
Video Upload Options
An electroencephalogram (EEG) is a graph of the difference in voltage between brain scalp locations over time. Since EEG signals are non-stationary and random, they usually present low signal intensity and high noise. Therefore, the development of advanced electrodes to obtain high-quality EEG signals is a very attractive but challenging research topic. Ag/AgCl wet electrodes have been widely used to record brain potentials in real life. However, the use of conductive gels still faces many challenges. Advanced electrodes such as semi-dry, dry contact, dry non-contact, and microneedle array electrodes have been developed to overcome the issues. In this entry, the physical features and EEG signal acquisition performances of these advanced EEG electrodes are introduced in view of the differences in contact between the skin and electrodes. Specifically, contact features, biofeatures, impedance, signal quality, and artifacts are discussed.
1. Introduction
2. Feature and Performance
2.1. Contact Feature
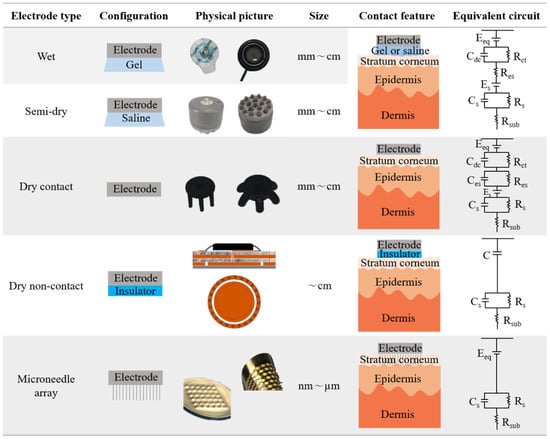
2.2. Biofeature
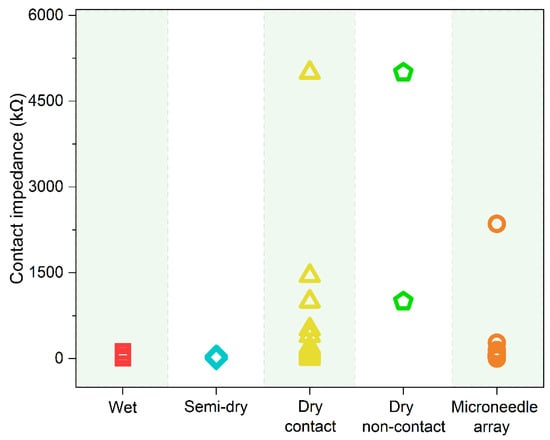
2.3. Impedance
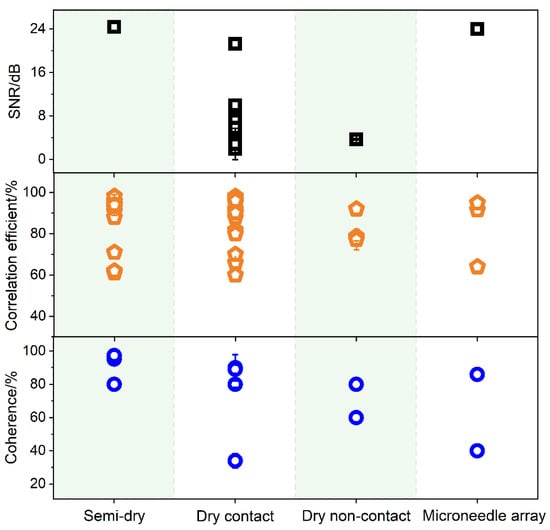
2.4. Signal Quality
2.5. Artifacts
References
- Fiscon, G.; Weitschek, E.; Felici, G.; Bertolazzi, P.; Salvo, S.D.; Bramanti, P.; Cola, M.C.D. Alzheimer’s disease patients classification through EEG signals processing. In Proceedings of the 2014 IEEE Symposium on Computational Intelligence and Data Mining (CIDM), Orlando, FL, USA, 9–12 December 2014; pp. 105–112.
- Jacob, J.E.; Nair, G.K. EEG entropies as estimators for the diagnosis of encephalopathy. Analog. Integr. Circuits Signal Process. 2019, 101, 463–474.
- Kalpakam, N.V.; Venkataramanan, S. EEG signal processing for modern wireless patient monitoring. In Proceedings of the IEEE 31st Annual Northeast Bioengineering Conference, Hoboken, NJ, USA, 2–3 April 2005; pp. 67–68.
- Sharanreddy, M.; Kulkarni, P.K. Automated EEG signal analysis for identification of epilepsy seizures and brain tumour. J. Med. Eng. Technol. 2013, 37, 511–519.
- Zetterberg, L.H. Estimation of parameters for a linear difference equation with application to EEG analysis. Math. Biosci. 1969, 5, 227–275.
- Jia, H. EEG Signal Analysis Method and Brain-Computer Interface Technology; Science Press: Beijing, China, 2016.
- Ruffini, G.; Dunne, S.; Farrés, E.; Marco-Pallarés, J.; Ray, C.; Mendoza, E.; Silva, R.; Grau, C. A dry electrophysiology electrode using CNT arrays. Sens. Actuators A Phys. 2006, 132, 34–41.
- Lopez-Gordo, M.A.; Sanchez-Morillo, D.; Valle, F.P. Dry EEG Electrodes. Sensors 2014, 14, 12847–12870.
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry Electrodes for Human Bioelectrical Signal Monitoring. Sensors 2020, 20, 3651.
- Burke, M.J.; Gleeson, D.T. A micropower dry-electrode ECG preamplifier. IEEE Trans. Biomed. Eng. 2000, 47, 155–162.
- Lee, S.M.; Kim, J.H.; Park, C.; Hwang, J.Y.; Hong, J.S.; Lee, K.H.; Lee, S.H. Self-Adhesive and Capacitive Carbon Nanotube-Based Electrode to Record Electroencephalograph Signals From the Hairy Scalp. IEEE Trans. Biomed. Eng. 2016, 63, 138–147.
- Zhang, L.; Kumar, K.S.; He, H.; Cai, C.J.; He, X.; Gao, H.; Yue, S.; Li, C.; Seet, R.C.; Ren, H.; et al. Fully organic compliant dry electrodes self-adhesive to skin for long-term motion-robust epidermal biopotential monitoring. Nat. Commun. 2020, 11, 4683.
- Hou, Y.; Li, Z.; Wang, Z.; Yu, H. Miura-ori structured flexible microneedle array electrode for biosignal recording. Microsyst. Nanoeng. 2021, 7, 53.
- Li, G.; Wang, S.; Li, M.; Duan, Y.Y. Towards real-life EEG applications: Novel superporous hydrogel-based semi-dry EEG electrodes enabling automatically “charge-discharge” electrolyte. J. Neural Eng. 2021, 18, 046016.
- Nandi, R.; Agam, Y.; Amdursky, N. A Protein-Based Free-Standing Proton-Conducting Transparent Elastomer for Large-Scale Sensing Applications. Adv. Mater. 2021, 33, 2101208.
- Tang, W.; Zhou, Y.; Chen, S.; Yu, S.; Yang, Y.; Lin, J.; Yin, S.; Ma, Y.; Hu, B. Delamination-Resistant Imperceptible Bioelectrode for Robust Electrophysiological Signals Monitoring. ACS Mater. Lett. 2021, 3, 1385–1393.
- Zhao, Y.; Zhang, S.; Yu, T.; Zhang, Y.; Ye, G.; Cui, H.; He, C.; Jiang, W.; Zhai, Y.; Lu, C.; et al. Ultra-conformal skin electrodes with synergistically enhanced conductivity for long-time and low-motion artifact epidermal electrophysiology. Nat. Commun. 2021, 12, 4880.
- Yang, L.; Liu, Q.; Zhang, Z.; Gan, L.; Zhang, Y.; Wu, J. Materials for Dry Electrodes for the Electroencephalography: Advances, Challenges, Perspectives. Adv. Mater. Technol. 2022, 7, 2100612.
- Ren, L.; Liu, B.; Zhou, W.; Jiang, L. A Mini Review of Microneedle Array Electrode for Bio-Signal Recording: A Review. IEEE Sens. J. 2020, 20, 577–590.
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics 2019, 8, 479.
- Sun, Y.; Yu, X.B. Capacitive Biopotential Measurement for Electrophysiological Signal Acquisition: A Review. IEEE Sens. J. 2016, 16, 2832–2853.
- Li, G.L.; Wu, J.T.; Xia, Y.H.; He, Q.G.; Jin, H.G. Review of semi-dry electrodes for EEG recording. J. Neural Eng. 2020, 17, 051004.
- Hua, H.; Tang, W.; Xu, X.; Feng, D.D.; Shu, L. Flexible Multi-Layer Semi-Dry Electrode for Scalp EEG Measurements at Hairy Sites. Micromachines 2019, 10, 518.
- Portelli, A.J.; Nasuto, S.J. Design and Development of Non-Contact Bio-Potential Electrodes for Pervasive Health Monitoring Applications. Biosensors 2017, 7, 2.
- Ren, L.; Xu, S.; Gao, J.; Lin, Z.; Chen, Z.; Liu, B.; Liang, L.; Jiang, L. Fabrication of Flexible Microneedle Array Electrodes for Wearable Bio-Signal Recording. Sensors 2018, 18, 1191.
- Sun, Y.; Ren, L.; Jiang, L.; Tang, Y.; Liu, B. Fabrication of Composite Microneedle Array Electrode for Temperature and Bio-Signal Monitoring. Sensors 2018, 18, 1193.
- Mota, A.R.; Duarte, L.; Rodrigues, D.; Martins, A.C.; Machado, A.V.; Vaz, F.; Fiedler, P.; Haueisen, J.; Nóbrega, J.M.; Fonseca, C. Development of a quasi-dry electrode for EEG recording. Sens. Actuators A Phys. 2013, 199, 310–317.
- Umar, A.H.; Othman, M.A.; Harun, F.K.C.; Yusof, Y. Dielectrics for Non-Contact ECG Bioelectrodes: A Review. IEEE Sens. J. 2021, 21, 18353–18367.
- Chi, Y.M.; Jung, T.; Cauwenberghs, G. Dry-Contact and Noncontact Biopotential Electrodes: Methodological Review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119.
- McAdams, E.T.; Jossinet, J. Nonlinear transient response of electrode-electrolyte interfaces. Med. Biol. Eng. Comput. 2000, 38, 427–432.
- Beckmann, L.; Neuhaus, C.; Medrano, G.; Jungbecker, N.; Walter, M.; Gries, T.; Leonhardt, S. Characterization of textile electrodes and conductors using standardized measurement setups. Physiol. Meas. 2010, 31, 233–247.
- Yoo, H.-J.; Van Hoof, C. Bio-Medical CMOS ICs; Springer: Berlin/Heidelberg, Germany, 2011.
- Li, G.; Qin, Z.; Xia, Y.; Wu, W.; Tian, Y.; Liu, J.; He, Q. A novel porous ceramics-based semi-dry EEG electrode. Packag. J. 2019, 11, 39–46.
- Li, G.; Wang, S.; Duan, Y. Towards conductive-gel-free electrodes: Understanding the wet electrode, semi-dry electrode and dry electrode-skin interface impedance using electrochemical impedance spectroscopy fitting. Sens. Actuators B Chem. 2018, 277, 250–260.
- 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. ISO: London, UK, 2009.
- 10993-10:2021; Biological Evaluation of Medical Devices Part 10: Tests for Skin Sensitization. ISO: London, UK, 2021.
- Xing, X.; Pei, W.; Wang, Y.; Guo, X.; Zhang, H.; Xie, Y.; Gui, Q.; Wang, F.; Chen, H. Assessing a novel micro-seepage electrode with flexible and elastic tips for wearable EEG acquisition. Sens. Actuators A Phys. 2018, 270, 262–270.
- Wang, L.-F.; Liu, J.-Q.; Yan, X.-X.; Yang, B.; Yang, C.-S. A MEMS-based pyramid micro-needle electrode for long-term EEG measurement. Microsyst. Technol. 2012, 19, 269–276.
- Grozea, C.; Voinescu, C.D.; Fazli, S. Bristle-sensors—Low-cost flexible passive dry EEG electrodes for neurofeedback and BCI applications. J. Neural Eng. 2011, 8, 025008.
- Fiedler, P.; Muhle, R.; Griebel, S.; Pedrosa, P.; Fonseca, C.; Vaz, F.; Zanow, F.; Haueisen, J. Contact Pressure and Flexibility of Multipin Dry EEG Electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 750–757.
- Fiedler, P.; Strohmeier, D.; Hunold, A.; Griebel, S.; Mühle, R.; Schreiber, M.; Pedrosa, P.; Vasconcelos, B.; Fonseca, C.; Vaz, F.; et al. Modular multipin electrodes for comfortable dry EEG. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 5705–5708.
- Ferree, T.C.; Luu, P.; Russell, G.S.; Tucker, D.M. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544.
- Vanlerberghe, F.; De Volder, M.; de Beeck, M.O.; Penders, J.; Reynaerts, D.; Puers, R.; Van Hoof, C. 2-Scale Topography Dry Electrode for Biopotential Measurements. In Proceedings of the 33rd Annual International Conference of the IEEE EMBS, Boston, MA, USA, 30 August–3 September 2011; pp. 1892–1895.
- Chen, Y.H.; de Beeck, M.O.; Vanderheyden, L.; Carrette, E.; Mihajlovic, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; Van Hoof, C. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors 2014, 14, 23758–23780.
- Wang, F.; Li, G.; Chen, J.; Duan, Y.; Zhang, D. Novel semi-dry electrodes for brain-computer interface applications. J. Neural Eng. 2016, 13, 046021.
- Gao, K.-P.; Yang, H.-J.; Wang, X.-L.; Yang, B.; Liu, J.-Q. Soft pin-shaped dry electrode with bristles for EEG signal measurements. Sens. Actuators A Phys. 2018, 283, 348–361.
- Baek, H.J.; Lee, H.J.; Lim, Y.G.; Park, K.S. Conductive polymer foam surface improves the performance of a capacitive EEG electrode. IEEE Trans. Biomed. Eng. 2012, 59, 3422–3431.
- Chen, Y.-C.; Lin, B.-S.; Pan, J.-S. Novel Noncontact Dry Electrode With Adaptive Mechanical Design for Measuring EEG in a Hairy Site. IEEE Trans. Instrum. Meas. 2015, 64, 3361–3368.
- Chi, Y.M.; Ng, P.; Cauwenberghs, G. Wireless noncontact ECG and EEG biopotential sensors. ACM Trans. Embed. Comput. Syst. 2013, 12, 1–19.
- Liu, S.; Zhu, M.; Liu, X.; Samuel, O.W.; Wang, X.; Huang, Z.; Wu, W.; Chen, S.; Li, G. Flexible noncontact electrodes for comfortable monitoring of physiological signals. Int. J. Adapt. Control Signal Process. 2019, 33, 1307–1318.
- Ren, L.; Jiang, Q.; Chen, K.; Chen, Z.; Pan, C.; Jiang, L. Fabrication of a Micro-Needle Array Electrode by Thermal Drawing for Bio-Signals Monitoring. Sensors 2016, 16, 908.
- Arai, M.; Nishinaka, Y.; Miki, N. Electroencephalogram measurement using polymer-based dry microneedle electrode. Jpn. J. Appl. Phys. 2015, 54, 06FP14.
- Liao, L.D.; Wang, I.J.; Chen, S.F.; Chang, J.Y.; Lin, C.T. Design, fabrication and experimental validation of a novel dry-contact sensor for measuring electroencephalography signals without skin preparation. Sensors 2011, 11, 5819–5834.
- Wang, Y.; Pei, W.; Guo, K.; Gui, Q.; Li, X.; Chen, H.; Yang, J. Dry electrode for the measurement of biopotential signals. Sci. China Inf. Sci. 2011, 54, 2435–2442.
- Ko, D.; Lee, C.; Lee, E.-J.; Lee, S.-H.; Jung, K.-Y. A dry and flexible electrode for continuous-EEG monitoring using silver balls based polydimethylsiloxane (PDMS). Biomed. Eng. Lett. 2012, 2, 18–23.
- Tsukada, S.; Nakashima, H.; Torimitsu, K. Conductive polymer combined silk fiber bundle for bioelectrical signal recording. PLoS ONE 2012, 7, e33689.
- Hsu, L.S.; Tung, S.W.; Kuo, C.H.; Yang, Y.J. Developing barbed microtip-based electrode arrays for biopotential measurement. Sensors 2014, 14, 12370–12386.
- Arai, M.; Kudo, Y.; Miki, N. Polymer-based candle-shaped microneedle electrodes for electroencephalography on hairy skin. Jpn. J. Appl. Phys. 2016, 55, 06GP16
- Gao, K.P.; Yang, H.J.; Liao, L.L.; Jiang, C.P.; Zhao, N.; Wang, X.L.; Li, X.Y.; Chen, X.; Yang, B.; Liu, J. A Novel Bristle-Shaped Semi-Dry Electrode With Low Contact Impedance and Ease of Use Features for EEG Signal Measurements. IEEE Trans. Biomed. Eng. 2020, 67, 750–761.
- Volosyak, I.; Valbuena, D.; Malechka, T.; Peuscher, J.; Graser, A. Brain-computer interface using water-based electrodes. J. Neural Eng. 2010, 7, 066007
- Lin, S.; Liu, J.; Li, W.; Wang, D.; Huang, Y.; Jia, C.; Li, Z.; Murtaza, M.; Wang, H.; Song, J.; et al. A Flexible, Robust, and Gel-Free Electroencephalogram Electrode for Noninvasive Brain-Computer Interfaces. Nano Lett. 2019, 19, 6853–6861.
- Krishnan, A.; Kumar, R.; Venkatesh, P.; Kelly, S.; Grover, P. Low-Cost Carbon Fiber-Based Conductive Silicone Sponge EEG Electrodes. IEEE Access 2018, 2018, 1287–1290.
- Peng, H.-L.; Liu, J.-Q.; Tian, H.-C.; Dong, Y.-Z.; Yang, B.; Chen, X.; Yang, C.-S. A novel passive electrode based on porous Ti for EEG recording. Sens. Actuators B Chem. 2016, 226, 349–356.
- Li, G.; Zhang, D.; Wang, S.; Duan, Y.Y. Novel passive ceramic based semi-dry electrodes for recording electroencephalography signals from the hairy scalp. Sens. Actuators B Chem. 2016, 237, 167–178.
- Kim, D.Y.; Ku, Y.; Ahn, J.W.; Kwon, C.; Kim, H.C. Electro-deposited Nanoporous Platinum Electrode for EEG Monitoring. J. Korean Med Sci. 2018, 33, e154.
- Pedrosa, P.; Fiedler, P.; Pestana, V.; Vasconcelos, B.; Gaspar, H.; Amaral, M.H.; Freitas, D.; Haueisen, J.; Nobrega, J.M.; Fonseca, C. In-service characterization of a polymer wick-based quasi-dry electrode for rapid pasteless electroencephalography. Biomed. Technol. 2018, 63, 349–359.
- Pasion, R.; Paiva, T.O.; Pedrosa, P.; Gaspar, H.; Vasconcelos, B.; Martins, A.C.; Amaral, M.H.; Nobrega, J.M.; Pascoa, R.; Fonseca, C.; et al. Assessing a novel polymer-wick based electrode for EEG neurophysiological research. J. Neurosci. Methods 2016, 267, 126–131.
- Yang, L.; Gan, L.; Zhang, Z.; Zhang, Z.; Yang, H.; Zhang, Y.; Wu, J. Insight into the Contact Impedance between the Electrode and the Skin Surface for Electrophysical Recordings. ACS Omega 2022, 7, 13906–13912.
- Li, Z.; Guo, W.; Huang, Y.; Zhu, K.; Yi, H.; Wu, H. On-skin graphene electrodes for large area electrophysiological monitoring and human-machine interfaces. Carbon 2020, 164, 164–170.
- Li, G.; Wu, J.; Xia, Y.; Wu, Y.; Tian, Y.; Liu, J.; Chen, D.; He, Q. Towards emerging EEG applications: A novel printable flexible Ag/AgCl dry electrode array for robust recording of EEG signals at forehead sites. J. Neural Eng. 2020, 17, 026001.
- Eickenscheidt, M.; Schafer, P.; Baslan, Y.; Schwarz, C.; Stieglitz, T. Highly Porous Platinum Electrodes for Dry Ear-EEG Measurements. Sensors 2020, 20, 3176.
- Velcescu, A.; Lindley, A.; Cursio, C.; Krachunov, S.; Beach, C.; Brown, C.A.; Jones, A.K.P.; Casson, A.J. Flexible 3D-Printed EEG Electrodes. Sensors 2019, 19, 1650.
- Shu, L.; Xu, T.; Xu, X. Multilayer Sweat-Absorbable Textile Electrode for EEG Measurement in Forehead Site. IEEE Sens. J. 2019, 19, 5995–6005.
- Shao, L.; Guo, Y.; Liu, W.; Sun, T.; Wei, D. A flexible dry electroencephalogram electrode based on graphene materials. Mater. Res. Express 2019, 6, 085619.
- Krachunov, S.; Casson, A.J. 3D Printed Dry EEG Electrodes. Sensors 2016, 16, 1635.
- Liu, J.; Liu, X.; He, E.; Gao, F.; Li, Z.; Xiao, G.; Xu, S.; Cai, X. A novel dry-contact electrode for measuring electroencephalography signals. Sens. Actuators A Phys. 2019, 294, 73–80.
- Yuan, W.; Wu, X.; Gu, W.; Lin, J.; Cui, Z. Printed stretchable circuit on soft elastic substrate for wearable application. J. Semicond. 2018, 39, 015002.
- Wang, Z.; Chen, C.; Li, W.; Yuan, W.; Han, T.; Sun, C.; Tao, L.; Zhao, Y.; Chen, W. A Multichannel EEG Acquisition System With Novel Ag NWs PDMS Flexible Dry Electrodes. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 1299–1302.
- Kappel, S.L.; Rank, M.L.; Toft, H.O.; Andersen, M.; Kidmose, P. Dry-Contact Electrode Ear-EEG. IEEE Trans. Biomed. Eng. 2019, 66, 150–158.
- Lee, J.H.; Hwang, J.Y.; Zhu, J.; Hwang, H.R.; Lee, S.M.; Cheng, H.; Lee, S.H.; Hwang, S.W. Flexible Conductive Composite Integrated with Personal Earphone for Wireless, Real-Time Monitoring of Electrophysiological Signs. ACS Appl. Mater. Interfaces 2018, 10, 21184–21190.
- Song, Y.; Li, P.; Li, M.; Li, H.; Li, C.; Sun, D.; Yang, B. Fabrication of chitosan/Au-TiO2 nanotube-based dry electrodes for electroencephalography recording. Mater. Sci. Eng. 2017, 79, 740–747.
- Muthukumar, N.; Thilagavathi, G.; Kannaian, T. Polyaniline-coated foam electrodes for electroencephalography (EEG) measurement. J. Text. Inst. 2015, 107, 283–290.
- Huang, Y.J.; Wu, C.Y.; Wong, A.M.; Lin, B.S. Novel active comb-shaped dry electrode for EEG measurement in hairy site. IEEE Trans. Biomed. Eng. 2015, 62, 256–263.
- Lin, B.S.; Pan, J.S.; Chu, T.Y.; Lin, B.S. Development of a Wearable Motor-Imagery-Based Brain-Computer Interface. J. Med Syst. 2016, 40, 71.
- Han, M.-F.; Liao, L.-D.; Liu, Y.-H.; Wang, W.-R.; Lin, B.-S.; Lin, C.-T. Performance optimized of the novel dry EEG electrodes by using the Non-Dominated Sorting Genetic Algorithms (NSGA-II). In Proceedings of the TENCON 2010—2010 IEEE Region 10 Conference, Fukuoka, Japan, 21–24 November 2010; pp. 1710–1715
- Xing, X.; Wang, Y.; Pei, W.; Guo, X.; Liu, Z.; Wang, F.; Ming, G.; Zhao, H.; Gui, Q.; Chen, H. A High-Speed SSVEP-Based BCI Using Dry EEG Electrodes. Sci. Rep. 2018, 8, 14708.
- Ma, R.; Kim, D.H.; McCormick, M.; Coleman, T.; Rogers, J. A stretchable electrode array for non-invasive, skin-mounted measurement of electrocardiography (ECG), electromyography (EMG) and electroencephalography (EEG). In Proceedings of the 32nd Annual International Conference of the IEEE EMBS, Buenos Aires, Argentina, 31 August–4 September 2010.
- Fiedler, P.; Fonseca, C.; Pedrosa, P.; Martins, A.; Vaz, F.; Griebel, S.; Haueisen, J. Novel flexible Dry multipin electrodes for EEG Signal quality and interfacial impedance of Ti and TiN coatings. In Proceedings of the 35th Annual International Conference of the IEEE EMBS, Osaka, Japan, 3–7 July 2013; pp. 547–550.
- Liao, L.-D.; Chen, C.-Y.; Wang, I.-J.; Chen, S.-F.; Li, S.-Y.; Chen, B.-W.; Chang, J.-W.; Lin, C.-T. Gaming control using a wearable and wireless. J. Neuroeng. Rehabil. 2012, 9, 5.
- Fiedler, P.; Pedrosa, P.; Griebel, S.; Fonseca, C.; Vaz, F.; Zanow, F.; Haueisen, J. Novel flexible dry PU TiN-multipin electrodes First application in EEG measurements. In Proceedings of the 33rd Annual International Conference of the IEEE EMBS, Boston, MA, USA, 30 August–3 September 2011; pp. 55–58.
- Wang, L.-F.; Liu, J.-Q.; Yang, B.; Yang, C.-S. PDMS-Based Low Cost Flexible Dry Electrode for Long-Term EEG Measurement. IEEE Sens. J. 2012, 12, 2898–2904.
- Fiedler, P.; Pedrosa, P.; Griebel, S.; Fonseca, C.; Vaz, F.; Supriyanto, E.; Zanow, F.; Haueisen, J. Novel Multipin Electrode Cap System for Dry Electroencephalography. Brain Topogr. 2015, 28, 647–656.
- Lin, C.T.; Liao, L.D.; Liu, Y.H.; Wang, I.J.; Lin, B.S.; Chang, J.Y. Novel dry polymer foam electrodes for long-term EEG measurement. IEEE Trans. Biomed. Eng. 2011, 58, 1200–1207.
- Fiedler, P.; Griebel, S.; Pedrosa, P.; Fonseca, C.; Vaz, F.; Zentner, L.; Zanow, F.; Haueisen, J. Multichannel EEG with novel Ti/TiN dry electrodes. Sens. Actuators A Phys. 2015, 221, 139–147.
- Nikulin, V.V.; Kegeles, J.; Curio, G. Miniaturized electroencephalographic scalp electrode for optimal wearing comfort. Clin. Neurophysiol. 2010, 121, 1007–1014.
- Lee, J.H.; Lee, S.M.; Byeon, H.J.; Hong, J.S.; Park, K.S.; Lee, S.H. CNT/PDMS-based canal-typed ear electrodes for inconspicuous EEG recording. J. Neural Eng. 2014, 11, 046014.
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D printed dry electrode for ECG/EEG recording. Sens. Actuators A Phys. 2012, 174, 96–102.
- Tautan, A.M.; Mihajlovic, V.; Chen, Y.H.; Grundlehner, B.; Penders, J.; Serdijn, W.A. Signal Quality in Dry Electrode EEG and the Relation to Skin-electrode Contact Impedance Magnitude. In Proceedings of the International Conference on Biomedical Electronics and Devices, Angers, France, 3–6 March 2014; pp. 12–22.
- Guo, S.; Lin, R.; Wang, L.; Lau, S.; Wang, Q.; Liu, R. Low melting point metal-based flexible 3D biomedical microelectrode array by phase transition method. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 735–739.
- Liu, W.; Zhou, W.; Liu, S.; Zhang, C.; Huang, S.; Li, Y.; Hui, K.S. Electrical impedance performance of metal dry bioelectrode with different surface coatings. Sens. Actuators A Phys. 2018, 269, 515–523.
- Zhou, W.; Liu, W.; Liu, S.; Zhang, C.; Shen, Z.; Zhang, G. Characterization of impedance properties of metal dry bioelectrodes with surface microstructure arrays. Sens. Actuators A Phys. 2017, 263, 252–258.
- Wang, R.; Jiang, X.; Wang, W.; Li, Z. A microneedle electrode array on flexible substrate for long-term EEG monitoring. Sens. Actuators B Chem. 2017, 244, 750–758.
- Ren, L.; Jiang, Q.; Chen, Z.; Chen, K.; Xu, S.; Gao, J.; Jiang, L. Flexible microneedle array electrode using magnetorheological drawing lithography for bio-signal monitoring. Sens. Actuators A Phys. 2017, 268, 38–45.
- Stavrinidis, G.; Michelakis, K.; Kontomitrou, V.; Giannakakis, G.; Sevrisarianos, M.; Sevrisarianos, G.; Chaniotakis, N.; Alifragis, Y.; Konstantinidis, G. SU-8 microneedles based dry electrodes for Electroencephalogram. Microelectron. Eng. 2016, 159, 114–120.
- Zhang, H.; Pei, W.; Chen, Y.; Guo, X.; Wu, X.; Yang, X.; Chen, H. A Motion Interference-Insensitive Flexible Dry Electrode. IEEE Trans. Biomed. Eng. 2016, 63, 1136–1144.
- Sun, M.; Jia, W.; Liang, W.; Sclabassi, R.J. A low-impedance, skin-grabbing, and gel-free EEG electrode. In Proceedings of the 34th Annual International Conference of the IEEE EMBS, San Diego, CA, USA, 28 August–1 September 2012; pp. 1992–1995.
- Arai, M.; Nishinaka, Y.; Miki, N. Long-term electroencephalogram measurement using polymer-based dry microneedle electrode. In Proceedings of the 2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 81–84.
- Forvi, E.; Bedoni, M.; Carabalona, R.; Soncini, M.; Mazzoleni, P.; Rizzo, F.; O’Mahony, C.; Morasso, C.; Cassarà, D.G.; Gramatica, F. Preliminary technological assessment of microneedles-based dry electrodes for biopotential monitoring in clinical examinations. Sens. Actuators A Phys. 2012, 180, 177–186.
- Chen, Y.; Pei, W.; Chen, S.; Wu, X.; Zhao, S.; Wang, H.; Chen, H. Poly(3,4-ethylenedioxythiophene) (PEDOT) as interface material for improving electrochemical performance of microneedles array-based dry electrode. Sens. Actuators B Chem. 2013, 188, 747–756.
- Srivastava, A.K.; Bhartia, B.; Mukhopadhyay, K.; Sharma, A. Long term biopotential recording by body conformable photolithography fabricated low cost polymeric microneedle arrays. Sens. Actuators A Phys. 2015, 236, 164–172.
- Ng, W.C.; Seet, H.L.; Lee, K.S.; Ning, N.; Tai, W.X.; Sutedja, M.; Fuh, J.Y.H.; Li, X.P. Micro-spike EEG electrode and the vacuum-casting technology for mass production. J. Mater. Process. Technol. 2009, 209, 4434–4438.
- Kawana, T.; Yoshida, Y.; Kudo, Y.; Iwatani, C.; Miki, N. Design and Characterization of an EEG-Hat for Reliable EEG Measurements. Micromachines 2020, 11, 635.
- Kawana, T.; Yoshida, Y.; Kudo, Y.; Miki, N. EEG-Hat with Candle-like Microneedle Electrode. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 1111–1114.
- Guevara, M.A.; Corsi-Cabrera, M. EEG coherence or EEG correlation? Int. J. Psychophysiol. 1996, 23, 145–153.
- Shaw, J.C. An introduction to the coherence function and its use in EEG signal analysis. J. Med. Eng. Technol. 1981, 5, 279–288.
- Bradford, J.C.; Burke, B.; Nguyen, C.; Slipher, G.A.; Mrozek, R.; Hairston, D. Performance of conformable, dry EEG sensors. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4957–4960.
- Slipher, G.A.; Hairston, W.D.; Bradford, J.C.; Bain, E.D.; Mrozek, R.A. Carbon nanofiber-filled conductive silicone elastomers as soft, dry bioelectronic interfaces. PLoS ONE 2018, 13, e0189415.
- Kabiri Ameri, S.; Ho, R.; Jang, H.; Tao, L.; Wang, Y.; Wang, L.; Schnyer, D.M.; Akinwande, D.; Lu, N. Graphene Electronic Tattoo Sensors. ACS Nano 2017, 11, 7634–7641.
- Yu, Y.H.; Chen, S.H.; Chang, C.L.; Lin, C.T.; Hairston, W.D.; Mrozek, R.A. New Flexible Silicone-Based EEG Dry Sensor Material Compositions Exhibiting Improvements in Lifespan, Conductivity, and Reliability. Sensors 2016, 16, 1826.
- Lee, J.S.; Han, C.M.; Kim, J.H.; Park, K.S. Reverse-curve-arch-shaped dry EEG electrode for increased skin–electrode contact area on hairy scalps. Biomed. Technol. 2015, 51, 1643–1645.
- Matiko, J.W.; Wei, Y.; Torah, R.; Grabham, N.; Paul, G.; Beeby, S.; Tudor, J. Wearable EEG headband using printed electrodes and powered by energy harvesting for emotion monitoring in ambient assisted living. Smart Mater. Struct. 2015, 24, 125028.
- Uriguen, J.A.; Garcia-Zapirain, B. EEG artifact removal—State-of-the-art and guidelines. J. Neural Eng. 2015, 12, 031001.
- Sweeney, K.T.; Ward, T.E.; McLoone, S.F. Artifact removal in physiological signals—Practices and possibilities. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 488–500.
- Kierkels, J.J.; van Boxtel, G.J.; Vogten, L.L. A model-based objective evaluation of eye movement correction in EEG recordings. IEEE Trans. Biomed. Eng. 2006, 53, 246–253.
- Romero, S.; Mananas, M.A.; Barbanoj, M.J. A comparative study of automatic techniques for ocular artifact reduction in spontaneous EEG signals based on clinical target variables: A simulation case. Comput. Biol. Med. 2008, 38, 348–360.
- Mannan, M.M.N.; Kamran, M.A.; Jeong, M.Y. Identification and Removal of Physiological Artifacts From Electroencephalogram Signals: A Review. IEEE Access 2018, 6, 30630–30652.
- Mäki, H.; Ilmoniemi, R.J. Projecting out muscle artifacts from TMS-evoked EEG. NeuroImage 2011, 54, 2706–2710.
- Khatun, S.; Mahajan, R.; Morshed, B.I. Comparative Study of Wavelet-Based Unsupervised Ocular Artifact Removal Techniques for Single-Channel EEG Data. IEEE J. Transl. Eng. Health Med. 2016, 4, 2000108.
- Benigno, G.B.; Menon, R.S.; Serrai, H. Schrödinger filtering: A precise EEG despiking technique for EEG-fMRI gradient artifact. NeuroImage 2021, 226, 117525.
- Wyckoff, S.N.; Sherlin, L.H.; Ford, N.L.; Dalke, D. Validation of a wireless dry electrode system for electroencephalography. J. Neuroeng. Rehabil. 2015, 12, 95.
- Matsumoto, J.H.; McArthur, D.L.; Szeliga, C.W.; Lerner, J.T.; Rao, L.M.; Hussain, S.A.; Wu, J.Y.; Nuwer, M.R.; Sankar, R. Conductive Plastic Electrodes Reduce EEG Artifact During Pediatric ECMO Therapy. J. Clin. Neurophysiol. 2016, 33, 426–430.
- Aghaei-Lasboo, A.; Inoyama, K.; Fogarty, A.S.; Kuo, J.; Meador, K.J.; Walter, J.J.; Le, S.T.; Graber, K.D.; Razavi, B.; Fisher, R.S. Tripolar concentric EEG electrodes reduce noise. Clin. Neurophysiol. 2020, 131, 193–198.
- Xu, J.; Yazicioglu, R.F.; Grundlehner, B.; Harpe, P.; Makinwa, K.A.; Van Hoof, C. A 160 muW 8-Channel Active Electrode System for EEG Monitoring. IEEE Trans. Biomed. Circuits Syst. 2011, 5, 555–567.
- Guerrero, F.N.; Spinelli, E.M. A Two-Wired Ultra-High Input Impedance Active Electrode. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 437–445.
- Ives, J.R.; Mirsattari, S.M.; Jones, D. Miniaturized, on-head, invasive electrode connector integrated EEG data acquisition system. Clin. Neurophysiol. 2007, 118, 1633–1638.
- Bonmassar, G.; Fujimoto, K.; Golby, A.J. PTFOS: Flexible and absorbable intracranial electrodes for magnetic resonance imaging. PLoS ONE 2012, 7, e41187.
- Benovitski, Y.B.; Lai, A.; McGowan, C.C.; Burns, O.; Maxim, V.; Nayagam, D.A.X.; Millard, R.; Rathbone, G.D.; le Chevoir, M.A.; Williams, R.A.; et al. Ring and peg electrodes for minimally-Invasive and long-term sub-scalp EEG recordings. Epilepsy Res. 2017, 135, 29–37.
- Mahmood, M.; Kwon, S.; Kim, H.; Kim, Y.S.; Siriaraya, P.; Choi, J.; Otkhmezuri, B.; Kang, K.; Yu, K.J.; Jang, Y.C.; et al. Wireless Soft Scalp Electronics and Virtual Reality System for Motor Imagery-Based Brain-Machine Interfaces. Adv. Sci. 2021, 8, e21011298.
- Ball, T.; Kern, M.; Mutschler, I.; Aertsen, A.; Schulze-Bonhage, A. Signal quality of simultaneously recorded invasive and non-invasive EEG. Neuroimage 2009, 46, 708–716.
- Klovatch-Podlipsky, I.; Gazit, T.; Fahoum, F.; Tsirelson, B.; Kipervasser, S.; Kremer, U.; Ben-Zeev, B.; Goldberg-Stern, H.; Eisenstein, O.; Harpaz, Y.; et al. Dual array EEG-fMRI: An approach for motion artifact suppression in EEG recorded simsultaneously with fMRI. NeuroImage 2016, 142, 674–686.
- Boucousis, S.M.; Beers, C.A.; Cunningham, C.J.; Gaxiola-Valdez, I.; Pittman, D.J.; Goodyear, B.G.; Federico, P. Feasibility of an intracranial EEG-fMRI protocol at 3T: Risk assessment and image quality. Neuroimage 2012, 63, 1237–1248.
- Mancuso, M.; Sveva, V.; Cruciani, A.; Brown, K.; Ibanez, J.; Rawji, V.; Casula, E.; Premoli, I.; D’Ambrosio, S.; Rothwell, J.; et al. Transcranial Evoked Potentials Can Be Reliably Recorded with Active Electrodes. Brain Sci. 2021, 11, 145.
- Yucel, M.A.; Selb, J.; Boas, D.A.; Cash, S.S.; Cooper, R.J. Reducing motion artifacts for long-term clinical NIRS monitoring using collodion-fixed prism-based optical fibers. NeuroImage 2014, 85, 192–201.




