
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shannon Erhardt | -- | 1922 | 2023-01-19 20:57:49 | | | |
| 2 | Amina Yu | + 6 word(s) | 1928 | 2023-01-29 02:51:28 | | |
Video Upload Options
Neural crest cells (NCCs) are a vertebrate-specific, multipotent stem cell population that have the ability to migrate and differentiate into various cell populations throughout the embryo during embryogenesis. Based on the initial axial position and site of contribution, NCCs are divided into specific subpopulations, such as the cardiac neural crest (NC), which mainly contributes to the cardiac valves, interventricular septum, and both the aorta and pulmonary vessel. The heart is a muscular and complex organ whose primary function is to pump blood and nutrients throughout the body. Mammalian hearts, such as those of humans, lose their regenerative ability shortly after birth. However, a few vertebrate species, such as zebrafish, have the ability to self-repair/regenerate after cardiac damage. Recent research has discovered the potential functional ability and contribution of cardiac NCCs to cardiac regeneration through the use of various vertebrate species and pluripotent stem cell-derived NCCs. This potential regenerative capacity to cardiac tissue poses interesting avenues to advance the treatment of various cardiac diseases. Heart disease, a leading cause of death in the United States, results in death or severe damage to the function and/or structural integrity of the heart. Determining the contribution and regenerative capacity of the cardiac NC in mammalian systems is of high clinical significance. Here, the focus is on the NC’s regenerative capacity in various tissues and systems, and in particular, the characteristics of cardiac NCCs between species and their roles in cardiac regeneration are summarized. Emerging and future work to determine the potential contributions of NCCs for disease treatment will be further discussed.
1. Cardiac Neural Crest Contribution to the Heart Between Species
1.1. Mouse
Tang et al. examined mice that had NCCs marked using cytoplasmic GFP (Wnt1-Cre;ZsGreenfl/fl) and found that at embryonic day (E) 15.5, a large number of cells in the OFT, interventricular septum, and myocardium of the ventricles were NC positive[2]. The authors do note that the number of Wnt1-Cre-positive cells remains stable postnatally and does not undergo cell division or apoptosis, providing similar information that has been noted in both chicks and zebrafish, indicating an evolutionary role of the NC within these cardiac regions[2][11][12]. The finding of the NC population in both of the mouse ventricles warrants further investigation into the NC-specific function in these regions.
1.2. Zebrafish
Unlike the structure of the amniote heart, zebrafish (Danio rerio) hearts are tubular in structure, consisting of one atrium and one ventricle, and maintain a one-directional flow[12][13][14]. As the developing heart in zebrafish varies compared to amniote models, this suggests that NC contribution varies as well. In contrast to the mouse or chick, the zebrafish heart does not consist of a ventricular septum due to having a single ventricle, clearly indicating varying cardiac NC contribution[12][14]. Sato and Yost found that in zebrafish, cardiac NCCs contribute to the bulbus arteriosus, ventricle, atrioventricular junction, atrium, and muscle formation in the myocardium[11]. The Kirby group also found that by using cell marking, cardiac NCCs migrated to the myocardial wall of the heart tube, and laser ablation of the pre-migratory cardiac NCCs resulted in the loss of NC migration to the heart and failed heart looping, along with reducing the ejection fraction and cardiac output[12]. Although it has further been corroborated that NCCs integrate into the myocardium in the zebrafish heart, the Chen group found that NCCs in the zebrafish can be characterized into two populations: one that gives rise to cardiomyocytes and another that contributes to the endothelium and bulbus arteriosus[15]. Furthermore, the Chen group found that ablation leads to reduced heart rates, defective myocardial maturation, and failure to recruit progenitor cells from the second heart field, indicating the need for further investigation into the cell–cell communication between the various cardiac contributing cell populations[15].
2. Regenerative Capacity of the Neural Crest
2.1. Gastrointestinal Tract and Enteric Nervous System
One field of interest that has sparked an investigation into NC regenerative capacity is the gastrointestinal tract. The coordination of the gastrointestinal tract is regulated by the enteric nervous system (ENS), a network comprised of neurons and glial cells that arise from the NC[16][17]. To understand the contribution of the NC to this highly regenerative area, Kruger and colleagues investigated the gastrointestinal tract in adult rats and found the persistent existence of NCCs. These postnatal NCCs were able to self-renew extensively in culture but were overall not as extensive as NCC regeneration of the fetal gut[18]. Furthermore, the authors were able to determine that the NCCs of the adult gut were still active and able to give rise to neuro-transmitting neurons. However, it was determined that NCCs of the adult gut were unable to give rise to specific neural subtypes that are present in the fetal gut[18]. They suggest that this reduction in producing various neuronal subtypes involves a loss of BMP expression but an increased response to gliogenic factors at postnatal stages[18]. Although the complete functional significance of NCCs in the adult mammalian system is still unknown, these findings of NCCs in the adult gut suggest new possibilities for NCCs in regeneration.
2.2. Cranial Bones, Bone Marrow, and Teeth
One major contribution of the NC is to assist in cranial skeletal formation during embryogenesis, including cartilage and bones of the head[19][20][21][22]. Beyond cranial bones, studies have also indicated that NCCs contribute to bone marrow and tooth formation[23][24]. However, whether the NC can re-activate stem cell-like properties or NCCs maintain multipotency postnatally in such structures is still under investigation.
Although NCCs give rise to the majority of bone, cartilage, and connective tissues of the skull, little is known about the regenerative ability of the NCC lineage in cranial structures after injury or disease. However, regarding the reactivation of stem cell-like characteristics in adult bones, Ransom et al., with the use of a mandibular distraction osteogenesis mouse model, identified that after injury, NC development-related genes, including sox10, sox18, and elk3, were upregulated within newly forming bones of the jaw[25]. Furthermore, it was identified that post-migratory cranial NCCs were not only self-renewing and able to form bone matrix in culture, but that subcutaneous transplanted post-migratory cranial NCCs in mice were able to regain their ability to differentiate into osteocytes and adipocytes, along with assisting in calvaria bone formation[26]. However, the ability and corresponding mechanisms of cranial NCCs to re-active or maintain their multipotency within bones of the skull and to properly contribute to new bone formation have yet to be deciphered.
2.3. Peripheral and Central Nervous System
During development, NCCs from the trunk region give rise to numerous sub-lineages, including glial cells of the peripheral nervous system (PNS). Glial cells contribute to the structure of both the PNC and central nervous system (CNS), assisting in the protection and regulation of neurons, particularly through NCC-derived Schwann cells[27]. The regeneration of nerve fibers and their supporting cells has been a standing field of interest regarding functional recovery to structures such as the spinal cord after injury.
Recent advances regarding PNS regeneration have been made possible due to the use of NC-like stem cells derived from human embryonic stem cells or iPSCs. For example, Huang and colleagues used NC stem cells derived from iPSCs to construct nerve conduits that, when implanted into a rat sciatic nerve transection model, were able to increase functional nerve recovery[28]. Supporting studies using sciatic nerve defect models and NC-derived human iPSCs found that in vivo, grafted cells proliferated and successfully migrated throughout the conduit after transplantation[29]. Furthermore, Kimura et al. discovered that grafted NC-derived iPSCs also contributed to the increased strength of the leg muscle, indicating functional recovery of the sciatic nerve after injury[29]. Similar conclusions have been made by other groups, indicating that NC-derived iPSCs or NC-derived embryonic stem cells are a valuable tool that can contribute to nerve regeneration. However, the mechanisms of this ability have yet to be determined[30][31][32].
3. Cardiac Neural Crest in Cardiac Regeneration
During early mammalian development, the heart maintains its regenerative capacity; however, shortly after birth, this ability is lost. Postnatal cardiac progenitors remain a challenging and controversial issue in the cardiac field. Recent studies have begun to investigate the potential ability of the heart to re-activate regenerative ability, particularly through the NC, to assist in cardiac regeneration after injury.
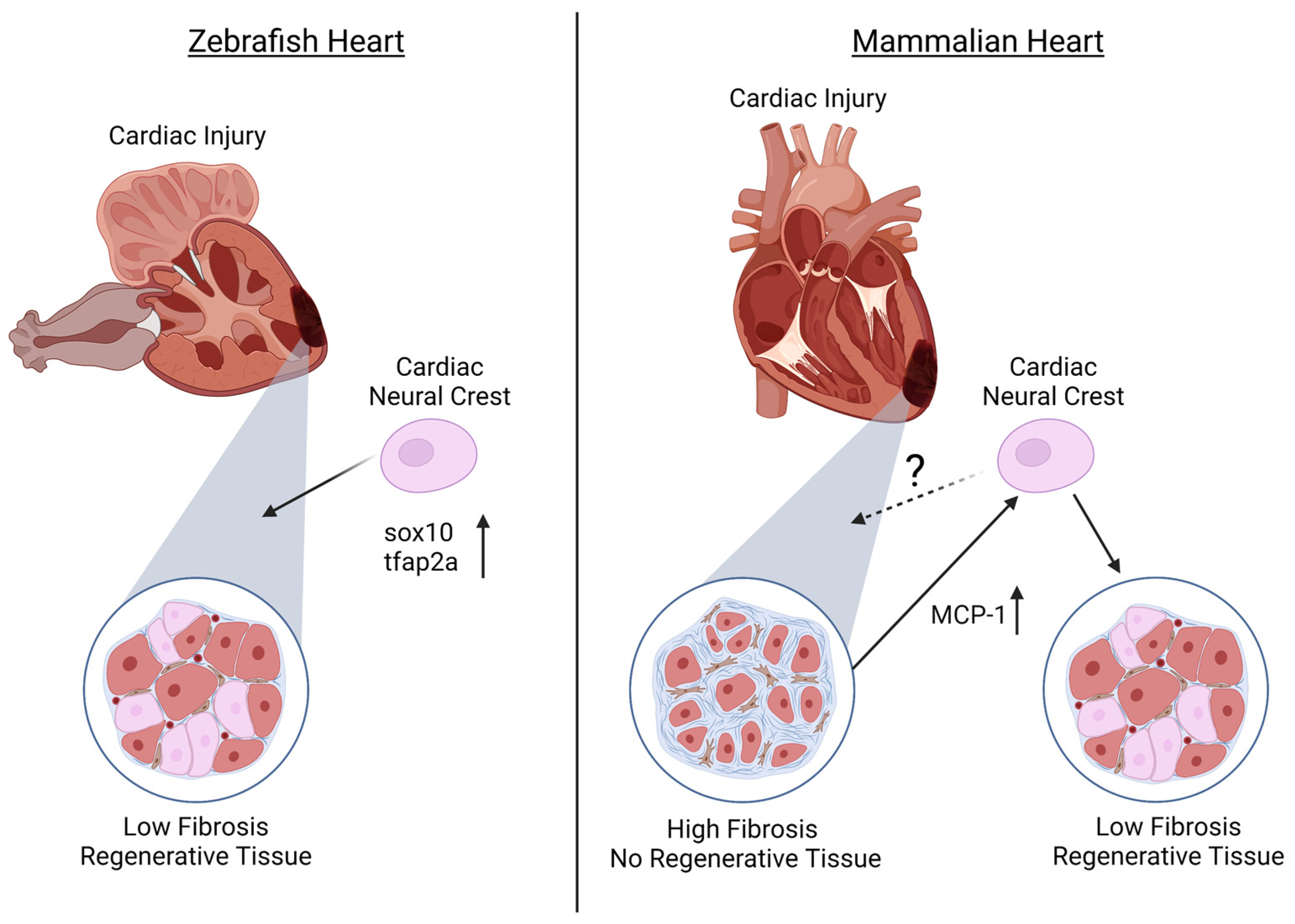
It has previously been established that zebrafish maintain regenerative abilities throughout their systems, including the fins and retina[33][34][35][36]. Furthermore, it has been identified that adult zebrafish hearts are able to regenerate ventricular myocardium, without scarring, through cardiomyocyte dedifferentiation and proliferation[37][38]. However, until recently, it was unknown whether the NC population assists in this regeneration capacity of the heart. In addition to the established NCC contributions to cardiovascular development, numerous groups recently determined that NCCs in the zebrafish heart also contribute to the cardiomyocyte population[2][39][40]. Based on this, Tang and colleagues further investigated whether the NC population of the zebrafish heart also plays a role in cardiac regeneration. Using the zebrafish transgenic line Tg(-4.9sox10:eGFP) to examine NCCs, it was found that although sox10 expression is down-regulated after NCCs reach the heart, the removal of a portion of the adult ventricular apex stimulates sox10-GFP expression, along with the NC marker tfap2a, in cardiomyocytes near the injury site, suggesting the reactivation of a NC-like state for cardiac regeneration[2] (Figure 1). Furthermore, Sande-Melón et al. determined that pre-existing sox10-positive NCCs not only contributed to the zebrafish adult heart but that after ventricular cryoinjury, the number of sox10-expressing cells significantly increased in the myocardium near the injured area[39] (Figure 1).
Although regeneration in zebrafish has shown promising roles for NCCs in cardiac regeneration, less is known about the contribution of the NC during mammalian cardiac regeneration. Similar to zebrafish, it was identified that NCCs are present in the postnatal mouse heart and can differentiate into cardiomyocytes[3][4][5][6][7]. Tamura and colleagues found that after myocardial infarction in adult mice, GFP-expressed NC-derived cells migrated to the border of the infarcted region and were able to differentiate into cardiomyocytes, contributing to the regeneration of the myocardium[5]. The authors suggest that this migration after myocardial infarction is due to monocyte chemoattractant protein-1 (MCP-1) expression in the infarcted area that provides guidance cues to NCC derivatives[5] (Figure 1). In contrast, although Hatzistergos and colleagues found that a population of NCC lineages generate cardiomyocytes postnatally, these cells were not proliferative and had lost their regenerative capacity[3].
References
- Chan, W.Y.; Cheung, C.S.; Yung, K.M.; Copp, A.J. Cardiac neural crest of the mouse embryo: axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development 2004, 131, 3367-3379, doi:10.1242/dev.01197.
- Tang, W.; Martik, M.L.; Li, Y.; Bronner, M.E. Cardiac neural crest contributes to cardiomyocytes in amniotes and heart regeneration in zebrafish. eLife 2019, 8, doi:10.7554/eLife.47929.
- Hatzistergos, K.E.; Durante, M.A.; Valasaki, K.; Wanschel, A.; Harbour, J.W.; Hare, J.M. A novel cardiomyogenic role for Isl1(+) neural crest cells in the inflow tract. Sci Adv 2020, 6, doi:10.1126/sciadv.aba9950.
- Lewis, A.E.; Vasudevan, H.N.; O'Neill, A.K.; Soriano, P.; Bush, J.O. The widely used Wnt1-Cre transgene causes developmental phenotypes by ectopic activation of Wnt signaling. Developmental biology 2013, 379, 229-234, doi:10.1016/j.ydbio.2013.04.026.
- Tamura, Y.; Matsumura, K.; Sano, M.; Tabata, H.; Kimura, K.; Ieda, M.; Arai, T.; Ohno, Y.; Kanazawa, H.; Yuasa, S.; et al. Neural crest-derived stem cells migrate and differentiate into cardiomyocytes after myocardial infarction. Arterioscler Thromb Vasc Biol 2011, 31, 582-589, doi:10.1161/ATVBAHA.110.214726.
- Tomita, Y.; Matsumura, K.; Wakamatsu, Y.; Matsuzaki, Y.; Shibuya, I.; Kawaguchi, H.; Ieda, M.; Kanakubo, S.; Shimazaki, T.; Ogawa, S.; et al. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol 2005, 170, 1135-1146, doi:10.1083/jcb.200504061.
- Jiang, X.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 2000, 127, 1607-1616, doi:10.1242/dev.127.8.1607.
- Simon, C.; Lickert, H.; Gotz, M.; Dimou, L. Sox10-iCreERT2 : a mouse line to inducibly trace the neural crest and oligodendrocyte lineage. Genesis 2012, 50, 506-515, doi:10.1002/dvg.22003.
- Macatee, T.L.; Hammond, B.P.; Arenkiel, B.R.; Francis, L.; Frank, D.U.; Moon, A.M. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development 2003, 130, 6361-6374, doi:10.1242/dev.00850.
- Epstein, J.A.; Li, J.; Lang, D.; Chen, F.; Brown, C.B.; Jin, F.; Lu, M.M.; Thomas, M.; Liu, E.; Wessels, A.; et al. Migration of cardiac neural crest cells in Splotch embryos. Development 2000, 127, 1869-1878, doi:10.1242/dev.127.9.1869.
- Sato, M.; Yost, H.J. Cardiac neural crest contributes to cardiomyogenesis in zebrafish. Developmental biology 2003, 257, 127-139, doi:10.1016/s0012-1606(03)00037-x.
- Li, Y.X.; Zdanowicz, M.; Young, L.; Kumiski, D.; Leatherbury, L.; Kirby, M.L. Cardiac neural crest in zebrafish embryos contributes to myocardial cell lineage and early heart function. Dev Dyn 2003, 226, 540-550, doi:10.1002/dvdy.10264.
- Beffagna, G. Zebrafish as a Smart Model to Understand Regeneration After Heart Injury: How Fish Could Help Humans. Front Cardiovasc Med 2019, 6, 107, doi:10.3389/fcvm.2019.00107.
- George, R.M.; Maldonado-Velez, G.; Firulli, A.B. The heart of the neural crest: cardiac neural crest cells in development and regeneration. Development 2020, 147, doi:10.1242/dev.188706.
- Cavanaugh, A.M.; Huang, J.; Chen, J.N. Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart. Developmental biology 2015, 404, 103-112, doi:10.1016/j.ydbio.2015.06.002.
- Nagy, N.; Goldstein, A.M. Enteric nervous system development: A crest cell's journey from neural tube to colon. Semin Cell Dev Biol 2017, 66, 94-106, doi:10.1016/j.semcdb.2017.01.006.
- Young, H.M.; Newgreen, D. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. The Anatomical record 2001, 262, 1-15, doi:10.1002/1097-0185(20010101)262:1<1::AID-AR1006>3.0.CO;2-2.
- Kruger, G.M.; Mosher, J.T.; Bixby, S.; Joseph, N.; Iwashita, T.; Morrison, S.J. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 2002, 35, 657-669, doi:10.1016/s0896-6273(02)00827-9.
- Mishina, Y.; Snider, T.N. Neural crest cell signaling pathways critical to cranial bone development and pathology. Exp Cell Res 2014, 325, 138-147, doi:10.1016/j.yexcr.2014.01.019.
- Zhao, X.; Le, T.P.; Erhardt, S.; Findley, T.O.; Wang, J. Hippo-Yap Pathway Orchestrates Neural Crest Ontogenesis. Front Cell Dev Biol 2021, 9, 706623, doi:10.3389/fcell.2021.706623.
- Zhao, X.; Tang, L.; Le, T.P.; Nguyen, B.H.; Chen, W.; Zheng, M.; Yamaguchi, H.; Dawson, B.; You, S.; Martinez-Traverso, I.M.; et al. Yap and Taz promote osteogenesis and prevent chondrogenesis in neural crest cells in vitro and in vivo. Sci Signal 2022, 15, eabn9009, doi:10.1126/scisignal.abn9009.
- Abe, M.; Ruest, L.B.; Clouthier, D.E. Fate of cranial neural crest cells during craniofacial development in endothelin-A receptor-deficient mice. The International journal of developmental biology 2007, 51, 97-105, doi:10.1387/ijdb.062237ma.
- Miletich, I.; Sharpe, P.T. Neural crest contribution to mammalian tooth formation. Birth Defects Res C Embryo Today 2004, 72, 200-212, doi:10.1002/bdrc.20012.
- Nagoshi, N.; Shibata, S.; Kubota, Y.; Nakamura, M.; Nagai, Y.; Satoh, E.; Morikawa, S.; Okada, Y.; Mabuchi, Y.; Katoh, H.; et al. Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell 2008, 2, 392-403, doi:10.1016/j.stem.2008.03.005.
- Ransom, R.C.; Carter, A.C.; Salhotra, A.; Leavitt, T.; Marecic, O.; Murphy, M.P.; Lopez, M.L.; Wei, Y.; Marshall, C.D.; Shen, E.Z.; et al. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature 2018, 563, 514-521, doi:10.1038/s41586-018-0650-9.
- Chung, I.H.; Yamaza, T.; Zhao, H.; Choung, P.H.; Shi, S.; Chai, Y. Stem cell property of postmigratory cranial neural crest cells and their utility in alveolar bone regeneration and tooth development. Stem Cells 2009, 27, 866-877, doi:10.1002/stem.2.
- Jessen, K.R.; Mirsky, R. Schwann Cell Precursors; Multipotent Glial Cells in Embryonic Nerves. Front Mol Neurosci 2019, 12, 69, doi:10.3389/fnmol.2019.00069.
- Huang, C.W.; Huang, W.C.; Qiu, X.; Fernandes Ferreira da Silva, F.; Wang, A.; Patel, S.; Nesti, L.J.; Poo, M.M.; Li, S. The Differentiation Stage of Transplanted Stem Cells Modulates Nerve Regeneration. Sci Rep 2017, 7, 17401, doi:10.1038/s41598-017-17043-4.
- Kimura, H.; Ouchi, T.; Shibata, S.; Amemiya, T.; Nagoshi, N.; Nakagawa, T.; Matsumoto, M.; Okano, H.; Nakamura, M.; Sato, K. Stem cells purified from human induced pluripotent stem cell-derived neural crest-like cells promote peripheral nerve regeneration. Sci Rep 2018, 8, 10071, doi:10.1038/s41598-018-27952-7.
- Lv, Y.; Nan, P.; Chen, G.; Sha, Y.; Xia, B.; Yang, L. In vivo repair of rat transected sciatic nerve by low-intensity pulsed ultrasound and induced pluripotent stem cells-derived neural crest stem cells. Biotechnol Lett 2015, 37, 2497-2506, doi:10.1007/s10529-015-1939-5.
- Jones, I.; Novikova, L.N.; Novikov, L.N.; Renardy, M.; Ullrich, A.; Wiberg, M.; Carlsson, L.; Kingham, P.J. Regenerative effects of human embryonic stem cell-derived neural crest cells for treatment of peripheral nerve injury. J Tissue Eng Regen Med 2018, 12, e2099-e2109, doi:10.1002/term.2642.
- Li, W.; Huang, L.; Zeng, J.; Lin, W.; Li, K.; Sun, J.; Huang, W.; Chen, J.; Wang, G.; Ke, Q.; et al. Characterization and transplantation of enteric neural crest cells from human induced pluripotent stem cells. Mol Psychiatry 2018, 23, 499-508, doi:10.1038/mp.2016.191.
- Lahne, M.; Brecker, M.; Jones, S.E.; Hyde, D.R. The Regenerating Adult Zebrafish Retina Recapitulates Developmental Fate Specification Programs. Front Cell Dev Biol 2020, 8, 617923, doi:10.3389/fcell.2020.617923.
- Powell, C.; Cornblath, E.; Elsaeidi, F.; Wan, J.; Goldman, D. Zebrafish Muller glia-derived progenitors are multipotent, exhibit proliferative biases and regenerate excess neurons. Sci Rep 2016, 6, 24851, doi:10.1038/srep24851.
- Lee, H.J.; Hou, Y.; Chen, Y.; Dailey, Z.Z.; Riddihough, A.; Jang, H.S.; Wang, T.; Johnson, S.L. Regenerating zebrafish fin epigenome is characterized by stable lineage-specific DNA methylation and dynamic chromatin accessibility. Genome Biol 2020, 21, 52, doi:10.1186/s13059-020-1948-0.
- Thompson, J.D.; Ou, J.; Lee, N.; Shin, K.; Cigliola, V.; Song, L.; Crawford, G.E.; Kang, J.; Poss, K.D. Identification and requirements of enhancers that direct gene expression during zebrafish fin regeneration. Development 2020, 147, doi:10.1242/dev.191262.
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188-2190, doi:10.1126/science.1077857.
- Jopling, C.; Sleep, E.; Raya, M.; Marti, M.; Raya, A.; Izpisua Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606-609, doi:10.1038/nature08899.
- Sande-Melon, M.; Marques, I.J.; Galardi-Castilla, M.; Langa, X.; Perez-Lopez, M.; Botos, M.A.; Sanchez-Iranzo, H.; Guzman-Martinez, G.; Ferreira Francisco, D.M.; Pavlinic, D.; et al. Adult sox10(+) Cardiomyocytes Contribute to Myocardial Regeneration in the Zebrafish. Cell reports 2019, 29, 1041-1054 e1045, doi:10.1016/j.celrep.2019.09.041.
- Abdul-Wajid, S.; Demarest, B.L.; Yost, H.J. Loss of embryonic neural crest derived cardiomyocytes causes adult onset hypertrophic cardiomyopathy in zebrafish. Nature communications 2018, 9, 4603, doi:10.1038/s41467-018-07054-8.




