Front. Immunol. 2019, 10, 2654.
- Huang, J.; Peng, J.; Pearson, J.A.; Efthimiou, G.; Hu, Y.; Tai, N.; Xing, Y.; Zhang, L.; Gu, J.; Jiang, J.; et al. Toll-like Receptor 7 Deficiency Suppresses Type 1 Diabetes Development by Modulating B-Cell Differentiation and Function. Cell Mol. Immunol. 2021, 18, 328–338.
- Lee, A.S.; Ghoreishi, M.; Cheng, W.K.; Chang, T.-Y.E.; Zhang, Y.Q.; Dutz, J.P. Toll-like Receptor 7 Stimulation Promotes Autoimmune Diabetes in the NOD Mouse. Diabetologia 2011, 54, 1407–1416.
- Tomita, T. Apoptosis of Pancreatic β-Cells in Type 1 Diabetes. Bosn J. Basic Med. Sci. 2017, 17, 183–193.
- Eizirik, D.L.; Mandrup-Poulsen, T. A Choice of Death—The Signal-Transduction of Immune-Mediated Beta-Cell Apoptosis. Diabetologia 2001, 44, 2115–2133.
- Wang, Y.; Roussel-Queval, A.; Chasson, L.; Hanna Kazazian, N.; Marcadet, L.; Nezos, A.; Sieweke, M.H.; Mavragani, C.; Alexopoulou, L. TLR7 Signaling Drives the Development of Sjögren’s Syndrome. Front. Immunol. 2021, 12, 676010.
- Alexopoulou, L. Nucleic Acid-Sensing Toll-like Receptors: Important Players in Sjögren’s Syndrome. Front. Immunol. 2022, 13, 980400.
- Karlsen, M.; Jonsson, R.; Brun, J.G.; Appel, S.; Hansen, T. TLR-7 and -9 Stimulation of Peripheral Blood B Cells Indicate Altered TLR Signalling in Primary Sjögren’s Syndrome Patients by Increased Secretion of Cytokines. Scand. J. Immunol. 2015, 82, 523–531.
- Rakoff-Nahoum, S.; Medzhitov, R. Toll-like Receptors and Cancer. Nat. Rev. Cancer 2009, 9, 57–63.
- Javaid, N.; Choi, S. Toll-like Receptors from the Perspective of Cancer Treatment. Cancers 2020, 12, 297.
- Urban-Wojciuk, Z.; Khan, M.M.; Oyler, B.L.; Fåhraeus, R.; Marek-Trzonkowska, N.; Nita-Lazar, A.; Hupp, T.R.; Goodlett, D.R. The Role of TLRs in Anti-Cancer Immunity and Tumor Rejection. Front. Immunol. 2019, 10, 2388.
- Zhou, J.; Xu, Y.; Wang, G.; Mei, T.; Yang, H.; Liu, Y. The TLR7/8 Agonist R848 Optimizes Host and Tumor Immunity to Improve Therapeutic Efficacy in Murine Lung Cancer. Int J Oncol 2022, 61, 81.
- Grimmig, T.; Matthes, N.; Hoeland, K.; Tripathi, S.; Chandraker, A.; Grimm, M.; Moench, R.; Moll, E.-M.; Friess, H.; Tsaur, I.; et al. TLR7 and TLR8 Expression Increases Tumor Cell Proliferation and Promotes Chemoresistance in Human Pancreatic Cancer. Int. J. Oncol. 2015, 47, 857–866.
- Fukata, M.; Abreu, M.T. Role of Toll-like Receptors in Gastrointestinal Malignancies. Oncogene 2008, 27, 234–243.
- Zhang, M.; Yan, Z.; Wang, J.; Yao, X. Toll-like Receptors 7 and 8 Expression Correlates with the Expression of Immune Biomarkers and Positively Predicts the Clinical Outcome of Patients with Melanoma. OTT 2017, 10, 4339–4346.
- Sheyhidin, I. Overexpression of TLR3, TLR4, TLR7 and TLR9 in Esophageal Squamous Cell Carcinoma. WJG 2011, 17, 3745.
- Keshavarz, A.; Pourbagheri-Sigaroodi, A.; Zafari, P.; Bagheri, N.; Ghaffari, S.H.; Bashash, D. Toll-like Receptors (TLRs) in Cancer; with an Extensive Focus on TLR Agonists and Antagonists. IUBMB Life 2021, 73, 10–25.
- Stark, M.; Nicolai, M.; Tatura, M.; Keber, C.U.; Kaufmann, A.; Chung, H.; Slater, E.P.; Heeschen, C.; Lawlor, R.T.; Scarpa, A.; et al. Dissecting the Role of Toll‐like Receptor 7 in Pancreatic Cancer. Cancer Med 2023, cam4.5606.
- Chatterjee, S.; Crozet, L.; Damotte, D.; Iribarren, K.; Schramm, C.; Alifano, M.; Lupo, A.; Cherfils-Vicini, J.; Goc, J.; Katsahian, S.; et al. TLR7 Promotes Tumor Progression, Chemotherapy Resistance, and Poor Clinical Outcomes in Non–Small Cell Lung Cancer. Cancer Res. 2014, 74, 5008–5018.
- Caron, G.; Duluc, D.; Frémaux, I.; Jeannin, P.; David, C.; Gascan, H.; Delneste, Y. Direct Stimulation of Human T Cells via TLR5 and TLR7/8: Flagellin and R-848 Up-Regulate Proliferation and IFN-γ Production by Memory CD4+ T Cells. J. Immunol. 2005, 175, 1551–1557.
- Liu, B.; Qu, L.; Yan, S. Cyclooxygenase-2 Promotes Tumor Growth and Suppresses Tumor Immunity. Cancer Cell Int. 2015, 15, 106.
- Rouanet, M.; Hanoun, N.; Hubert Lulka; Ferreira, C.; Garcin, P.; Sramek, M.; Jacquemin, G.; Coste, A.; Pagan, D.; Valle, C.; et al. The Antitumoral Activity of TLR7 Ligands Is Corrupted by the Microenvironment of Pancreatic Tumors. Mol Ther 2022, 30, 1553–1563.
- Meås, H.Z.; Haug, M.; Beckwith, M.S.; Louet, C.; Ryan, L.; Hu, Z.; Landskron, J.; Nordbø, S.A.; Taskén, K.; Yin, H.; et al. Sensing of HIV-1 by TLR8 Activates Human T Cells and Reverses Latency. Nat. Commun. 2020, 11, 147.
- Fugger, L.; Jensen, L.T.; Rossjohn, J. Challenges, Progress, and Prospects of Developing Therapies to Treat Autoimmune Diseases. Cell 2020, 181, 63–80.
- Patamawenu, A.A.; Wright, N.E.; Shofner, T.; Evans, S.; Manion, M.M.; Doria-Rose, N.; Migueles, S.A.; Mendoza, D.; Peterson, B.; Wilhelm, C.; et al. Toll-like Receptor 7-Adapter Complex Modulates Interferon-α Production in HIV-Stimulated Plasmacytoid Dendritic Cells. PLoS ONE 2019, 14, e0225806.
- Dominguez-Villar, M.; Gautron, A.-S.; de Marcken, M.; Keller, M.J.; Hafler, D.A. TLR7 Induces Anergy in Human CD4+ T Cells. Nat. Immunol. 2015, 16, 118–128.
- Fraietta, J.A.; Mueller, Y.M.; Yang, G.; Boesteanu, A.C.; Gracias, D.T.; Do, D.H.; Hope, J.L.; Kathuria, N.; McGettigan, S.E.; Lewis, M.G.; et al. Type I Interferon Upregulates Bak and Contributes to T Cell Loss during Human Immunodeficiency Virus (HIV) Infection. PLoS Pathog. 2013, 9, e1003658.
- Simmons, R.P.; Scully, E.P.; Groden, E.E.; Arnold, K.B.; Chang, J.J.; Lane, K.; Lifson, J.; Rosenberg, E.; Lauffenburger, D.A.; Altfeld, M. HIV-1 Infection Induces Strong Production of IP-10 through TLR7/9-Dependent Pathways. AIDS 2013, 27, 2505–2517.
- Gu, Y.; Zuo, X.; Zhang, S.; Ouyang, Z.; Jiang, S.; Wang, F.; Wang, G. The Mechanism behind Influenza Virus Cytokine Storm. Viruses 2021, 13, 1362.
- Liu, Q.; Zhou, Y.; Yang, Z. The Cytokine Storm of Severe Influenza and Development of Immunomodulatory Therapy. Cell Mol. Immunol. 2016, 13, 3–10.
- Rappe, J.C.F.; Finsterbusch, K.; Crotta, S.; Mack, M.; Priestnall, S.L.; Wack, A. A TLR7 Antagonist Restricts Interferon-Dependent and -Independent Immunopathology in a Mouse Model of Severe Influenza. J. Exp. Med. 2021, 218, e20201631.
- Xu, H.; Huang, L.; Luo, Q.; Tu, Q.; Liu, J.; Yu, R.; Huang, J.; Chen, T.; Yin, Y.; Cao, J. Absence of Toll-like receptor 7 protects mice against Pseudomonas aeruginosa pneumonia. Int. Immunopharmacol. 2021, 96, 107739.
- Mancuso, G.; Gambuzza, M.; Midiri, A.; Biondo, C.; Papasergi, S.; Akira, S.; Teti, G.; Beninati, C. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat. Immunol. 2009, 10, 587–594.
- Lee, C.Y.Q.; Chan, Y.T.; Cheok, Y.Y.; Tan, G.M.Y.; Tang, T.F.; Cheong, H.C.; Vadivelu, J.; Abdullah, S.; Looi, C.Y.; Wong, W.F. Helicobacter pylori Infection Elicits Type I Interferon Response in Human Monocytes via Toll-Like Receptor 8 Signaling. J. Immunol. Res. 2022, 2022, 3861518.
- Sluis, R.M.; Cham, L.B.; Gris‐Oliver, A.; Gammelgaard, K.R.; Pedersen, J.G.; Idorn, M.; Ahmadov, U.; Hernandez, S.S.; Cé-malovic, E.; Godsk, S.H.; et al. TLR2 and TLR7 Mediate Distinct Immunopathological and Antiviral Plasmacytoid Dendritic Cell Responses to SARS‐CoV‐2 Infection. The EMBO Journal 2022, 41.
- Liu, L.; Wang, Y.; Wang, W.; Ying, W.; Sun, B.; Wang, X.; Sun, J. Increased Expression of the TLR7/9 Signaling Pathways in Chronic Active EBV Infection. Front. Pediatr. 2022, 10, 1091571.
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.-Y. MicroRNAs Are Ligands of Toll-like Receptors. RNA 2013, 19, 737–739.
- Hoshikawa, N.; Sakai, A.; Takai, S.; Suzuki, H. Targeting Extracellular MiR-21-TLR7 Signaling Provides Long-Lasting Analgesia in Osteoarthritis. Mol. Ther. Nucleic Acids 2020, 19, 199–207.
- Salagianni, M.; Galani, I.E.; Lundberg, A.M.; Davos, C.H.; Varela, A.; Gavriil, A.; Lyytikäinen, L.-P.; Lehtimäki, T.; Sigala, F.; Folkersen, L.; et al. Toll-Like Receptor 7 Protects From Atherosclerosis by Constraining “Inflammatory” Macrophage Activation. Circulation 2012, 126, 952–962.
- Shafeghat, M.; Kazemian, S.; Aminorroaya, A.; Aryan, Z.; Rezaei, N. Toll-like Receptor 7 Regulates Cardiovascular Diseases. Int Immunopharmacol 2022, 113, 109390.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revision:
1 time
(View History)
Update Date:
30 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





