
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexey Chubarov | -- | 2646 | 2023-01-25 06:11:44 | | | |
| 2 | Dean Liu | -2 word(s) | 2644 | 2023-01-29 03:39:39 | | |
Video Upload Options
Magnetic nanocomposites (MNCs) combine the features of magnetic nanoparticles and a second material, which provide distinct physical, chemical, and biological properties. The magnetic core for nanocomposite synthesis is extensively used due to its high saturation magnetization, chemical stability, large surface area, and easy functionalization. Moreover, magnetic nanoparticles (MNPs) have great potential for magnetic resonance imaging (MRI), magnetic particle imaging (MPI), hyperthermia, and targeted drug and gene delivery by an external magnetic field. Numerous composing units exist, which leads to the outstanding application of composites. The authors focused on nucleic acid-based bioapplications of MNCs with polymeric, organic, inorganic, biomolecules, and bioinspared surface coating. The unique types of nanocomposites as magnetic molecularly imprinted polymer (MMIP) properties are presented. The authors aim to discuss the features of nucleic acid-based MNC information available to researchers in this field and guide them through some problems in the area, structure variation, and surface functionalization possibilities. The advancements of MNCs and imprinted polymers in nucleic acid-based therapy, diagnostics, theranostics, magnetic separation, biocatalytic, and biosensing are introduced.
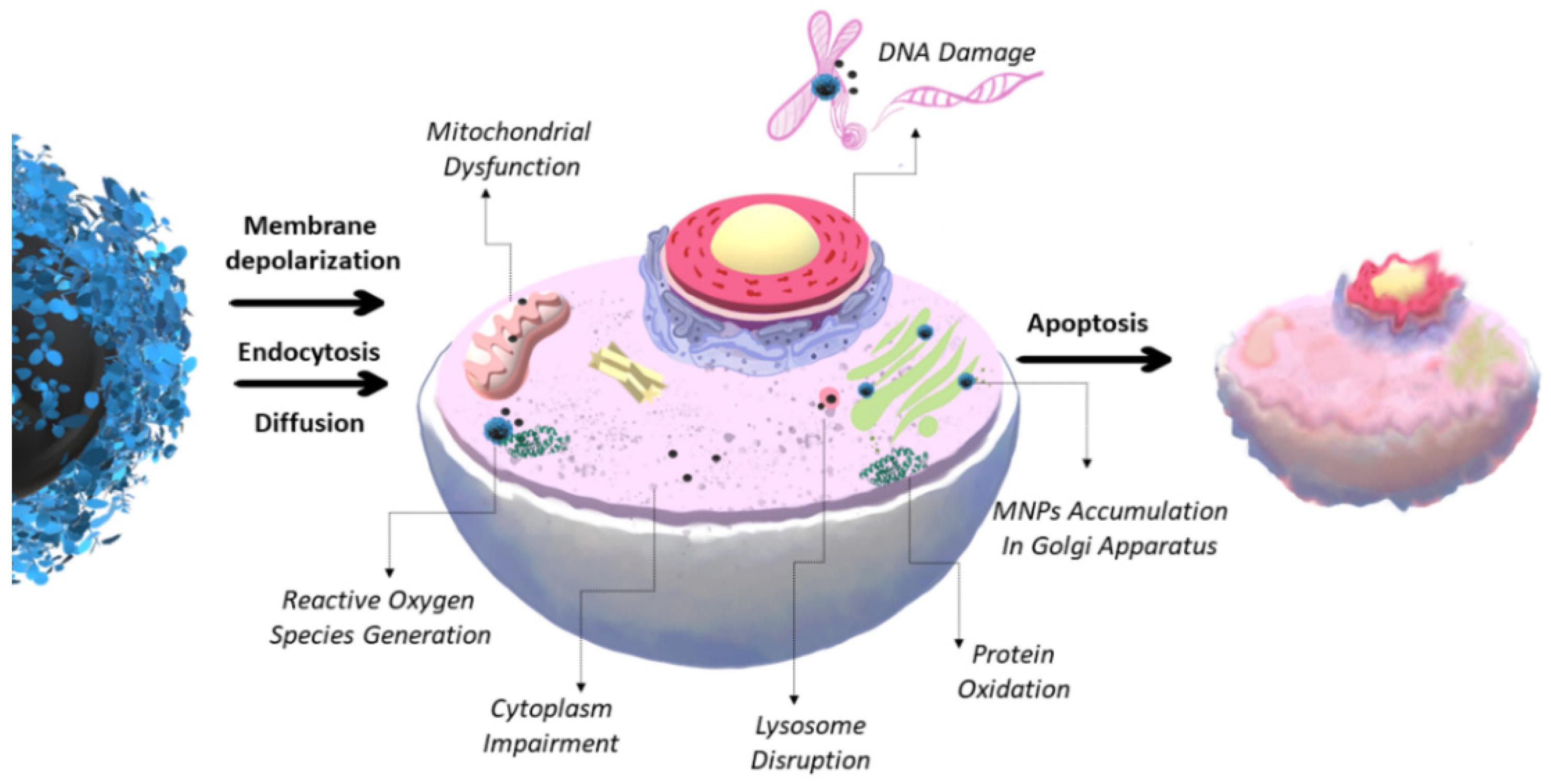
1. Toxicity of MNCs
2. Drug and Gene Delivery, Therapy, and Diagnostics (Theranostics)
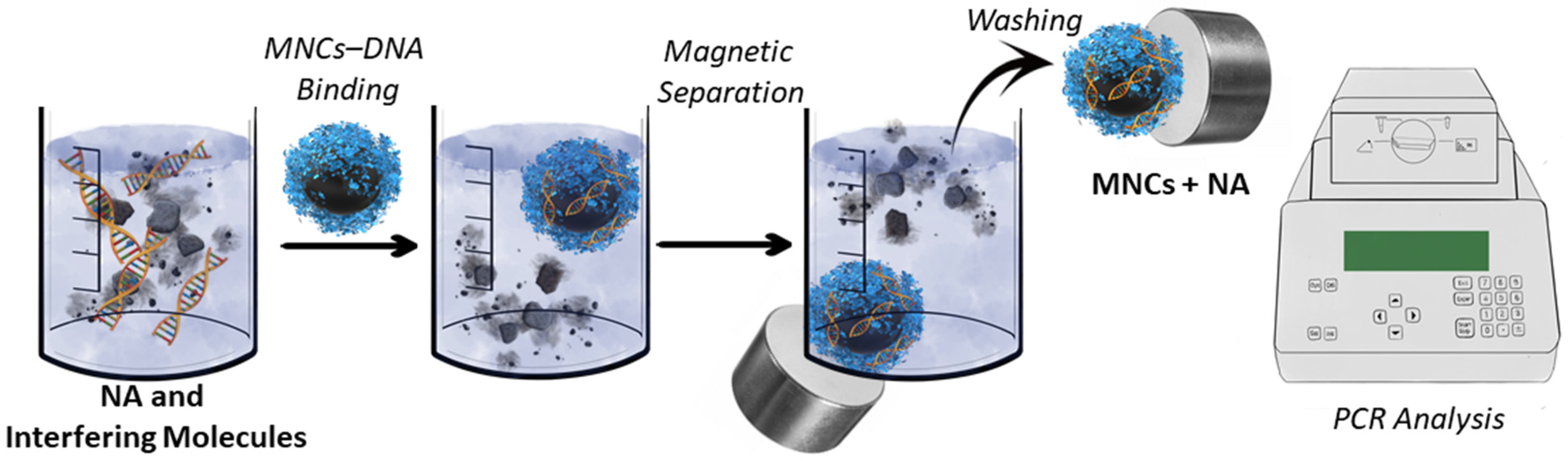
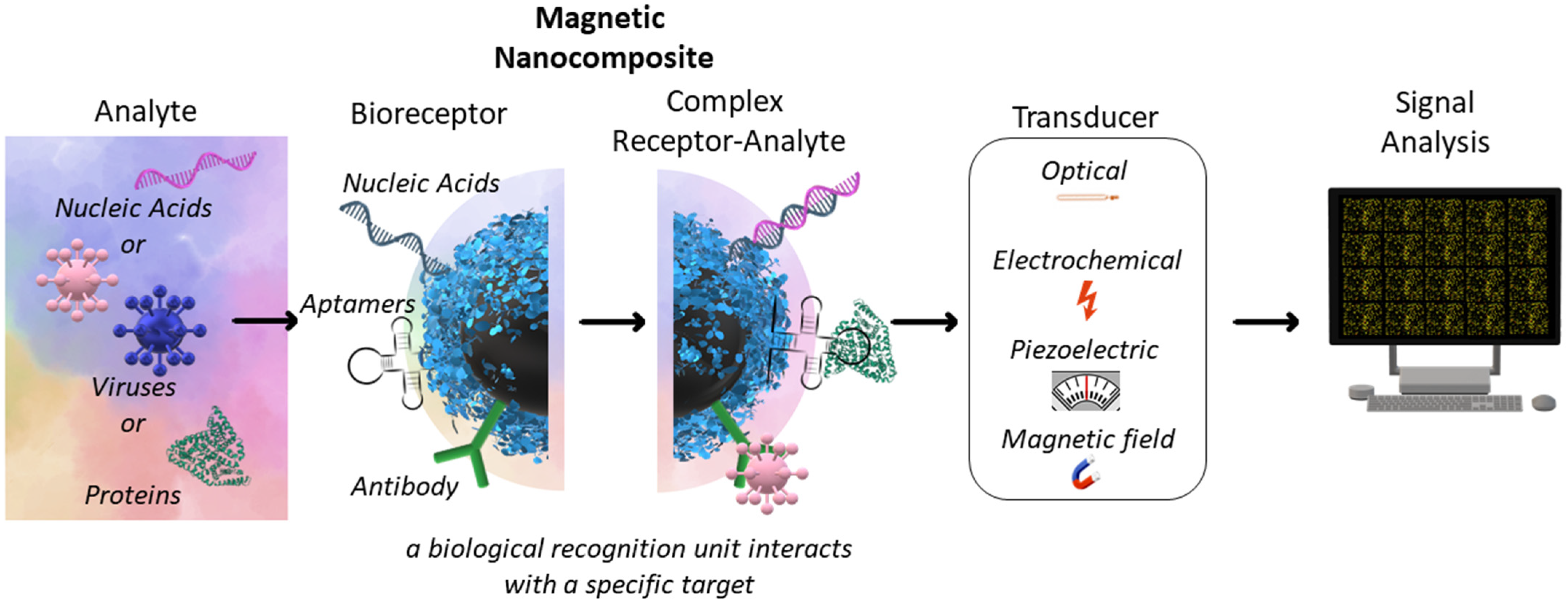
3. Magnetic Separation and Biosensors
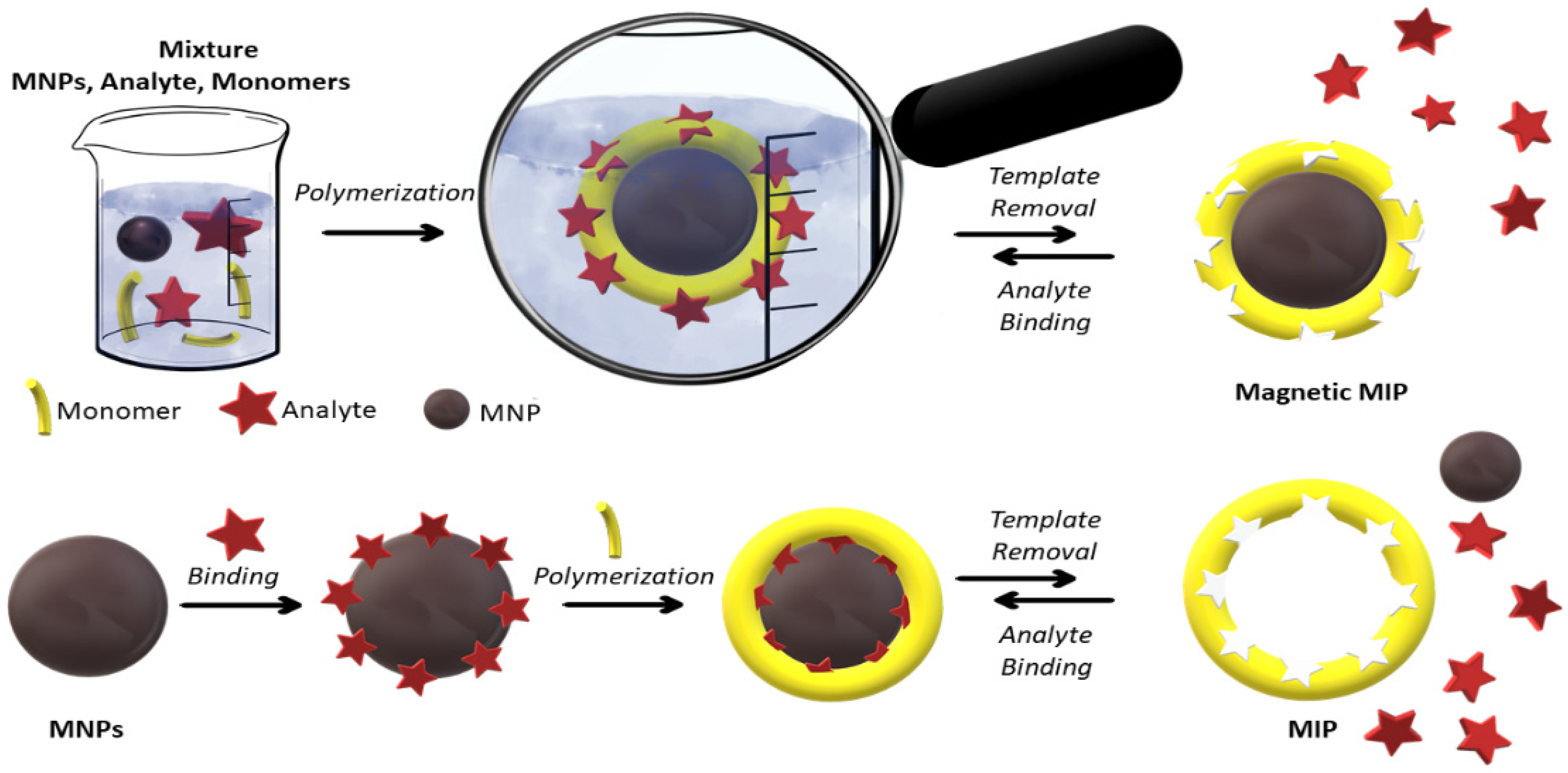
4. Magnetic Molecularly Imprinted Polymers
| Application Area | MNCs Type | NA-Based Application | Reference |
|---|---|---|---|
| Biosensing and diagnostics | MNP@Ag-amine-modified anti-miR-155 | miR-155 detection through resveratrol interaction (electrochemical label) | [90] |
| MNP@Au | Ultrasensitive colorimetric and electrochemical miRNA detection | [91][92] | |
| MNP@graphene | Electrochemical miRNA detection | [92] | |
| MNP@SiO2 | DNA and RNA extraction from Hepatocellular Carcinoma, virus RNA extraction and detection by RT-PCR, Taq polymerase fixation for long-term enzyme activity for PCR | [57][93] | |
| MNP-oleic acid | DNA detection by PCR | [57] | |
| MNP-NH2 | DNA extraction from blood and detection by PCR | [93] | |
| MNP-COOH | DNA extraction from staphylococcus aureus bacteriophages, mRNA isolation from mammalian cells | [93] | |
| MNP-OH/-NH2/-COOH | Hybrid NA separation from animal tissue samples | [94] | |
| MMIP | DNA detection | [86] | |
| MNP—rabbit antigoat immunoglobulin | Immunoglobulin (IgG) detection | [95] | |
| Therapy and diagnostics | MNP@PEI | micro-RNA intracellular delivery for MYCN inhibition in neuroblastoma | [96] |
| MNP-chitosan | Gene delivery | [97] | |
| MNP-Hyaluronic acid | Gene delivery | [98] | |
| MNP-lipids | siRNA delivery | [31] | |
| MNP lipoplex | Theranostics, imaging guided (MRI) delivery of NA | [98] | |
| Magnetic separation | MNP@Ag | mRNA extraction | [99] |
| MNP@Au | mRNA, dsDNA extraction | [99][100] | |
| MNP@graphene | dsDNA extraction | [100] | |
| MNP@SiO2 | DNA/RNA extraction | [46][93][99][100] | |
| MNP@SiO2 | NA capture from lysed white blood cells, B. subtilis, E. coli, and Rift Valley fever viruses | [101] | |
| MNP@SiO2 | viral NA extraction from serum | [93] | |
| MNP@SiO2-organic halide | DNA extraction | [46] | |
| MNP@SiO2-NH2 | DNA extraction | [46] | |
| MNP@polydopamine | genomic DNA extraction | [102] | |
| MNP-Nylon-6 | RNA extraction | [103] | |
| MNP-Streptavidin | DNA/RNA extraction, aptamer-based cell separation | [58][101] | |
| MNP-CD138 (syndecan-1) antibody conjugated | Endothelial cells (HUVEC) separation |
[95] | |
| MNP@PEI | dsDNA extraction | [100] | |
| MNP-thermosensitive polymer, poly(N-isopropylacrylamide-co-2-aminoethyl methacrylate) | DNA extraction | [52] | |
| MNP-N-isopropylacrylamide and allyl glycidyl ether, 3,5-difluoro-4-formylphenylboronic acid | S. aureus and Salmonella spp. separation | [104] |
References
- Petrov, K.D.; Chubarov, A.S. Magnetite Nanoparticles for Biomedical Applications. Encyclopedia 2022, 2, 1811–1828.
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflore, O.B.; Ger, T.R.; Hsiao, C. Der Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159.
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-specific toxicity of magnetic iron oxide-based nanoparticles. Nanotoxicology 2021, 15, 167–204.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63.
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays. Methods in Molecular Biology; Springer: Cham, Switzerland, 2017; pp. 1–17.
- Attarilar, S.; Yang, J.; Ebrahimi, M.; Wang, Q.; Liu, J.; Tang, Y.; Yang, J. The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review From the Biomedical Perspective. Front. Bioeng. Biotechnol. 2020, 8, 822.
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545.
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of iron oxide nanoparticles: Size and coating effects. J. Biochem. Mol. Toxicol. 2018, 32, e22225.
- Żuk, M.; Podgórski, R.; Ruszczyńska, A.; Ciach, T.; Majkowska-Pilip, A.; Bilewicz, A.; Krysiński, P. Multifunctional Nanoparticles Based on Iron Oxide and Gold-198 Designed for Magnetic Hyperthermia and Radionuclide Therapy as a Potential Tool for Combined HER2-Positive Cancer Treatment. Pharmaceutics 2022, 14, 1680.
- De Simone, U.; Roccio, M.; Gribaldo, L.; Spinillo, A.; Caloni, F.; Coccini, T. Human 3D cultures as models for evaluating magnetic nanoparticle CNS cytotoxicity after short- and repeated long-term exposure. Int. J. Mol. Sci. 2018, 19, 1993.
- Kappes, M.; Friedrich, B.; Pfister, F.; Huber, C.; Friedrich, R.P.; Stein, R.; Braun, C.; Band, J.; Schreiber, E.; Alexiou, C.; et al. Superparamagnetic Iron Oxide Nanoparticles for Targeted Cell Seeding: Magnetic Patterning and Magnetic 3D Cell Culture. Adv. Funct. Mater. 2022, 32, 2203672.
- Nguyen, K.; Nuß, B.; Mühlberger, M.; Unterweger, H.; Friedrich, R.P.; Alexiou, C.; Janko, C. Superparamagnetic iron oxide nanoparticles carrying chemotherapeutics improve drug efficacy in monolayer and spheroid cell culture by enabling active accumulation. Nanomaterials 2020, 10, 1577.
- Anisimov, R.A.; Gorin, D.A.; Abalymov, A.A. 3D Cell Spheroids as A Tool for Evaluating the Effectiveness of Carbon Nanotubes as A Drug Delivery and Photothermal Therapy Agents. C 2022, 8, 56.
- Henrique, R.B.L.; Lima, R.R.M.; Monteiro, C.A.P.; Oliveira, W.F.; Pereira, G.; Cabral Filho, P.E.; Fontes, A. Advances in the study of spheroids as versatile models to evaluate biological interactions of inorganic nanoparticles. Life Sci. 2022, 302, 120657.
- Juarez-Moreno, K.; Chávez-García, D.; Hirata, G.; Vazquez-Duhalt, R. Monolayer (2D) or spheroids (3D) cell cultures for nanotoxicological studies? Comparison of cytotoxicity and cell internalization of nanoparticles. Toxicol. Vitr. 2022, 85, 105461.
- Hepel, M. Magnetic nanoparticles for nanomedicine. Magnetochemistry 2020, 6, 3.
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2021, 14, 53.
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13.
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305.
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic nanoparticles for biomedical purposes: Modern trends and prospects. Magnetochemistry 2020, 6, 30.
- Nelson, N.; Port, J.; Pandey, M. Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review. J. Nanotheranostics 2020, 1, 105–135.
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Prim. 2022, 2, 1–21.
- Huang, R.-Y.; Liu, Z.-H.; Weng, W.-H.; Chang, C.-W. Magnetic nanocomplexes for gene delivery applications. J. Mater. Chem. B 2021, 9, 4267–4286.
- Sizikov, A.A.; Kharlamova, M.V.; Nikitin, M.P.; Nikitin, P.I.; Kolychev, E.L. Nonviral locally injected magnetic vectors for in vivo gene delivery: A review of studies on magnetofection. Nanomaterials 2021, 11, 1078.
- Laurent, N.; Sapet, C.; Gourrierec, L.L.; Bertosio, E.; Zelphati, O. Nucleic acid delivery using magnetic nanoparticles: The MagnetofectionTM technology. Ther. Deliv. 2011, 2, 471–482.
- Kami, D.; Takeda, S.; Itakura, Y.; Gojo, S.; Watanabe, M.; Toyoda, M. Application of magnetic nanoparticles to gene delivery. Int. J. Mol. Sci. 2011, 12, 3705–3722.
- Bakshi, S.; Zakharchenko, A.; Minko, S.; Kolpashchikov, D.; Katz, E. Towards Nanomaterials for Cancer Theranostics: A System of DNA-Modified Magnetic Nanoparticles for Detection and Suppression of RNA Marker in Cancer Cells. Magnetochemistry 2019, 5, 24.
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; et al. Smart Nanocarriers as an Emerging Platform for Cancer Therapy: A Review. Molecules 2022, 27, 146.
- Leach, J.C.; Wang, A.; Ye, K.; Jin, S. A RNA-DNA hybrid aptamer for nanoparticle-based prostate tumor targeted drug delivery. Int. J. Mol. Sci. 2016, 17, 380.
- Taghavi Pourianazar, N.; Gunduz, U. CpG oligodeoxynucleotide-loaded PAMAM dendrimer-coated magnetic nanoparticles promote apoptosis in breast cancer cells. Biomed. Pharmacother. 2016, 78, 81–91.
- Bassetto, M.; Sen, M.; Poulhes, F.; Arango-Gonzalez, B.; Bonvin, E.; Sapet, C.; Ueffing, M.; Zelphati, O. New Method for Efficient siRNA Delivery in Retina Explants: Reverse Magnetofection. Bioconjug. Chem. 2021, 32, 1078–1093.
- Gozuacik, D.; Akkoc, Y.; Kosar, A.; Dogan-Ekici, A.I.; Ekici, S. Anticancer Use of Nanoparticles as Nucleic Acid Carriers. J. Biomed. Nanotechnol. 2014, 10, 1751–1783.
- Creţu, B.E.B.; Dodi, G.; Shavandi, A.; Gardikiotis, I.; Şerban, I.L.; Balan, V. Imaging constructs: The rise of iron oxide nanoparticles. Molecules 2021, 26, 3437.
- Ganapathe, L.S.; Mohamed, M.A.; Yunus, R.M.; Berhanuddin, D.D. Magnetite (Fe3O4) nanoparticles in biomedical application: From synthesis to surface functionalisation. Magnetochemistry 2020, 6, 68.
- Baki, A.; Remmo, A.; Löwa, N.; Wiekhorst, F.; Bleul, R. Albumin-coated single-core iron oxide nanoparticles for enhanced molecular magnetic imaging (Mri/mpi). Int. J. Mol. Sci. 2021, 22, 6235.
- Kostevšek, N. A review on the optimal design of magnetic nanoparticle-based t2 mri contrast agents. Magnetochemistry 2020, 6, 11.
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—A review. Pharmaceutics 2019, 11, 601.
- Ellis, C.M.; Pellico, J.; Davis, J.J. Magnetic Nanoparticles Supporting Bio-responsive T1/T2 Magnetic Resonance Imaging. Materials 2019, 12, 4096.
- Bruno, F.; Granata, V.; Bellisari, F.C.; Sgalambro, F.; Tommasino, E.; Palumbo, P.; Arrigoni, F.; Cozzi, D.; Grassi, F.; Brunese, M.C.; et al. Advanced Magnetic Resonance Imaging (MRI) Techniques: Technical Principles and Applications in Nanomedicine. Cancers 2022, 14, 1626.
- Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V.V. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67.
- Gavilán, H.; Simeonidis, K.; Myrovali, E.; Mazarío, E.; Chubykalo-Fesenko, O.; Chantrell, R.; Balcells, L.; Angelakeris, M.; Morales, M.P.; Serantes, D. How size, shape and assembly of magnetic nanoparticles give rise to different hyperthermia scenarios. Nanoscale 2021, 13, 15631–15646.
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107.
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022.
- Wang, X.; Tu, M.; Tian, B.; Yi, Y.; Wei, Z.Z.; Wei, F. Synthesis of tumor-targeted folate conjugated fluorescent magnetic albumin nanoparticles for enhanced intracellular dual-modal imaging into human brain tumor cells. Anal. Biochem. 2016, 512, 8–17.
- Jiao, W.; Zhang, T.; Peng, M.; Yi, J.; He, Y.; Fan, H. Design of Magnetic Nanoplatforms for Cancer Theranostics. Biosensors 2022, 12, 38.
- Li, P.; Li, M.; Yue, D.; Chen, H. Solid-phase extraction methods for nucleic acid separation. A review. J. Sep. Sci. 2022, 45, 172–184.
- Wang, L.; He, K.; Sadak, O.; Wang, X.; Wang, Q.; Xu, X. Visual detection of in vitro nucleic acid replication by submicro- and nano-sized materials. Biosens. Bioelectron. 2020, 169, 112602.
- Gessner, I.; Fries, J.W.U.; Brune, V.; Mathur, S. Magnetic nanoparticle-based amplification of microRNA detection in body fluids for early disease diagnosis. J. Mater. Chem. B 2021, 9, 9–22.
- Pang, Y.; Wang, C.; Wang, J.; Sun, Z.; Xiao, R.; Wang, S. Fe3O4@Ag magnetic nanoparticles for microRNA capture and duplex-specific nuclease signal amplification based SERS detection in cancer cells. Biosens. Bioelectron. 2016, 79, 574–580.
- Pivetal, J.; Frénéa-Robin, M.; Haddour, N.; Vézy, C.; Zanini, L.F.; Ciuta, G.; Dempsey, N.M.; Dumas-Bouchiat, F.; Reyne, G.; Bégin-Colin, S.; et al. Development and applications of a DNA labeling method with magnetic nanoparticles to study the role of horizontal gene transfer events between bacteria in soil pollutant bioremediation processes. Environ. Sci. Pollut. Res. 2015, 22, 20322–20327.
- Ali, R.S.; Meng, H.; Li, Z. Zinc-Based Metal-Organic Frameworks in Drug Delivery, Cell Imaging, and Sensing. Molecules 2022, 27, 100.
- Hossain, S.; Rahman, M.; Nahar, Y.; Rahman, A.; Sharafat, M.K.; Hossain, M.; Ochiai, B.; Elaissari, A.; Ahmad, H. A simple in situ synthesis of iron oxide magnetic nanoparticles embedded in thermosensitive polymer for DNA capture. J. Mater. Res. 2020, 35, 2441–2450.
- Damavandi, F.; Wang, W.; Shen, W.Z.; Cetinel, S.; Jordan, T.; Jovel, J.; Montemagno, C.; Wong, G.K.S. Enrichment of low abundance DNA/RNA by oligonucleotide-clicked iron oxide nanoparticles. Sci. Rep. 2021, 11, 1–10.
- Li, B.; Mou, X.; Chen, Z.; Chen, H.; Deng, Y.; Li, S.; Su, E.; He, L.; He, N. The development of a rapid high-quality universal nucleic acid extraction kit based on magnetic separation. Sci. China Chem. 2017, 60, 1602–1608.
- Pinchon, E.; Leon, F.; Temurok, N.; Morvan, F.; Vasseur, J.J.; Clot, M.; Foulongne, V.; Cantaloube, J.F.; Perre, P.V.; Daynès, A.; et al. Rapid and specific DNA detection by magnetic field-enhanced agglutination assay. Talanta 2020, 219, 121344.
- Camacho-Fernández, J.C.; Jin, M.; Liu, X.; Berg, A.V.D.; Zhou, G. Ultrasensitive DNA detection based on two-step quantitative amplification on magnetic nanoparticles. Nanotechnology 2016, 27, 335102.
- Yang, Z.; Shen, B.; Yue, L.; Miao, Y.; Hu, Y.; Ouyang, R. Application of Nanomaterials to Enhance Polymerase. Molecules 2022, 27, 8854.
- Frenea-Robin, M.; Marchalot, J. Basic Principles and Recent Advances in Magnetic Cell Separation. Magnetochemistry 2022, 8, 11.
- Krishnan, S.; Goud, K.Y. Magnetic Particle Bioconjugates: A Versatile Sensor Approach. Magnetochemistry 2019, 5, 64.
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36.
- Sayad, A.; Skafidas, E.; Kwan, P. Magneto-impedance biosensor sensitivity: Effect and enhancement. Sensors 2020, 20, 5213.
- Ramin, N.A.; Ramachandran, M.R.; Saleh, N.M.; Mat Ali, Z.M.; Asman, S. Magnetic Nanoparticles Molecularly Imprinted Polymers: A Review. Curr. Nanosci. 2022, 18, 1–29.
- Meseguer-Lloret, S.; Torres-Cartas, S.; Gómez-Benito, C.; Herrero-Martínez, J.M. Magnetic molecularly imprinted polymer for the simultaneous selective extraction of phenoxy acid herbicides from environmental water samples. Talanta 2022, 239, 123082.
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly imprinted polymer micro- And nano-particles: A review. Molecules 2020, 25, 4740.
- Dmitrienko, E.V.; Pyshnaya, I.A.; Martyanov, O.N.; Pyshnyi, D.V. Molecularly imprinted polymers for biomedical and biotechnological applications. Russ. Chem. Rev. 2016, 85, 513–536.
- Ariani, M.D.; Zuhrotun, A.; Manesiotis, P.; Hasanah, A.N. Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products. Polymers 2022, 14, 1389.
- Dmitrienko, E.V.; Bulushev, R.D.; Haupt, K.; Kosolobov, S.S.; Latyshev, A.V.; Pyshnaya, I.A.; Pyshnyi, D.V. A simple approach to prepare molecularly imprinted polymers from nylon-6. J. Mol. Recognit. 2013, 26, 368–375.
- Dinc, M.; Esen, C.; Mizaikoff, B. Recent advances on core–shell magnetic molecularly imprinted polymers for biomacromolecules. Trends Anal. Chem. 2019, 114, 202–217.
- Dinc, M.; Basan, H.; Diemant, T.; Behm, R.J.; Lindén, M.; Mizaikoff, B. Inhibitor-assisted synthesis of silica-core microbeads with pepsin-imprinted nanoshells. J. Mater. Chem. B 2016, 4, 4462–4469.
- Gao, S.; Wang, W.; Wang, B. Molecularly imprinted polymers as recognition elements in optical sensors. In Molecularly Imprinted Materials; CRC: Boca Raton, FL, USA, 2008; pp. 701–726.
- Huynh, T.P.; Pieta, P.; D’Souza, F.; Kutner, W. Molecularly imprinted polymer for recognition of 5-fluorouracil by RNA-type nucleobase pairing. Anal. Chem. 2013, 85, 8304–8312.
- Babamiri, B.; Salimi, A.; Hallaj, R. A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens. Bioelectron. 2018, 117, 332–339.
- Slinchenko, O.; Rachkov, A.; Miyachi, H.; Ogiso, M.; Minoura, N. Imprinted polymer layer for recognizing double-stranded DNA. Biosens. Bioelectron. 2004, 20, 1091–1097.
- Huang, L.; Wang, X.; Xie, X.; Xie, W.; Li, X.; Gong, X.; Long, S.; Guo, H.; Liu, Z. Synthesis and DNA Adsorption of Poly(2-Vinyl-4,6-Diamino-1,3,5-Triazine) Coated Polystyrene Microspheres. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2018, 33, 999–1006.
- Rutkowska, M.; Płotka-Wasylka, J.; Morrison, C.; Wieczorek, P.P.; Namieśnik, J.; Marć, M. Application of molecularly imprinted polymers in analytical chiral separations and analysis. Trends Anal. Chem. 2018, 102, 91–102.
- Ding, X.; Heiden, P.A. Recent developments in molecularly imprinted nanoparticles by surface imprinting techniques. Macromol. Mater. Eng. 2014, 299, 268–282.
- Whitcombe, M.J.; Kirsch, N.; Nicholls, I.A. Molecular imprinting science and technology: A survey of the literature for the years 2004–2011. J. Mol. Recognit. 2014, 27, 297–401.
- Garnier, M.; Sabbah, M.; Ménager, C.; Griffete, N. Hybrid molecularly imprinted polymers: The future of nanomedicine? Nanomaterials 2021, 11, 3091.
- Niu, M.; Pham-Huy, C.; He, H. Core-shell nanoparticles coated with molecularly imprinted polymers: A review. Microchim. Acta 2016, 183, 2677–2695.
- Huang, S.; Xu, J.; Zheng, J.; Zhu, F.; Xie, L.; Ouyang, G. Synthesis and application of magnetic molecularly imprinted polymers in sample preparation. Anal. Bioanal. Chem. 2018, 410, 3991–4014.
- Li, J.; Wang, Y.; Yu, X. Magnetic Molecularly Imprinted Polymers: Synthesis and Applications in the Selective Extraction of Antibiotics. Front. Chem. 2021, 9, 1–17.
- Li, J.; Zhou, Q.; Yuan, Y.; Wu, Y. Iron-based magnetic molecular imprinted polymers and their application in removal and determination of di-n-pentyl phthalate in aqueous media. R. Soc. Open Sci. 2017, 4, 170672.
- Goyal, G.; Bhakta, S.; Mishra, P. Surface Molecularly Imprinted Biomimetic Magnetic Nanoparticles for Enantioseparation. ACS Appl. Nano Mater. 2019, 2, 6747–6756.
- Dai, Q.; Wang, Y.; Xu, W.; Liu, Y.; Zhou, Y. Adsorption and specific recognition of DNA by using imprinted polymer layers grafted onto ionic liquid functionalized magnetic microspheres. Microchim. Acta 2017, 184, 4433–4441.
- Sanadgol, N.; Wackerlig, J. Developments of smart drug-delivery systems based on magnetic molecularly imprinted polymers for targeted cancer therapy: A short review. Pharmaceutics 2020, 12, 831.
- Nawaz, N.; Abu Bakar, N.K.; Mahmud, H.N.M.E.; Jamaludin, N.S. Molecularly imprinted polymers-based DNA biosensors. Anal. Biochem. 2021, 630, 114328.
- Zhang, Z.; Liu, J. Molecularly Imprinted Polymers with DNA Aptamer Fragments as Macromonomers. ACS Appl. Mater. Interfaces 2016, 8, 6371–6378.
- Brahmbhatt, H.; Poma, A.; Pendergraff, H.M.; Watts, J.K.; Turner, N.W. Improvement of DNA recognition through molecular imprinting: Hybrid oligomer imprinted polymeric nanoparticles (oligoMIP NPs). Biomater. Sci. 2016, 4, 281–287.
- Zhang, Z.; Liu, J. Molecular Imprinting with Functional DNA. Small 2019, 15, 1805246.
- Yazdanparast, S.; Benvidi, A.; Azimzadeh, M.; Tezerjani, M.D.; Ghaani, M.R. Experimental and theoretical study for miR-155 detection through resveratrol interaction with nucleic acids using magnetic core-shell nanoparticles. Microchim. Acta 2020, 187, 1–10.
- Wang, L.; Liu, Z.J.; Cao, H.X.; Liang, G.X. Ultrasensitive colorimetric miRNA detection based on magnetic 3D DNA walker and unmodified AuNPs. Sens. Actuators B Chem. 2021, 337, 3–9.
- Masud, M.K.; Umer, M.; Hossain, M.S.A.; Yamauchi, Y.; Nguyen, N.T.; Shiddiky, M.J.A. Nanoarchitecture Frameworks for Electrochemical miRNA Detection. Trends Biochem. Sci. 2019, 44, 433–452.
- Chen, Y.; Liu, Y.; Shi, Y.; Ping, J.; Wu, J.; Chen, H. Magnetic particles for integrated nucleic acid purification, amplification and detection without pipetting. TrAC Trends Anal. Chem. 2020, 127, 115912.
- Li, P.; Li, M.; Zhang, F.; Wu, M.; Jiang, X.; Ye, B.; Zhao, Z.; Yue, D.; Fan, Q.; Chen, H. High-efficient nucleic acid separation from animal tissue samples via surface modified magnetic nanoparticles. Sep. Purif. Technol. 2021, 262, 118348.
- Yang, Q.; Dong, Y.; Qiu, Y.; Yang, X.; Cao, H.; Wu, Y. Design of Functional Magnetic Nanocomposites for Bioseparation. Colloids Surf. B Biointerfaces 2020, 191, 111014.
- Mdlovu, N.V.; Lin, K.S.; Chen, Y.; Wu, C.M. Formulation of magnetic nanocomposites for intracellular delivery of micro-RNA for MYCN inhibition in neuroblastoma. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126264.
- Lawai, V.; Ngaini, Z. Chitosan magnetic nanocomposites for gene delivery. In Polysaccharide-Based Nanocomposites for Gene Delivery and Tissue Engineering; Woodhead: Sawston, UK, 2021; p. 335.
- Do, H.D.; Ménager, C.; Michel, A.; Seguin, J.; Korichi, T.; Dhotel, H.; Marie, C.; Doan, B.T.; Mignet, N. Development of theranostic cationic liposomes designed for image-guided delivery of nucleic acid. Pharmaceutics 2020, 12, 854.
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 1–19.
- Szymczyk, A.; Drozd, M.; Kamińska, A.; Matczuk, M.; Trzaskowski, M.; Mazurkiewicz-Pawlicka, M.; Ziółkowski, R.; Malinowska, E. Comparative Evaluation of Different Surface Coatings of Fe3O4-Based Magnetic Nano Sorbent for Applications in the Nucleic Acids Extraction. Int. J. Mol. Sci. 2022, 23, 8860.
- Emaus, M.N.; Varona, M.; Eitzmann, D.R.; Hsieh, S.A.; Zeger, V.R.; Anderson, J.L. Nucleic acid extraction: Fundamentals of sample preparation methodologies, current advancements, and future endeavors. TrAC Trends Anal. Chem. 2020, 130, 115985.
- Zhang, M.; Li, L.; Li, B.; Tian, N.; Yang, M.; Zhang, H.; You, C.; Zhang, J. Adsorption of DNA by using polydopamine modified magnetic nanoparticles based on solid-phase extraction. Anal. Biochem. 2019, 579, 9–17.
- Bulgakova, A.; Chubarov, A.; Dmitrienko, E. Magnetic Nylon 6 Nanocomposites for the Microextraction of Nucleic Acids from Biological Samples. Magnetochemistry 2022, 8, 85.
- Zheng, H.; Lin, H.; Chen, X.; Sui, J.; Ullah Khan, M.; Ramesh Pavase, T.; Han, X.; Cao, L. Tailor-made magnetic nanocomposite with pH and thermo-dual responsive copolymer brush for bacterial separation. Food Chem. 2021, 358, 129907.




