
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ambroz Pusnik | -- | 1777 | 2023-01-22 11:09:43 | | | |
| 2 | Xhevat Lumi | + 7 word(s) | 1784 | 2023-01-25 09:54:17 | | | | |
| 3 | Xhevat Lumi | -14 word(s) | 1770 | 2023-01-27 13:06:30 | | | | |
| 4 | Catherine Yang | -1 word(s) | 1769 | 2023-01-28 04:04:20 | | |
Video Upload Options
Dysphotopsias are unwanted visual phenomena that occur after cataract surgery. They represent some of the most common reasons for patient dissatisfaction after uncomplicated surgery for cataract phacoemulsification with in-the-bag intraocular lens (IOL) implantation. Depending on the form of the optical phenomenon and the effect it poses on vision, dysphotopsias are divided into positive and negative type. Positive dysphotopsias are usually described by patients as glare, light streaks, starbursts, light arcs, rings, haloes, or flashes of light. Negative dysphotopsias (ND) are manifested as an arc-shaped shadow or line usually located in the temporal part of the visual field, similar to a temporal scotoma. ND is evoked by an external light source that is typically temporally oriented. Patients most commonly experience this phenomenon in photopic conditions when the pupil is narrow. ND is a diagnosis of exclusion where other possible ocular and neuro-ophthalmological pathologies should be excluded. The etiology of ND is not clearly defined, and the cause seems to be multifactorial. Holladay and Simpson categorized the risk factors for ND development into three groups: anatomic characteristics (pupil size, hyperopia, and angle kappa), IOL properties (IOL surface steepness, edge design, dioptricpower, and refraction index), and surgical technique for cataract removal (optic–haptic junction orientation and position of nasal anterior capsule to the IOL surface).
1. The Illumination Gap Theory
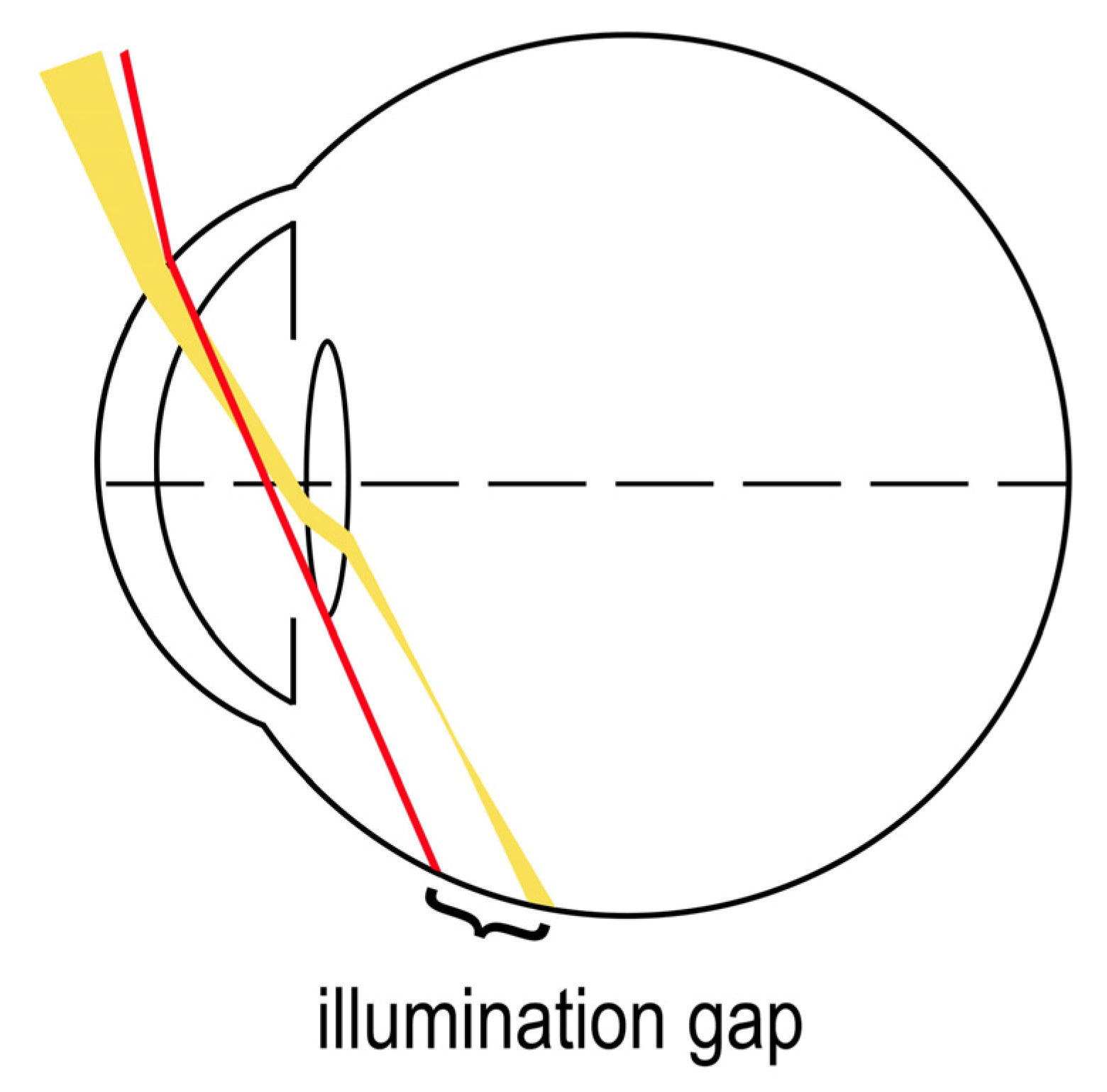
The most supported working theory for temporal visual field shadow occurrence in pseudophakic patients with ND is the illumination gap of the nasal retina [1]. The illumination gap is caused by different refraction of rays that hit the IOL optic periphery to those that miss the IOL (Figure 1) [1][2][3]. The illumination gap is bounded posteriorly by the rays refracting on IOL optic periphery and anteriorly by the rays missing the IOL which are not refracted [2][3][4]. The location of the illumination gap usually corelates well with the ND symptoms described by patients [1][2][3].
2. Visual Field Defects
A study of eleven patients with ND showed that symptoms may be objectively evaluated by kinetic perimetry testing as statistically significant constrictions of the peripheral temporal and inferior visual field [5]. Similar findings were observed by Masket et al. [6]. Since kinetic perimetry measures the extension of the visual field up to 90 degrees and the visual field of a normal individual can extend up to 110 degrees temporally, it is possible that scotomas reaching to the extreme periphery of the temporal visual field are being underestimated [5]. Masket et al. found that translucent or opaque occlusion of the fellow eye resulted in subjective improvement of symptoms [6]. Furthermore, a pilot kinetic perimetry investigation on four ND patients found inferotemporal peripheral scotoma to be larger in extent with both eyes fully opened compared to a peripherally occluding contact lens being applied to the contralateral eye [7]. These findings raise the possibility of a neuroadaptive component to the ND [6].
3. Patient Anatomy
A study by Osher RH found that permanent ND symptoms could be a result of interaction between the IOL edge and anatomical predisposition of patients [8]. One of the fundamental predispositions for an illumination gap to be perceived as a temporal arching shadow is the presence of functional nasal retina, which extends more anteriorly compared to the temporal retina [8][9][10]. Other anatomical factors presented together include prominent eyeball, shallow orbit, hyperopia and large angle kappa, smaller and decentered pupil, and large angle alpha, which can also increase the incidence of ND [1][8][10][11]. A ray-tracing analysis by Holladay et al. showed that the distance between IOL and iris, ranging from 0.06 to 1.23 mm for acrylic and 0.06 to 0.62 mm for silicon IOLs, may be a factor for ND development [9]. However, the claim that a larger distance between iris and IOL increases the rate of ND has not been confirmed by later studies [12][13][14].
A ray tracing analysis study on computer eye models by Holladay and Simpson showed that a smaller photopic pupil is a significant factor for temporal shadow occurrence [1]. The retinal shadow occurred in pseudophakic conditions with a small 2.5 mm pupil diameter, while the shadow disappeared when the pupil was 5 mm wide [1]. This was attributed mostly to larger ray dispersion in wider pupils which reduces the chance for an illumination gap to occur [1][3]. A 2011 study by Masket and Fram noted an increase in ND symptoms with miotic agents and their improvement after the application of mydriatic agents [12]. Additionally, there are case reports of patients complaining of more intensive ND symptoms in bright photopic conditions [15]. Nasal location of the pupil relative to the eye’s optical axis (>2.6° or 0.3 mm on the cornea) can be the cause of exposure of the nasal retina to light rays [3][16].
Another study on 95 patients showed that hyperopic subjects might be more susceptible for ND development [17]. Large angle kappa—the angle between the visual axis (an imaginary line connecting the point of gaze fixation and the fovea) and the pupil axis (an imaginary line running through the pupil center perpendicular to the cornea)—might also contribute to ND development [1][18]. Angle kappa is larger in hyperopic patients as significant correlation exists between angle kappa values and positive refractive errors [19]. Furthermore, patients’ angle alpha—the angle between the visual axis and the optical center of the cornea—could be another factor for ND development [3][16]. A large angle alpha causes the eye to be turned more temporally and thus increases the exposure of functional nasal retina [3][16]. A complex interplay of all the factors mentioned above seems to increase ND incidence.
4. IOL Properties
ND can occur with different types of IOLs, irrespective of shape and material [2][12][15]. Hydrophobic, hydrophilic, acrylic, and silicon IOLs can all be associated with ND [15][20][13]. However, ND could be more commonly associated with acrylic IOLs with a sharp-edge design and less commonly with silicone IOLs with a rounded-edge design [21][16][22]. According to Holladay et al., silicone IOLs reduce the width of the illumination gap and move it more anteriorly [3]. It is thus less likely for the illumination gap to form on the functional retina [3]. Edge shape could also be an important factor, since rounded edges disperse the rays and thus reduce or eliminate the illumination gap [3]. An irregular IOL edge could also eliminate the gap by causing sufficient ray scatter [23]. The effect of the IOL shape on ND development has been further highlighted in a computer ray-tracing analysis by Holladay and Simpson [1]. The same research mentioned a number of IOL properties that could affect ND incidence, including high refractive index (RI), higher dioptric power, equi-biconvex or plano-convex shape, negative aspheric surface, and IOL diameter [1]. Lower RI of the optic material, in particular, silicone IOLs, moves forward the anterior and the posterior border of the shadow reducing its width compared to acrylic IOLs. [3][16]. Modification of the IOL design and diameter could also reduce ND [24][25][26]. A concave region on the peripheral posterior surface of a biconvex IOL may prevent ND by increasing the area of illuminated peripheral retina and narrowing the illumination gap [25]. A ray-tracing analysis suggested that 7.0 mm optic diameter IOLs enlarge and shift the illumination gap more peripherally compared to 6.0 mm diameter IOLs [26]. This effect was, however, more pronounced with a lower RI IOL [26]. That way, it could be less likely for the shifted illumination gap to fall on the functional retina and be perceived as troublesome [26]. A study on 86 patients comparing two hydrophilic acrylic IOLs with the same RI showed the 7.0 mm optic diameter IOL to have reduced ND incidence compared to the 6.0 mm diameter IOL [24].
5. Surgical Techniques
ND occurrence has been reported after IOL implantation in the capsular bag but not after ciliary sulcus or anterior chamber implantations [12]. One study suggests that a nasal anterior capsule overlying the anterior nasal part of the IOL optic could be a factor determining the presence of ND [1]. ND could, thus, be alleviated if IOL optic covered the anterior capsulotomy edge [12]. A surgical technique of reverse optic capture was developed, where the edge of the IOL optic is secondarily elevated above the anterior capsulorhexis while leaving the IOL haptics in the capsular bag [12][20]. A study by Masket et al. showed this technique to be highly successful in eliminating or preventing ND [20]. However, the intervention can be linked to postoperative complications, such as earlier opacification of the posterior lens capsule, capsular block syndrome, iris chafing, and postoperative myopic refractive error (myopic shift) [12][20]. Case reports of successful ND treatment by laser capsulotomy of the nasal anterior capsule further suggests that ND is likely caused by the anterior capsulotomy edge with in-the-capsular-bag implantations [27][28].
Optic–haptic junction positioning could also affect ND development. A study on 305 patients found a 2.3-fold decrease in ND incidence one day after cataract surgery when one of the two optic–haptic junctions of the IOL was positioned inferotemporally compared to the control group with vertical positioning of the junctions [22]. However, one month after surgery, the difference in ND incidence was no longer statistically significant [22]. A study by Holladay et al. also found horizontal haptic positioning to reduce ND incidence [3]. Another study by Manasseh et al. found ND incidence 4 weeks after surgery to be decreased from 16% to 8% when optic–haptic junctions were horizontally oriented [29]. A ray-tracing analysis by Erie et al. suggested that light rays missing the IOL optic but hitting the optic–haptic junction are completely internally reflected, thus not forming the anterior boundary of the illumination gap on the peripheral retina [4].
Osher RH proposed temporal corneal incision causing localized corneal edema to explain transient, but not persistent, ND symptoms [8]. Osher RH observed a crescent-shaped shadow near the pupil when light was passing through the incision from a temporal angle [8]. The disappearance of ND symptoms weeks after surgery could be associated with resolution of corneal edema [8]. Similarly, a 5-year follow-up study on 320 patients showed hydration of the temporal corneal wound at the end of surgery to possibly increase the risk for transient ND [30]. In this study, 13% of the patients who received wound hydration experienced ND compared to 5% who did not receive wound hydration [30].
6. Preventive and Treatment Measures
Adaptation might play a role in long-term decrease of ND symptoms [8][31]. Thick-rimmed glasses or sunglasses may reduce symptoms by blocking the temporal field of view [8][31]. Symptoms can also be alleviated by pharmacologic mydriasis which increases the illumination of the peripheral retina [12][32]. Surgical measures may be considered if troublesome ND symptoms persist for several months or more [20][33]. Reverse optic capture technique, sulcus placement of the IOL, and implantation of a secondary “piggy-back” IOL might improve ND symptoms [12]. An IOL exchange can alleviate symptoms, although it is not always successful [13][20]. An IOL exchange for a sulcus-fixated round-edged silicon IOL may also be successful [10][34][35].
References
- Holladay, J.T.; Simpson, M.J. Negative dysphotopsia: Causes and rationale for prevention and treatment. J. Cataract Refract. Surg. 2017, 43, 263–275.
- Masket, S.; Fram, N.R. Pseudophakic Dysphotopsia: Review of Incidence, Cause, and Treatment of Positive and Negative Dysphotopsia. Ophthalmology 2021, 128, e195–e205.
- Holladay, J.T.; Zhao, H.; Reisin, C.R. Negative dysphotopsia: The enigmatic penumbra. J. Cataract Refract. Surg. 2012, 38, 1251–1265.
- Erie, J.C.; Simpson, M.J.; Bandhauer, M.H. Influence of the intraocular lens optic-haptic junction on illumination of the peripheral retina and negative dysphotopsia. J. Cataract Refract. Surg. 2019, 45, 1335–1339.
- Makhotkina, N.Y.; Berendschot, T.T.J.M.; Nuijts, R.M.M.A. Objective evaluation of negative dysphotopsia with Goldmann kinetic perimetry. J. Cataract Refract. Surg. 2016, 42, 1626–1633.
- Masket, S.; Rupnik, Z.; Fram, N.R. Neuroadaptive changes in negative dysphotopsia during contralateral eye occlusion. J. Cataract Refract. Surg. 2019, 45, 242–243.
- Masket, S.; Rupnik, Z.M.; Fram, N.R.; Vikesland, R.J. Binocular Goldmann visual field testing of negative dysphotopsia. J. Cataract Refract. Surg. 2020, 46, 147–148.
- Osher, R.H. Negative dysphotopsia: Long-term study and possible explanation for transient symptoms. J. Cataract Refract. Surg. 2008, 34, 1699–1707.
- Holladay, J.T.; Zhao, H.; Reisin, C.R. Negative dysphotopsia: The enigmatic penumbra. J. Cataract Refract. Surg. 2012, 38, 1251–1265.
- Henderson, B.A.; Geneva, I.I. Negative dysphotopsia: A perfect storm. J. Cataract Refract. Surg. 2015, 41, 2291–2312.
- van Vught, L.; Luyten, G.P.M.; Beenakker, J.W.M. Distinct differences in anterior chamber configuration and peripheral aberrations in negative dysphotopsia. J. Cataract Refract. Surg. 2020, 46, 1007–1015.
- Masket, S.; Fram, N.R. Pseudophakic negative dysphotopsia: Surgical management and new theory of etiology. J. Cataract Refract. Surg. 2011, 37, 1199–1207.
- Vámosi, P.; Csákány, B.; Németh, J. Intraocular lens exchange in patients with negative dysphotopsia symptoms. J. Cataract Refract. Surg. 2010, 36, 418–424.
- van Vught, L.; Dekker, C.E.; Stoel, B.C.; Luyten, G.P.M.; Beenakker, J.W.M. Evaluation of intraocular lens position and retinal shape in negative dysphotopsia using high-resolution magnetic resonance imaging. J. Cataract Refract. Surg. 2021, 47, 1032–1038.
- Trattler, W.B.; Whitsett, J.C.; Simone, P.A. Negative dysphotopsia after intraocular lens implantation irrespective of design and material. J. Cataract Refract. Surg. 2005, 31, 841–845.
- Bhalla, J.S.; Gupta, S. Dysphotopsia—Unraveling the Enigma. Off. Sci. J. Delhi Ophthalmol. Soc. 2016, 27, 97–101.
- Makhotkina, N.Y.; Nijkamp, M.D.; Berendschot, T.T.J.M.; van den Borne, B.; Nuijts, R.M.M.A. Effect of active evaluation on the detection of negative dysphotopsia after sequential cataract surgery: Discrepancy between incidences of unsolicited and solicited complaints. Acta Ophthalmol. 2018, 96, 81–87.
- Karhanová, M.; Pluháček, F.; Mlčák, P.; Vláčil, O.; Šín, M.; Marešová, K. The importance of angle kappa evaluation for implantation of diffractive multifocal intra-ocular lenses using pseudophakic eye model. Acta Ophthalmol. 2015, 93, e123–e128.
- Basmak, H.; Sahin, A.; Yildirim, N.; Papakostas, T.D.; Kanellopoulos, A.J. Measurement of angle kappa with synoptophore and Orbscan II in a normal population. J. Refract. Surg. 2007, 23, 456–460.
- Masket, S.; Fram, N.R.; Cho, A.; Park, I.; Pham, D. Surgical management of negative dysphotopsia. J. Cataract Refract. Surg. 2018, 44, 6–16.
- Tester, R.; Pace, N.L.; Samore, M.; Olson, R.J. Dysphotopsia in phakic and pseudophakic patients: Incidence and relation to intraocular lens type (2). J. Cataract Refract. Surg. 2000, 26, 810–816.
- Henderson, B.A.; Yi, D.H.; Constantine, J.B.; Geneva, I.I. New preventative approach for negative dysphotopsia. J. Cataract Refract. Surg. 2016, 42, 1449–1455.
- Alapati, N.M.; Harocopos, G.J.; Sheybani, A. In-the-bag nasal intraocular lens optic truncation for treatment of negative dysphotopsia. J. Cataract Refract. Surg. 2016, 42, 1702–1706.
- Bonsemeyer, M.K.; Becker, E.; Liekfeld, A. Dysphotopsia and functional quality of vision after implantation of an intraocular lens with a 7.0 mm optic and plate haptic design. J. Cataract Refract. Surg. 2022, 48, 75–82.
- Erie, J.C.; Simpson, M.J.; Bandhauer, M.H. A modified intraocular lens design to reduce negative dysphotopsia. J. Cataract Refract. Surg. 2019, 45, 1013–1019.
- Erie, J.C.; Simpson, M.J.; Mahr, M.A. Effect of a 7.0 mm intraocular lens optic on peripheral retinal illumination with implications for negative dysphotopsia. J. Cataract Refract. Surg. 2022, 48, 95–99.
- Folden, D.V. Neodymium: YAG laser anterior capsulectomy: Surgical option in the management of negative dysphotopsia. J. Cataract Refract. Surg. 2013, 39, 1110–1115.
- Cooke, D.L.; Kasko, S.; Platt, L.O. Resolution of negative dysphotopsia after laser anterior capsulotomy. J. Cataract Refract. Surg. 2013, 39, 1107–1109.
- Manasseh, G.S.L.; Pritchard, E.W.J.; Rothwell, A.E.J.; Luck, J. Pseudophakic negative dysphotopsia and intraocular lens orientation: A prospective double-masked randomized controlled trial. Acta Ophthalmol. 2020, 98, 14368. Available online: https://onlinelibrary.wiley.com/doi/10.1111/aos.14368 (accessed on 24 November 2022).
- Sharma, P.; Kalia, S.; Chouhan, J.K. Incidence and causes of negative dysphotopsia after uncomplicated cataract surgery—A randomized clinical trial. Indian J. Ophthalmol. 2021, 69, 1786–1791.
- CRSTEurope. What Is the Best Approach to Negative Dysphotopsia? Available online: https://crstodayeurope.com/articles/2016-jan/what-is-the-best-approach-to-negative-dysphotopsia/ (accessed on 25 March 2022).
- Erie, J.C.; Simpson, M.J.; Bandhauer, M.H. Effect of a sulcus-fixated piggyback intraocular lens on negative dysphotopsia: Ray-tracing analysis. J. Cataract Refract. Surg. 2019, 45, 443–450.
- Makhotkina, N.Y.; Dugrain, V.; Purchase, D.; Berendschot, T.T.J.M.; Nuijts, R.M.M.A. Effect of supplementary implantation of a sulcus-fixated intraocular lens in patients with negative dysphotopsia. J. Cataract Refract. Surg. 2018, 44, 209–218.
- Vámosi, P.; Csákány, B.; Németh, J. Intraocular lens exchange in patients with negative dysphotopsia symptoms. J. Cataract Refract. Surg. 2010, 36, 418–424.
- Burke, T.R.; Benjamin, L. Sulcus-fixated intraocular lens implantation for the management of negative dysphotopsia. J. Cataract Refract. Surg. 2014, 40, 1469–1472.





