Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Van-An Duong | -- | 2438 | 2023-01-20 06:53:39 | | | |
| 2 | Catherine Yang | Meta information modification | 2438 | 2023-01-28 04:20:01 | | | | |
| 3 | Catherine Yang | Meta information modification | 2438 | 2023-01-29 09:19:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Duong, V.; Nguyen, T.; Maeng, H. Intranasal Liposomes for Vaccine Delivery. Encyclopedia. Available online: https://encyclopedia.pub/entry/40439 (accessed on 03 March 2026).
Duong V, Nguyen T, Maeng H. Intranasal Liposomes for Vaccine Delivery. Encyclopedia. Available at: https://encyclopedia.pub/entry/40439. Accessed March 03, 2026.
Duong, Van-An, Thi-Thao-Linh Nguyen, Han-Joo Maeng. "Intranasal Liposomes for Vaccine Delivery" Encyclopedia, https://encyclopedia.pub/entry/40439 (accessed March 03, 2026).
Duong, V., Nguyen, T., & Maeng, H. (2023, January 20). Intranasal Liposomes for Vaccine Delivery. In Encyclopedia. https://encyclopedia.pub/entry/40439
Duong, Van-An, et al. "Intranasal Liposomes for Vaccine Delivery." Encyclopedia. Web. 20 January, 2023.
Copy Citation
Liposomes are safe, biocompatible, and biodegradable spherical nanosized vesicles produced from cholesterol and phospholipids. Liposomes have been widely administered intranasally for systemic and brain delivery. Intranasal liposomes are also a potential approach for vaccine delivery. Liposomes can be used as a platform to load antigens and as vaccine adjuvants to induce a robust immune response.
liposome
intranasal
systemic
brain
phospholipid
bioavailability
1. Intranasal Vaccines
The nasal cavity has a large surface area that facilitates antigen absorption. An antigen can be taken up by microfold or dendritic cells and transferred to nasal-associated lymphoid tissue (NALT). Here, the antigen interacts with T and B cells, which can release IgA antibodies or pass through the blood to trigger cellular and IgG-based systemic immunity. Mucosal immunity against respiratory pathogens relies heavily on IgA-mediated protection [1]. FluMist, a live attenuated influenza vaccine, is the only IN vaccine approved by the US FDA (in 2003). In recent years, the IN vaccine has gained significant interest because it is particularly appropriate for various respiratory diseases (such as influenza and COVID-19). IN vaccination is noninvasive, convenient, and applicable to large populations [2]. Different IN vaccines have been developed and evaluated in phase 1 and phase 2 clinical trials [1].
2. Recent Advances in IN Liposomes for Vaccine Delivery
Liposomes have been used to encapsulate antigens or vaccine adjuvants to develop IN vaccines. An et al. developed an IN-liposome vaccine to control COVID-19 [3]. They first prepared liposomes containing 2′-3’-cyclic guanosine monophosphate adenosine monophosphate (cGAMP) in the core as adjuvants. The SARS-CoV-2 spike protein (S-protein) trimer was then adsorbed on the liposomes with 61.5% entrapment efficiency (Figure 1). In mice, a single IN-liposome administration elicited serum-neutralizing antibodies comparable to those elicited by other vaccine candidates. The IN-liposome vaccine also increased IgA responses in the nasal compartment and lungs and induced spike-specific T-cell response in the lungs and spleen.
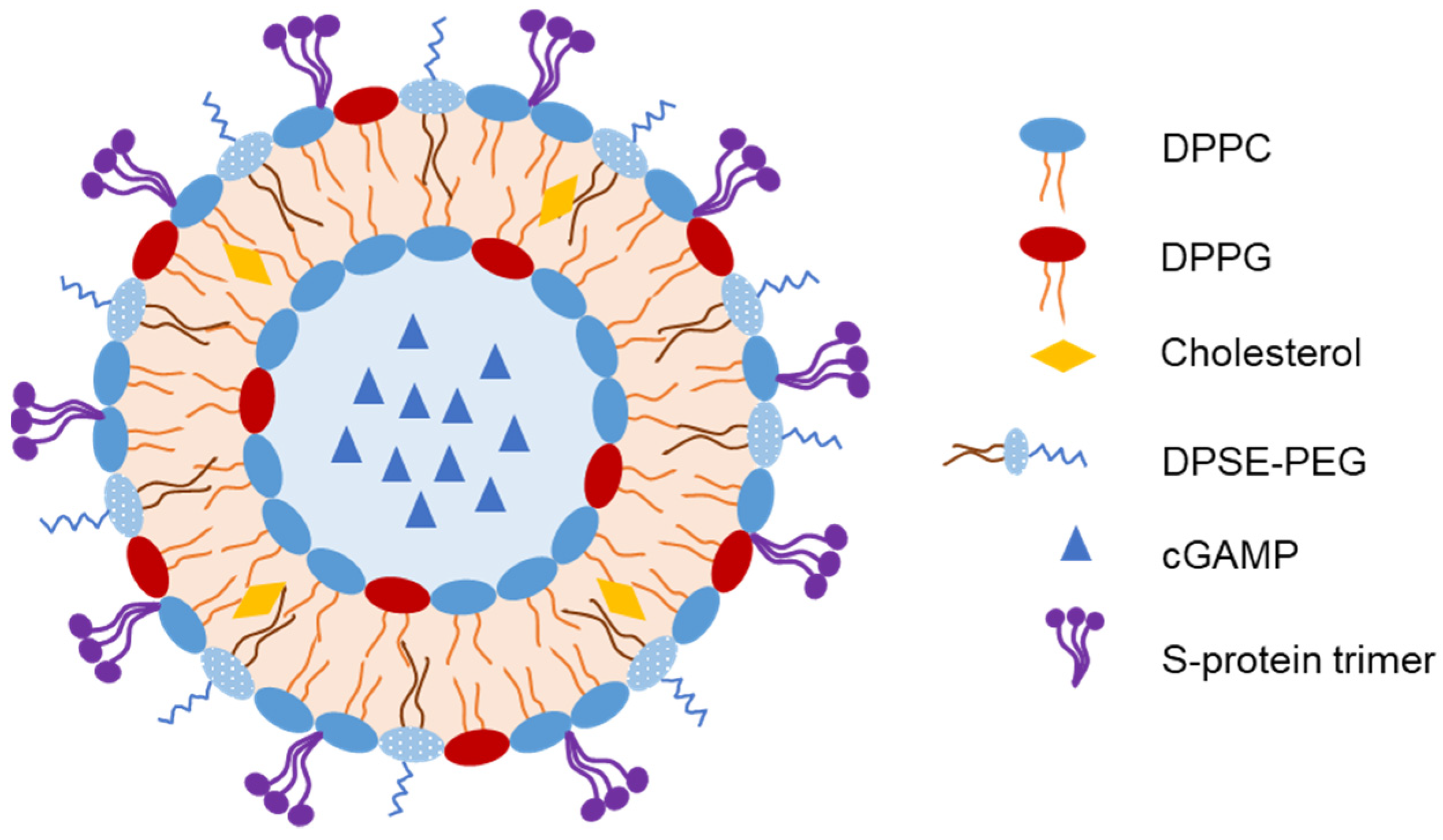
Figure 1. Representative structure of liposomes loaded with spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as an intranasal (IN) vaccine [3].
Huang et al. developed liposomes loaded with the SARS-CoV-2 spike glycoprotein receptor-binding domain (RBD) for IN vaccination [4]. Cobalt porphyrin–phospholipids were incorporated into the liposomes for stable RBD binding. They used K18 hACE2 mice expressing the human ACE2 receptor as an animal model. IN liposomes induced RBD-specific IgA production and RBD-specific cellular responses in the lungs. However, IM immunization with the same liposomes further increased RBD-specific IgG antibody levels in the serum. Moreover, both IM- and IN-immunized transgenic mice challenged with SARS-CoV-2 were completely protected against lethal viral infection.
A recent study has reported the development of a liposome vaccine adjuvant, CAF09b, containing the toll-like receptor 3 agonist polyinosinic: polycytidylic acid [5]. IN administration of liposomes to mice upregulates some type I interferon (IFN-I)-related genes. Pretreating mice with IN liposomes prevents death upon lethal challenge with mouse-adapted influenza A H1N1 PR8 virus.
Tada et al. developed cationic liposomes as a mucosal adjuvant for co-administration with pneumococcal surface protein A (PspA) [6]. This IN vaccine induced protective PspA-specific antibodies (nasal, lung, and vaginal IgA; lung and serum IgG; and serum IgG1 and IgG2a) against lethal S. pneumoniae inhalation better than IN PspA alone. The survival rate of IN liposome-vaccinated mice after S. pneumoniae challenge was 100%, whereas that of the IN antigen-vaccinated mice was <35%. IN liposomes also induced PspA-specific IL-17+ T cells (Th17 cells) and increased PspA uptake by nasal dendritic cells. Thus, IN PspA–liposomes may be an efficient vaccine against pneumococcal infection.
Dai et al. developed an IN-liposome vaccine containing the lipopeptide LCP-1 for immunization against Group A Streptococcus [7]. Liposomes were coated with polyethylenimine to promote cellular uptake and improve the immune response. In mice, IN liposomes elicited significant systemic and mucosal immune responses. The produced IgA and IgG antibodies effectively opsonized multiple isolates of clinically isolated Group A Streptococcus. Yang et al. developed another IN-liposome vaccine against Group A Streptococcus by decorating the liposomes with a CPP to enhance their permeability [8]. Among several CPPs evaluated, IN Tat47–57 and KALA-coated liposomes induced the highest production of antibodies against Group A Streptococcus in mice.
An IN liposome-based vaccine against tuberculosis that contains bacterial T-cell peptides was developed [9]. It induced immune responses and long-lasting central memory responses as well as reduced the bacterial burden and tuberculosis recurrence risk in infected mice. In addition, Bacillus Calmette-Guerin vaccination, followed by IN-liposome immunization, significantly boosted immune responses against tuberculosis via enhanced antigen-specific Th1 and Th17 responses. Another IN-liposome vaccine designed to target CD44 using thioaptamers to boost host immunity against tuberculosis was developed by Singh et al. [10]. In Mycobacterium tuberculosis-injected mice with increased lung and spleen loads, IN liposomes preferentially accumulated in the lungs, reduced the bacterial colony-forming units by 10-fold, and increased resident memory T cells. Thus, CD44 thioaptamer-loaded IN liposomes effectively enhanced antitubercular immunity in this mouse model.
Kakhi et al. developed different liposome-based formulations (SUV, MLV, reverse-phase evaporation vesicles, and ultra-flexible liposomes) for use as vaccines with antitumor activity following IN administration [11]. The liposomes were loaded with ErbB2 peptide (a TCD8+ epitope derived from the ErbB2 protein), HA peptide (a TCD4+ epitope derived from influenza hemagglutinin), and lipopeptidic Pam2CAG as an adjuvant. These vaccines were administered intranasally to mice, followed by IV or subcutaneous (SC) implantation of ErbB2-surexpressing cancer cells. Different liposome-based vaccines have shown similar antitumor efficiencies in a lung tumor model. When administered to the SC implantation tumor model, the IN-SUV vaccine resulted in 20% tumor-free mice on day 50, whereas all control mice developed tumors within 8 days after SC implantation of cancer cells; however, the difference was non-significant. Thus, the tumor size and weight should be determined, which may indicate the efficiency of the IN-liposome vaccine.
Mai et al. developed another IN-liposome vaccine for cancer by loading liposome with mRNA encoding cytokeratin 19, a target antigen for immunotherapy against lung cancer [12]. Protamine was used to compress and completely encapsulate the mRNA in the liposomal core (Figure 2). IN immunization of mice with liposomes elicited a strong cellular immune response via cytokine secretion. More importantly, IN liposomes reduced tumor growth and induced IL-2 and IL-4 secretion in the spleen of Lewis lung cancer model mice.
Figure 2. Representative structure of liposomes loaded with mRNA encoding cytokeratin 19 as an intranasal vaccine [12].
Abhyankar et al. developed IN liposome adjuvant and combined it with Entamoeba histolytica Gal/GalNAc–lectin-derived antigen as a vaccine against diarrheal pathogens [13]. Toll-like receptor agonists were incorporated to enhance the immune response. IN liposomes induced a robust mucosal IgA response as well as systemic and cellular immune responses. In an intestinal amebiasis mouse model, the IN liposomes reduced the infection rate by 55%, and enhanced fecal IgA, serum IgG, systemic IFN-γ, and IL-17A levels, and cleared more than 80% cecal antigen upon challenge [14].
Fan et al. developed hyaluronic acid- and PEG-coated cationic liposomes to deliver a candidate recombinant antigen F1-V for Yersinia pestis, a causative agent of pneumonic plague transmitted through pulmonary inhalation, with approximately 100% death rate within a week of infection [15]. The surface decoration of cationic liposomes increased stability, prolonged antigen release (~40% in 3 weeks in vitro), and reduced cytotoxicity in bone marrow-derived dendritic cells by 20-fold. IN liposomes loaded with ovalbumin (a model antigen) and monophosphoryl lipid A (a molecular adjuvant) increased serum IgG and IgG1 levels as well as ovalbumin-specific CD8+ T cells in mice compared with IN antigen plus adjuvant solution. When F1-V and the adjuvant were loaded, the IN liposomes induced 11-, 23-, and 15-fold higher serum F1-V-specific total IgG, IgG1, and IgG2c, respectively, at day 77, compared with the IN antigen plus adjuvant solution. Thus, decorated liposomes are a promising IN vaccine against Y. pestis and other infectious pathogens.
Olsen et al. developed a vaccine against Chlamydia trachomatis [16]. They first produced recombinant proteins based on the VD4 region from the major outer membrane proteins of Chlamydia trachomatis. The antigens were combined with liposome adjuvant CAF01 to induce a strong immune response. In mice, the vaccine reduced the bacterial count in the vagina and prevented pathological changes in the upper genital tract. Mice with simultaneous IM and IN vaccination showed high IgA levels in the vaginal secretions. In the phase 1, first-in-human, double-blind, parallel, randomized, placebo-controlled trial, this vaccine was safe, well-tolerated, and immunogenic; however, the regimen consisted of three IM injections of liposome vaccine followed by two IN administrations of the antigen solution (not containing liposome adjuvant) [17].
Several studies have developed a liposome platform for vaccine delivery and loaded it with a model antigen (such as ovalbumin) to evaluate the immune response. Wang et al. developed galactose-modified liposomes for specific recognition by macrophages and encapsulated ovalbumin for IN immunization [18]. The authors synthesized α- and β-galactosyl lipids and incorporated them into the liposomes. Galactose molecules are recognized by macrophage galactose-type C-type lectins, which increase macrophage uptake and increase tumor necrosis factor-α and interleukin-6 levels. In mice, the IN-modified liposomes induced secretory IgA levels in the nasal and lung wash fluids and a serum IgG antibody response. Moreover, the modified liposomes increased antigen uptake by dendritic cells and induced higher levels of pro-inflammatory cytokines than unmodified liposomes in vitro [19]. Macrophage galactose-type C-type lectins are also expressed on immature dendritic cells in humans and mice, thus mediating modified liposome uptake and initiating the immune response in immature dendritic cells. IN β-galactosylated liposomes resulted in complete protection against EG7 tumor challenge in mice.
Wasan et al. developed cationic liposomes containing ovalbumin as an antigen and incorporated an adjuvant (TriAdj) to boost immune response [20]. IN liposomes induce IgG and IgA production in mice. Tada et al. developed cationic liposomes combined with ovalbumin for IN vaccination [21]. IN liposomes produced IgA in nasal tissues and increased serum IgG1 levels in mice. Cationic liposomes played a role in ovalbumin uptake into dendritic cells in NALT. Then, liposomes were modified with class B oligodeoxynucleotides containing immunostimulatory CpG motifs (CpG ODN), a potent mucosal adjuvant [22]. Loading CpG ODN into cationic liposomes could reduce their dose and cause adverse effects. The IN ovalbumin/CpG ODN/liposomes increased antigen-specific IgA production in mouse nasal mucosa 10-fold compared to the IN non-liposome formulation (only ovalbumin plus CpG ODN). In addition, serum IgG levels in mice vaccinated with IN ovalbumin/CpG ODN/liposomes and IN ovalbumin/liposomes were higher than those in mice vaccinated with IN ovalbumin.
Yusuf et al. developed PEGylated cationic liposomes as carriers for the IN vaccines [23]. These modifications increased the permeability and penetration of liposomes into bovine nasal tissue ex vivo. Serum IgG1, nasal IgA, and vaginal IgA levels in mice vaccinated with IN ovalbumin-loaded liposomes were higher than those in mice vaccinated with IM- and IN-free ovalbumin. Thus, the PEGylated cationic liposome system is a promising IN vaccine delivery system.
Table 1 summarizes the major features of the aforementioned IN-liposome vaccines.
Table 1. Major features of intranasal liposomes for vaccine delivery.
| Loading Agent | Disease/Pathogen | Composition | Size, PDI, ZP | Primary Outcomes | Year and Ref |
|---|---|---|---|---|---|
| SARS-CoV-2 S-protein trimer | COVID-19 | DPPC, DPPG, cholesterol, DPPE–PEG, and cGAMP | 105 nm, 0.24, −30 mV |
Systematic and mucosal immune responses in mice; spike-specific IgA responses in nasal compartment and lungs. | 2021 [3] |
| SARS-CoV-2 RBD spike glycoprotein | COVID-19 | DOPC, cholesterol, CoPoPs, and 3D6A–PHAD | N/A, N/A, N/A |
Induced RBD-specific IgA production and RBD-specific cellular responses in the lungs; full protection against lethal virus in K18 hACE2 transgenic mice. | 2022 [4] |
| None | Influenza virus disease | DDA, MMG, and polyinosinic: polycytidylic acid | 150–200 nm, N/A, +40 mV |
Upregulation of some IFN-I-related genes; reduced mice death. | 2022 [5] |
| Pneumococcal surface protein A (PspA) | Streptococcus pneumoniae | DOTAP, and DC-chol | 138 nm, N/A, +4 mV |
Higher antibodies in nasal, lung, and vagina vs. IN protein alone; survival rate = 100% in infected mice; induced PspA-specific IL-17+ T cells (Th17 cells); increased uptake of PspA by nasal dendritic cells. | 2018 [6] |
| Lipopeptide LCP-1 | Group A Streptococcus | DPPC, cholesterol, and polyethylenimine | 138 nm, 0.09, +39 mV |
Elicited production of IgA and IgG antibodies. | 2020 [7] |
| Lipopeptide LCP-1 | Group A Streptococcus | DPPC, cholesterol, and CPP | 112 nm, 0.08, +38 mV |
IN Tat47–57 and KALA-coated liposomes resulted in the highest production of antibodies against Group A Streptococcus in mice. | 2021 [8] |
| T-cell peptides | Tuberculosis | N/A | N/A, N/A, N/A |
Induced immune responses and long-lasting central memory responses; reduced bacterial burden and risk of recurrence in infected mice | 2021 [9] |
| CD44 thioaptamer | Tuberculosis | Soybean PC, cholesterol, and DSPE–PEG | 205 nm, N/A, −23 mV |
Accumulated in lungs; reduced bacterial colony-forming units; increased resident memory T cells in Mycobacterium tuberculosis-injected mice. | 2022 [10] |
| ErbB2 peptide and HA peptide | Cancer | Egg PC, L-α-phosphatidylglycerol, and cholesterol | 68 nm, 0.2, −82 mV |
Efficient antitumor efficiency in lung tumor model for all different liposome-based vaccines. | 2016 [11] |
| mRNA-encoded CK19 | Lung cancer | DOTAP, cholesterol, DSPE-–PEG, and protamine | 170 nm, 0.18, +10 mV |
Elicited cytokine secretion; reduced tumor growth in tumor-bearing mice. | 2020 [12] |
| LecA protein | Diarrhea | DPPC, DPPE–PEG, and cholesterol | 65–130 nm, N/A, N/A |
Induced mucosal, systemic, and cellular immune response; reduced infection rate by 55%; enhanced fecal IgA, serum IgG, systemic IFN-γ, and IL-17A; cleared > 80% cecal antigen upon challenge. | 2017–2018 [13][14] |
| Recombinant antigen F1-V | Yersinia pestis | DOTAP, DOPE, hyaluronic acid, and PEG | N/A, N/A, N/A |
Prolonged release of antigen; increased stability; higher serum F1-V-specific IgG, IgG1, and IgG2c at day 77 (11-, 23-, and 15-fold vs. IN solution, respectively). | 2015 [15] |
| VD4-based recombinant proteins | Chlamydia trachomatis | DDA and TDB | N/A, N/A, N/A |
Reduced bacterial numbers in vagina; prevented pathological changes in upper genital tract; higher IgA levels in vaginal secretions in mice with simultaneous IM and IN vaccination. | 2015 [16] |
| Ovalbumin | None | PC, cholesterol, and β-galactosyl DLPE | ~1000 nm, 0.221, −14 mV |
Higher IgA (nasal and lung) and IgG (serum); complete protection against EG7 tumor challenge in mice. | 2013–2015 [18][19] |
| Ovalbumin | None | Egg PC, DDAB, and DOPE | <200 nm, 0.36, +50 mV |
IgG and IgA production. | 2019 [20] |
| Ovalbumin | None | DOTAP and DC-chol | 57 nm, N/A, +9 mV |
The IN liposomes produced IgA in nasal tissues and increased serum IgG1 levels in mice. | 2015 [21] |
| Ovalbumin | None | DOTAP, DC-chol, and class B CpG ODN | 138 nm, N/A, +4 mV |
Higher IgA in mouse nasal mucosa (10-fold) vs. IN nonliposome formulation; higher serum IgG vs. IN ovalbumin. | 2017 [22] |
| Ovalbumin | None | Soybean PC, and DDA, TPGS | 109 nm, N/A, +50 mV |
Higher serum IgG1, nasal IgA, and vaginal IgA levels in mice (vs. IM- and IN-free ovalbumin). | 2017 [23] |
3D6A–PHAD, monophosphoryl hexa-acyl lipid A and 3-deacyl; cGAMP, 2′-3″cyclic guanosine monophosphate adenosine monophosphate; CoPoPs, cobalt porphyrin–phospholipids; COVID-19, coronavirus disease 2019; CPP, cell-penetrating peptide; CpG ODN, oligodeoxynucleotide containing immunostimulatory CpG motifs; DC-chol, cholesteryl 3β-N-(dimethylaminoethyl) carbamate; DDA, dimethyldioctadecylammonium bromide; DDAB, didecyldimethyl ammonium bromide; DLPE, 1,2-didodecanoyl-sn-glycero-3-phosphoethanolamine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, 1,2-dioleoyl-3-trimethylammoniopropane; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DPPG, 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol); DPPE–PEG, dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)]; DSPE–PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino (polyethylene glycol)]; HA peptide, influenza virus hemagglutinin-derived peptide; IFN, interferon gamma; Ig, immunoglobulin; IL, interleukin; IM, intramuscular; IN, intranasal; MMG, monomycoloyl glycerol; N/A, not available; PC, phosphatidylcholine; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TDB, trehalose 6,6-dibehenate; and TPGS, d-alpha-tocopherol polyethylene glycol 1000 succinate.
References
- Nian, X.; Zhang, J.; Huang, S.; Duan, K.; Li, X.; Yang, X. Development of Nasal Vaccines and the Associated Challenges. Pharmaceutics 2022, 14, 1983.
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250.
- An, X.; Martinez-Paniagua, M.; Rezvan, A.; Sefat, S.R.; Fathi, M.; Singh, S.; Biswas, S.; Pourpak, M.; Yee, C.; Liu, X.; et al. Single-dose intranasal vaccination elicits systemic and mucosal immunity against SARS-CoV-2. iScience 2021, 24, 103037.
- Huang, W.-C.; Chiem, K.; Martinez-Sobrido, L.; Lovell, J.F. Intranasal Immunization with Liposome-Displayed Receptor-Binding Domain Induces Mucosal Immunity and Protection against SARS-CoV-2. Pathogens 2022, 11, 1035.
- Zimmermann, J.; Schmidt, S.T.; Trebbien, R.; Cox, R.J.; Zhou, F.; Follmann, F.; Pedersen, G.K.; Christensen, D. A Novel Prophylaxis Strategy Using Liposomal Vaccine Adjuvant CAF09b Protects against Influenza Virus Disease. Int. J. Mol. Sci. 2022, 23, 1850.
- Tada, R.; Suzuki, H.; Takahashi, S.; Negishi, Y.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Nasal vaccination with pneumococcal surface protein A in combination with cationic liposomes consisting of DOTAP and DC-chol confers antigen-mediated protective immunity against Streptococcus pneumoniae infections in mice. Int. Immunopharmacol. 2018, 61, 385–393.
- Dai, C.C.; Yang, J.; Hussein, W.M.; Zhao, L.; Wang, X.; Khalil, Z.G.; Capon, R.J.; Toth, I.; Stephenson, R.J. Polyethylenimine: An Intranasal Adjuvant for Liposomal Peptide-Based Subunit Vaccine against Group A Streptococcus. ACS Infect. Dis. 2020, 6, 2502–2512.
- Yang, J.; Firdaus, F.; Azuar, A.; Khalil, Z.G.; Marasini, N.; Capon, R.J.; Hussein, W.M.; Toth, I.; Skwarczynski, M. Cell-Penetrating Peptides-Based Liposomal Delivery System Enhanced Immunogenicity of Peptide-Based Vaccine against Group A Streptococcus. Vaccines 2021, 9, 499.
- Kumar, S.; Bhaskar, A.; Patnaik, G.; Sharma, C.; Singh, D.K.; Kaushik, S.R.; Chaturvedi, S.; Das, G.; Dwivedi, V.P. Intranasal immunization with peptide-based immunogenic complex enhances BCG vaccine efficacy in a murine model of tuberculosis. JCI Insight 2021, 6, e145228.
- Singh, V.K.; Chau, E.; Mishra, A.; DeAnda, A.; Hegde, V.L.; Sastry, J.K.; Haviland, D.; Jagannath, C.; Godin, B.; Khan, A. CD44 receptor targeted nanoparticles augment immunity against tuberculosis in mice. J. Control. Release 2022, 349, 796–811.
- Kakhi, Z.; Frisch, B.; Heurtault, B.; Pons, F. Liposomal constructs for antitumoral vaccination by the nasal route. Biochimie 2016, 130, 14–22.
- Mai, Y.; Guo, J.; Zhao, Y.; Ma, S.; Hou, Y.; Yang, J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell. Immunol. 2020, 354, 104143.
- Abhyankar, M.M.; Noor, Z.; Tomai, M.A.; Elvecrog, J.; Fox, C.B.; Petri, W.A. Nanoformulation of synergistic TLR ligands to enhance vaccination against Entamoeba histolytica. Vaccine 2017, 35, 916–922.
- Abhyankar, M.M.; Orr, M.T.; Lin, S.; Suraju, M.O.; Simpson, A.; Blust, M.; Pham, T.; Guderian, J.A.; Tomai, M.A.; Elvecrog, J.; et al. Adjuvant composition and delivery route shape immune response quality and protective efficacy of a recombinant vaccine for Entamoeba histolytica. NPJ Vaccines 2018, 3, 22.
- Fan, Y.; Sahdev, P.; Ochyl, L.J.; Akerberg, J.J.; Moon, J.J. Cationic liposome–hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J. Control. Release 2015, 208, 121–129.
- Olsen, A.W.; Follmann, F.; Erneholm, K.; Rosenkrands, I.; Andersen, P. Protection Against Chlamydia trachomatis Infection and Upper Genital Tract Pathological Changes by Vaccine-Promoted Neutralizing Antibodies Directed to the VD4 of the Major Outer Membrane Protein. J. Infect. Dis. 2015, 212, 978–989.
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: A first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2019, 19, 1091–1100.
- Wang, H.-W.; Jiang, P.-L.; Lin, S.-F.; Lin, H.-J.; Ou, K.-L.; Deng, W.-P.; Lee, L.-W.; Huang, Y.-Y.; Liang, P.-H.; Liu, D.-Z. Application of galactose-modified liposomes as a potent antigen presenting cell targeted carrier for intranasal immunization. Acta Biomater. 2013, 9, 5681–5688.
- Jiang, P.-L.; Lin, H.-J.; Wang, H.-W.; Tsai, W.-Y.; Lin, S.-F.; Chien, M.-Y.; Liang, P.-H.; Huang, Y.-Y.; Liu, D.-Z. Galactosylated liposome as a dendritic cell-targeted mucosal vaccine for inducing protective anti-tumor immunity. Acta Biomater. 2015, 11, 356–367.
- Wasan, E.K.; Syeda, J.; Strom, S.; Cawthray, J.; Hancock, R.E.; Wasan, K.M.; Gerdts, V. A lipidic delivery system of a triple vaccine adjuvant enhances mucosal immunity following nasal administration in mice. Vaccine 2019, 37, 1503–1515.
- Tada, R.; Hidaka, A.; Iwase, N.; Takahashi, S.; Yamakita, Y.; Iwata, T.; Muto, S.; Sato, E.; Takayama, N.; Honjo, E.; et al. Intranasal Immunization with DOTAP Cationic Liposomes Combined with DC-Cholesterol Induces Potent Antigen-Specific Mucosal and Systemic Immune Responses in Mice. PLoS ONE 2015, 10, e0139785.
- Tada, R.; Muto, S.; Iwata, T.; Hidaka, A.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Attachment of class B CpG ODN onto DOTAP/DC-chol liposome in nasal vaccine formulations augments antigen-specific immune responses in mice. BMC Res. Notes 2017, 10, 68.
- Yusuf, H.; Ali, A.A.; Orr, N.; Tunney, M.M.; McCarthy, H.O.; Kett, V.L. Novel freeze-dried DDA and TPGS liposomes are suitable for nasal delivery of vaccine. Int. J. Pharm. 2017, 533, 179–186.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
29 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





