Magnetic nanocomposites (MNCs) combine the features of magnetic nanoparticles and a second material, which provide distinct physical, chemical, and biological properties. The magnetic core for nanocomposite synthesis is extensively used due to its high saturation magnetization, chemical stability, large surface area, and easy functionalization. Moreover, magnetic nanoparticles (MNPs) have great potential for magnetic resonance imaging (MRI), magnetic particle imaging (MPI), hyperthermia, and targeted drug and gene delivery by an external magnetic field. Numerous composing units exist, which leads to the outstanding application of composites. This e authorsntry focuseds on nucleic acid-based bioapplications of MNCs with polymeric, organic, inorganic, biomolecules, and bioinspared surface coating. The unique types of nanocomposites as magnetic molecularly imprinted polymer (MMIP) properties are presented. The authors aimis entry aims to discuss the features of nucleic acid-based MNC information available to researchers in this field and guide them through some problems in the area, structure variation, and surface functionalization possibilities. The most recent advancements of MNCs and imprinted polymers in nucleic acid-based therapy, diagnostics, theranostics, magnetic separation, biocatalytic, and biosensing are introduced.
1. Toxicity of MNCs
The toxicity of MNPs is an essential factor for future healthcare applications
[1][2][3][31,223,224]. However, the studies on MNCs’ creation and safety assessment remained largely divided. While most of the safety studies have been focused on easy nanoparticles on the cell model, the material area goes forward with various smart constructions. However, most works usually present a primary cytotoxicity test, using an MTT compound on cancer cell lines
[4][5][225,226]. The MTT assay does not show the interaction with blood proteins, tissue media, and delayed toxicity of the degraded product. The cancer cells are highly adapted to ROS levels and unfavorable media conditions and have activated cell growth and survival systems. In this way, the toxicity of the MNCs is still less explored
[2][3][6][7][8][223,224,227,228,229]. Recently, studies on the toxicity of MNCs on spheroids were highlighted
[9][10][11][12][185,230,231,232]. Such three-dimensional (3D) cell aggregates can mimic the tumor microenvironment. In recent years, significant progress in the development of spheroids for use as a tumor model has been obtained. The cell viability in two-dimensional (2D) cell culture monolayers and spheroids has not shown the same results
[10][12][13][14][230,232,233,234]. Therefore, dose predictions from conventional cell experiments are often misleading for in vivo applications. Spheroids are a successful replacement for expensive and unethical animal experiments. However, extensive further studies are required for the stable manufacturing of various cancer spheroids and better tumor mimicking as sustainable cell growth, proliferating and non-proliferating cells, a hypoxic center, etc.
[14][15][234,235].
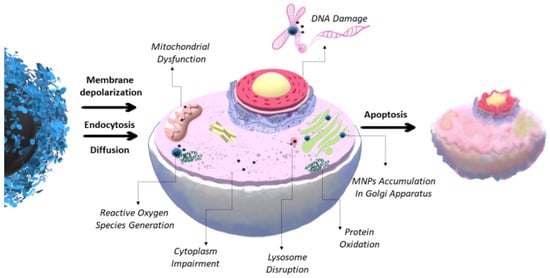
Herein, the possible cytotoxicity and organ-specific toxicity are discussed. A high indestructibility in biological liquids or low toxicity is not required for some purposes. For example, for magnetic separation, MNCs usually do not interact with human organisms and should only have good magnetic properties and the ability to interact with a target. For in vivo studies, MNCs must be non-toxic, stable in biological liquids, and biocompatible. Unstable MNPs may be extremely toxic due to the formation of reactive oxygen species (ROS), injury to the immune system, metabolic disorders, decrease in growth rate, or changes in alterations, inflammation, ulceration, etc. (
Figure 1)
[2][3][16][17][44,223,224,236]. A high ROS level leads to mitochondrial membrane damage, harmful cell proliferation, modulating gene transcription, dysregulation of ion channels, RNA destruction, and DNA and lipids oxidation with a subsequent formation of a point mutation. In extreme cases, significant damage leads to cell death (
Figure 1)
[7][17][228,236]. The oxidative stress mechanism can be provided by the release of ferrous ions due to the instability of the MNCs, direct ROS generation on the MNCs’ surface, and altering the mitochondrial function and signaling pathways
[7][17][228,236].
Figure 1. Possible toxicity mechanism of MNCs, leading cell processes’ dysregulation and triggering cell death (necrosis or apoptosis).
The MNCs’ size, shape, surface charge, coating, and surface modification highly influence biodistribution and toxicity
[2][223]. MNCs with a size less than 10 nm are quickly removed through renal clearance. Nanocomposites greater than 180–200 nm are filtrated by the spleen. Therefore, MNCs in the range from 10 to 150 nm are the most preferred. The surfaces of nanoparticles are rapidly covered in blood by various proteins. Surface chemistry highly influences nanocomposite biodistribution. For example, albumin coating usually prevents non-specific blood interaction and liver accumulation, prolongs circulation time, and moderates particle uptake in cancer tissue
[18][25]. The wrong coating of the nanocomposites may lead to the MNCs’ destabilization, aggregation, and precipitation
[19][29]. Inside the body, MNCs can be absorbed through interactions with proteins (e.g., protein albumin), blood components, and cells
[7][228]. Blood compatibility is essential for any in vivo application of MNCs. Lack of stability in the blood can trigger liver accumulation with further degradation and elimination from the body. In the worst case, the co-coagulation and precipitation of MNCs and the blood component may happen, activating thrombus formation.
The MNCs’ dose, initial concentration, biodistribution, and circulation time should be taken into account
[20][42]. The nanocomposites can be distributed into various organs and further metabolized. The overload of local MNCs may lead to high levels of free iron ion release and ROS generation in the tissue and cause aberrant cellular reactions and organ-specific toxicity
[2][3][21][223,224,237]. The in vivo toxicity experiment is an expensive and vast work, which has greatly shut down the progress in this area
[3][7][224,228]. Almost all organs are influenced by the toxic effects of MNCs. Among those are the heart, lungs, liver, kidney, and nervous and reproductive systems. For example, heart-specific toxicity provides contractile apparatus and endothelial damage, the violation of a conducting system, and ischemia
[3][224]. The MNCs’ organ-specific toxic effects may be associated with ROS generation, leading to changes in glutathione, superoxide dismutase, and coenzyme levels
[3][224]. Afterward, the in vivo interaction of MNCs and the biological system is quite complicated and dynamic. In the future, extended toxicity studies could help to bridge the gap between in vitro research results and successful clinical trials.
2. Drug and Gene Delivery, Therapy, and Diagnostics (Theranostics)
Nanoparticles and MNCs became extremely popular for cancer therapy due to the possible targeted delivery. MNCs provide drug or gene delivery to the cell or tissue by an external magnetic field. Magnetic transfection, or magnetofection, is a method that uses magnetic fields to transport NA-based MNCs to target cells. Magnetofection has been adapted to various NA types, including aptamers, siRNA, miRNA, shRNA, etc.
[22][23][24][25][26][27][76,77,78,238,239,240]. Such technology is a possibility to solve the drug resistance problem and the low efficiency of gene delivery through cell membranes
[28][241]. A combination of MNCs and siRNA or antisense oligonucleotides may be successfully used instead of a chemotherapeutic drug, resulting in a therapeutic effect
[22][25][29][30][31][32][76,238,242,243,244,245].
As stated above, the magnetic core of MNCs has multimodal advantages, such as possible tracking by magnetic resonance imaging (MRI) or magnetic particle imaging (MPI) and the hyperthermia effect. MRI and MPI are great non-invasive diagnostic techniques
[16][33][34][35][36][37][38][39][34,43,44,148,246,247,248,249]. MRI provides a high-resolution and easy image contrast manipulation. MNPs are usually known as T
2-contrast agents, which lead to a dark zone on the MRI image. However, the previous simple MNPs were withdrawn due to the side effects. There are many successful in vitro experiments and undergoing pre-clinical animal studies for MNCs
[33][34]. MPI is a relatively novel technology that was presented in 2005. MPI detects tracer MNCs selectively, providing the signal is observed without background with a high signal-to-noise ratio
[16][44]. The method possesses potential for tumor, metastases, and cell detection.
The hyperthermia effect is generated by an alternating magnetic field
[40][41][250,251]. In the presence of MNCs, heat appears in local regions, which damages tumor cells. The method is limited by the MNCs’ quality, size, morphology, and coating. The simultaneous drug, therapeutic NA, and hyperthermia using single-MNCs is a promising anticancer strategy. Recently, various MNCs to be used for multimodal imaging and theranostics have been developed
[16][33][42][34,40,44]. The combination of MRI, MPI, and primary used methods, such as single-photon emission computed tomography (SPECT), computed tomography (CT), positron emission tomography (PET), and optical imaging, have become known
[33][42][43][44][34,40,252,253]. However, theranostic synthesis is a complicated problem, which limits progress in the area
[45][88].
3. Magnetic Separation and Biosensors
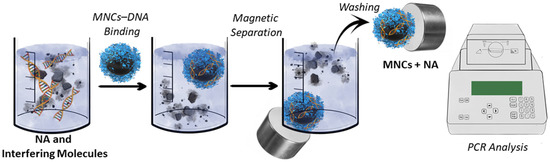
Solid-phase magnetic separation is a much more efficient protocol for NA isolation than traditional approaches
[46][47][48][49][50][51][52][53][54][55][56][62,69,71,187,254,255,256,257,258,259,260]. Magnetic separation is a relatively cheap, quick method for pure NA capture with a high yield. It usually requires ~10 min for NA isolation from the mixture using specific MNCs and a rack with magnets (
Figure 2). However, the high cost of commercially available MNCs limits their routine applications. Moreover, the specific “bind-release” of NA MNCs is extremely rare. One of the primarily used magnetic beads has an avidin/streptavidin coating and forms a specific complex with biotin-labeled oligonucleotide
[57][261]. Biotinylated oligonucleotide interacts with a targeted NA. Such systems are specific. However, the high binding constant of avidin to biotin hinders the separation of NA from MNPs and their further use. Other commercially available MNCs for NA isolation are not specific and usually bind all the NA in the probe with a certain degree of purity. Such MNCs work on the ionic interaction of NA to the MNC’s surface. Therefore, protein binding is possible. Finally, new cheap, high-capacity sorbents for the capture of NA are required.
Figure 2. Basic principles of NA magnetic separation.
Recently, magnetic cell separation has become a comprehensive technology for targeted cell population separation for various applications
[58][50]. Some MNCs for cell isolation work by using NA-based MNCs bearing aptamers. However, aptamer-based magnetic cell separation faces many obstacles, which makes it difficult to use such an approach in common practice
[58][50]. DNA aptamers can also provide specific recognition capabilities against many targets used for magnetic separation.
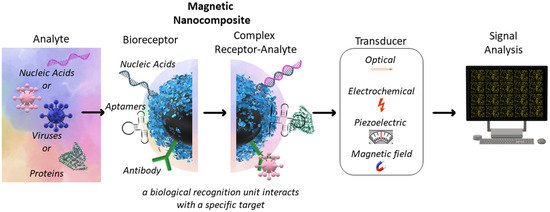
Selectively sensing a single NA allows for the discovery of rich information regarding human health
[48][71]. The separation of NA, with further analysis, is a laborious procedure that, in some cases, does not make sense. The new specific, sensitive methods are required for rapid diagnosis
[59][49]. The surface of the specific MNCs for NA detection may be modified by NA-specific molecules, including antibodies, aptamers, NA, proteins, etc. The transducer from “chemical” to “physical” signal may be electrochemical, optical, piezoelectric, etc.
[59][60][61][49,262,263] (
Figure 3). In some cases, the isolation of NA with subsequent analysis is required. This procedure may be performed using MNCs with further PCR analysis
[57][261]. For instance, MNCs can improve the sensitivity of the PCR with an extreme detection limit. Compared to traditional PCR approaches, an MNC-based PCR shows richness and a high potential
[57][261].
Figure 3. Basic principles of NA-based biosensors.
4. Magnetic Molecularly Imprinted Polymers
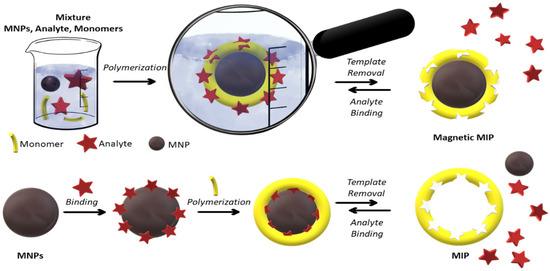
MNPs have been extensively developed for their excellent separation and extraction ability. A new innovative approach in this area is the use of MNPs with a molecularly imprinted polymer
[62][63][64][264,265,266]. Molecular imprinting is a technique to create molecule (template)-specific cavities in polymer matrices (
Figure 4).
Figure 4. Schematic representation of core–shell imprinting for MMIP preparation. The analyte is a template molecule that MIP has reversibly recognized. The number of cycles is proportional to the efficiency of MIPs.
The procedure is similar to the enzymes’ “lock and key” model. The resulting polymer is called molecularly imprinted polymers (MIPs)
[64][65][266,267]. For the magnetic core, magnetic molecularly imprinted polymers (MMIPs) have been developed
[62][66][264,268]. In the structure of MIPs, some regions can specifically interact with template molecules or structurally related molecules. Recognition can occur concerning the shape, size, or due to interactions between the functionalities of the template and polymer
[65][267]. Two types of MIPs are known
[67][269]. Among those, the classic version involves the polymerization of functional monomers, templates, and cross-linking agents (
Figure 4, top line). The second one is the change in the polymer state from liquid to solid in the presence of a template.
MIPs have many advantages, such as chemical and physical stability, easy synthesis, reusability, and cost-efficient preparation
[64][68][91,266]. Polymer matrices may consist of organic or inorganic compounds capable of recognizing molecules or ions
[62][64][65][66][264,266,267,268]. The synthesis methods of MIPs can be classified according to the format (two- or three-dimensional) and bonding type between the functional monomers and template (covalent or non-covalent) and by the nature of the functional monomers (organic or inorganic). MIPs are used in many areas, such as synthetic recognition elements, solid-phase extraction, liquid chromatography, electrochromatography, assays, drug delivery, theranostics, and biosensor production
[64][65][69][70][266,267,270,271]. MIPs are primarily prepared by bulk polymerization as monoliths (3D imprinting). The first templates for MIP recognition were low molecular weight biologically active compounds such as vitamins, hormones, toxins, drugs, nucleotides, NA, and their derivatives
[71][72][73][74][272,273,274,275]. However, this method has various drawbacks, such as a low amount of binding sites near the surface, inaccessible recognition sites within the polymer bulk, a wide range of particle sizes, and non-uniform morphology
[75][76][276,277]. The transition to the imprinting of biomolecules (nucleic acids, peptides, and proteins) requires significant changes in the existing imprinting protocols and the emergence of new ones
[64][266]. Obtaining MIPs for biomolecules remains a formidable challenge due to their large dimensions, low solubility and stability, complex structure, slow mass transfer, and structural flexibility in solution. The bulk polymerization is limited to the macromolecule and biomolecule imprinting, including peptides, proteins, NA, viruses, and bacteria. Nearly 1200 research articles were published annually on MIP-based biosensors, out of which only nearly 10% included the recognition of biomacromolecules
[77][278]. Surface molecular imprinting seems to be an alternative approach that can address some of the shortcomings of the synthesis of a primary MIP. Inorganic materials, such as silica, magnetic, gold, and silver nanoparticles, are especially widely used as a core for MIPs
[78][79][279,280]. The combination of MIPs and other materials combines features yielding smart core–shell MIP structures which allow for the control of the size and distribution of the synthesis. The hybrid magnetic MIP (MMIP) has the advantages of the technology of MIPs and MNPs.
The magnetic properties of the MMIP allow for magnetic separation, imaging, hyperthermia, and selective template release
[62][63][66][80][81][82][264,265,268,281,282,283]. MMIPs have shown a high potential in identifying a broad spectrum of analytes, from small molecule enantiomers to large proteins, NA, and macromolecules
[62][68][83][84][91,264,284,285]. The possibility to automatize this process by using magnetic properties is also an interesting feature for industrialization and mass production. Nowadays, MMIPs are widely used in various fields of biomedicine, such as biosensors, drug and gene delivery, and NA isolation
[65][78][84][267,279,285]. MMIPs show a high potential for use in cancer therapy due to targeted delivery by an external magnetic field, hyperthermia effect, and possible simultaneous drug and therapeutic NA delivery
[85][286]. The use of MMIPs for NA-based applications is also being extensively studied
[65][68][86][87][88][91,267,287,288,289]. NA can act as both templates and complex macromolecular functional monomers, which provide unique properties to the resulting MIPs
[89][290]. Consequently, combining MIPs with NA with magnetic properties provides a new class of smart synthetic NA receptors, i.e., NA-MMIPs
[86][287]. These materials open up new possibilities in this research area.
Table 1 summarizes NA-based MNC biomedical applications.
Table 1. Some examples of nucleic acid-based biomedical applications of MNCs.
| Application Area |
MNCs Type |
NA-Based Application |
Reference |
| Biosensing and diagnostics |
MNP@Ag-amine-modified anti-miR-155 |
miR-155 detection through resveratrol interaction (electrochemical label) |
[90][291] |
| MNP@Au |
Ultrasensitive colorimetric and electrochemical miRNA detection |
[91][92][292,293] |
| MNP@graphene |
Electrochemical miRNA detection |
[92][293] |
| MNP@SiO2 |
DNA and RNA extraction from Hepatocellular Carcinoma, virus RNA extraction and detection by RT-PCR, Taq polymerase fixation for long-term enzyme activity for PCR |
[57][93][122,261] |
| MNP-oleic acid |
DNA detection by PCR |
[57][261] |
| MNP-NH2 |
DNA extraction from blood and detection by PCR |
[93][122] |
| MNP-COOH |
DNA extraction from staphylococcus aureus bacteriophages, mRNA isolation from mammalian cells |
[93][122] |
| MNP-OH/-NH2/-COOH |
Hybrid NA separation from animal tissue samples |
[94][294] |
| |
MMIP |
DNA detection |
[86][287] |
| |
MNP—rabbit antigoat immunoglobulin |
Immunoglobulin (IgG) detection |
[95][295] |
| Therapy and diagnostics |
MNP@PEI |
micro-RNA intracellular delivery for MYCN inhibition in neuroblastoma |
[96][296] |
| MNP-chitosan |
Gene delivery |
[97][297] |
| MNP-Hyaluronic acid |
Gene delivery |
[98][298] |
| MNP-lipids |
siRNA delivery |
[31][244] |
| MNP lipoplex |
Theranostics, imaging guided (MRI) delivery of NA |
[98][298] |
| Magnetic separation |
MNP@Ag |
mRNA extraction |
[99][63] |
| MNP@Au |
mRNA, dsDNA extraction |
[99][63[100],114] |
| MNP@graphene |
dsDNA extraction |
[100][114] |
| MNP@SiO2 |
DNA/RNA extraction |
[46][93][99][100][62,63,114,122] |
| MNP@SiO2 |
NA capture from lysed white blood cells, B. subtilis, E. coli, and Rift Valley fever viruses |
[101][299] |
| MNP@SiO2 |
viral NA extraction from serum |
[93][122] |
| MNP@SiO2-organic halide |
DNA extraction |
[46][62] |
| MNP@SiO2-NH2 |
DNA extraction |
[46][62] |
| MNP@polydopamine |
genomic DNA extraction |
[102][171] |
| MNP-Nylon-6 |
RNA extraction |
[103][19] |
| MNP-Streptavidin |
DNA/RNA extraction, aptamer-based cell separation |
[58][101][50,299] |
| MNP-CD138 (syndecan-1) antibody conjugated |
Endothelial cells (HUVEC)
separation |
[95][295] |
| MNP@PEI |
dsDNA extraction |
[100][114] |
| MNP-thermosensitive polymer, poly(N-isopropylacrylamide-co-2-aminoethyl methacrylate) |
DNA extraction |
[52][256] |
| MNP-N-isopropylacrylamide and allyl glycidyl ether, 3,5-difluoro-4-formylphenylboronic acid |
S. aureus and Salmonella spp. separation |
[104][300] |