
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammed Bashir | -- | 3558 | 2023-01-18 12:03:20 | | | |
| 2 | Camila Xu | + 64 word(s) | 3622 | 2023-01-19 01:23:46 | | |
Video Upload Options
Algae-based biopolymers may be modified by adding additives, plasticizers, and compatibilizers to enhance the intermolecular force of contact between components, and boost material strength, flexibility, and durability. Moreover, biopolymers are widely used in cosmetics, medicines, and food packaging. Furthermore, algal biopolymer could be used as a food additive due to its high nutritional content.
1. Introduction
In comparison to petroleum-based synthetic polymers, microalgae-driven biopolymers are thought to be the most sustainable biomass feedstock for the synthesis of biopolymers in the direction of a global circular bioeconomy [1]. Recent research reports that when compared to petroleum-based polymers, algae-based biopolymers have better mechanical properties [2]. Additionally, algae-based biopolymers may be modified by adding additives, plasticizers, and compatibilizers to enhance the intermolecular force of contact between components, and boost material strength, flexibility, and durability [3][4]. Moreover, biopolymers are widely used in cosmetics, medicines, and food packaging. Furthermore, algal biopolymer could be used as a food additive due to its high nutritional content [5]. Utilizing innovative biopolymers such as chitosan dramatically improves processes such as medication delivery and tissue regeneration [6]. Biopolymers and their composites are intensively used in contemporary technologies such as 3D printing [7][8].
2. Biopolymers
2.1. Production of Biopolymers from Algae Biomass
2.1.1. Solvent Extraction
2.1.2. Microwave Assisted Extraction
2.1.3. Ultrasound Assisted Extraction
2.1.4. Subcritical Water Extraction
2.2. Biopolymers Produced from Algae Biomass
2.2.1. Poly Hydroxy Alkanoate (PHA)
2.2.2. Poly Hydroxy Butyrate (PHB)
2.2.3. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)
2.2.4. Polylactide (PLA) and Polyalcohol
2.2.5. Polysaccharides
2.2.6. Alginate
2.2.7. Fucoidan
2.2.8. Laminarin
2.2.9. Carrageenan
| Microalgae Species | Isolation Method | Solvent | Isolation Conditions | Biopolymer | Yield | Reference |
|---|---|---|---|---|---|---|
| Alaria esculenta, Saccharina latissima and Ascophyllum nodosum | Solvent extraction | Water | 0.2 M HCl and 0.1 M NaHCO3 | Alginate |
|
[47] |
| Ulva sp. | Solvent extraction | Dimethyl Sulfoxide |
|
PHA | 77.88% | [48] |
| Nizamuddinia zanardinii | Subcritical water extraction. | Water |
|
Fucoidan | 25.98% | [21] |
| Saccharica japonica | Subcritical water extraction. | Water |
|
Fucoidan | 13.65% | [22] |
2.3. Bio-Composite Polymers
2.4. Applications of Algae Biomass-Based Biopolymers
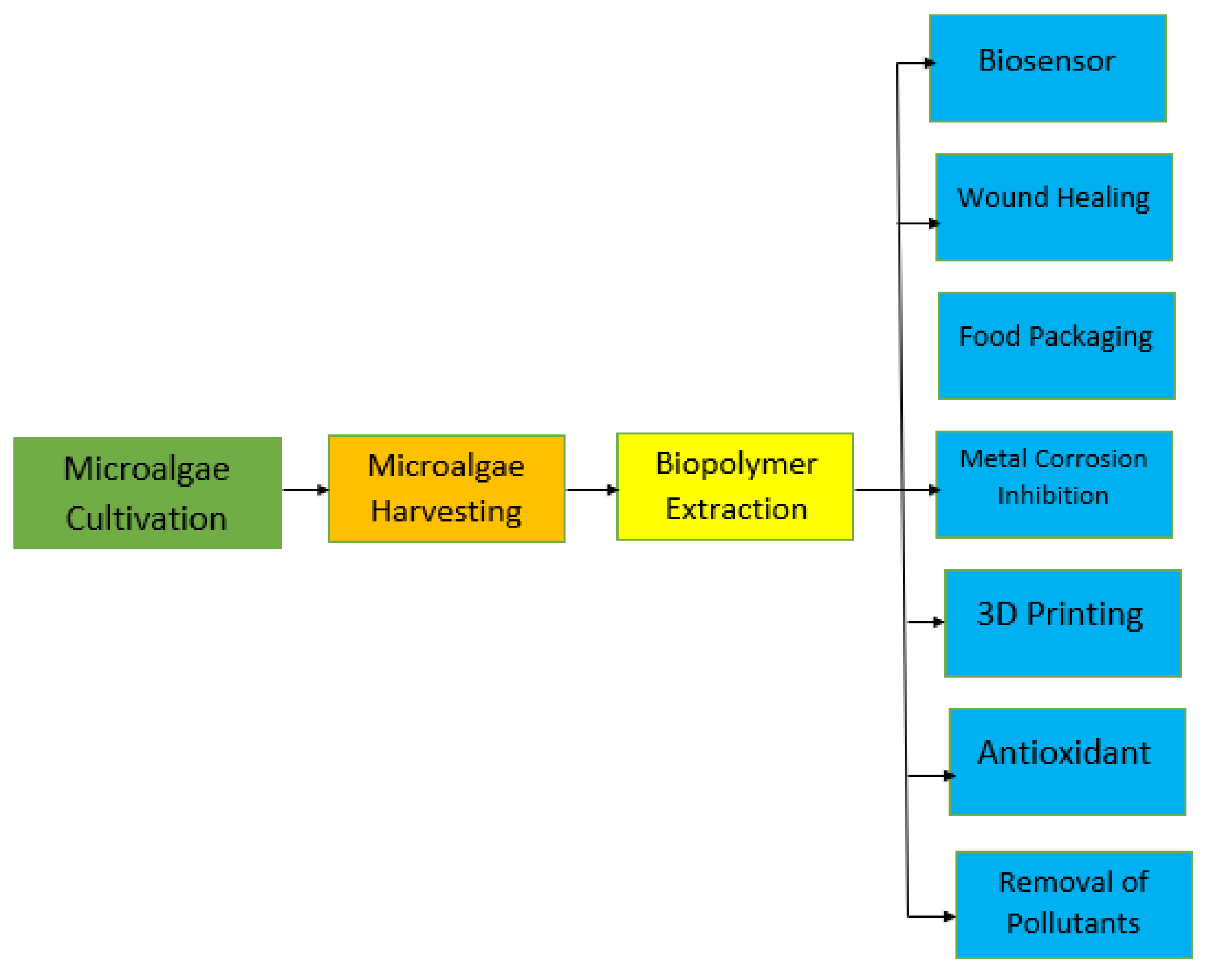
2.4.1. Biosensor
2.4.2. Removal of Pollutants
2.4.3. Biomedical Applications
2.4.4. 3D Printing
2.4.5. Antioxidant
2.5. Degradation of Bioplastics
References
- Das, S.K.; Sathish, A.; Stanley, J. Production of Biofuel and Bioplastic from Chlorella Pyrenoidosa. Mater. Today Proc. 2018, 5, 16774–16781.
- Beckstrom, B.D.; Wilson, M.H.; Crocker, M.; Quinn, J.C. Bioplastic Feedstock Production from Microalgae with Fuel Co-Products: A Techno-Economic and Life Cycle Impact Assessment. Algal Res. 2020, 46, 101769.
- Cinar, S.O.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842.
- Devadas, V.V.; Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Munawaroh, H.S.H.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Lee, K.T.; Show, P.L. Algae Biopolymer towards Sustainable Circular Economy. Bioresour. Technol. 2021, 325, 124702.
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; van Loey, A.M. The Potential of Microalgae and Their Biopolymers as Structuring Ingredients in Food: A Review. Biotechnol. Adv. 2019, 37, 107419.
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on Modification of Chitosan Biopolymer and Its Potential Applications. Int. J. Biol. Macromol. 2020, 152, 681–702.
- Kolan, K.; Liu, Y.; Baldridge, J.; Murphy, C.; Semon, J.; Day, D.; Leu, M. Solvent Based 3D Printing of Biopolymer/Bioactive Glass Composite and Hydrogel for Tissue Engineering Applications. Procedia CIRP 2017, 65, 38–43.
- Kartik, A.; Akhil, D.; Lakshmi, D.; Panchamoorthy Gopinath, K.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A Critical Review on Production of Biopolymers from Algae Biomass and Their Applications. Bioresour. Technol. 2021, 329, 124868.
- Kakarla, R.; Choi, J.W.; Yun, J.H.; Kim, B.H.; Heo, J.; Lee, S.; Cho, D.H.; Ramanan, R.; Kim, H.S. Application of High-Salinity Stress for Enhancing the Lipid Productivity of Chlorella Sorokiniana HS1 in a Two-Phase Process. J. Microbiol. 2018, 56, 56–64.
- Ramanan, R.; Tran, Q.G.; Cho, D.H.; Jung, J.E.; Kim, B.H.; Shin, S.Y.; Choi, S.H.; Liu, K.H.; Kim, D.S.; Lee, S.J.; et al. The Ancient Phosphatidylinositol 3-Kinase Signaling System Is a Master Regulator of Energy and Carbon Metabolism in Algae. Plant Physiol. 2018, 177, 1050–1065.
- Venkata Mohan, S.; Hemalatha, M.; Chakraborty, D.; Chatterjee, S.; Ranadheer, P.; Kona, R. Algal Biorefinery Models with Self-Sustainable Closed Loop Approach: Trends and Prospective for Blue-Bioeconomy. Bioresour. Technol. 2020, 295, 122128.
- Roja, K.; Ruben Sudhakar, D.; Anto, S.; Mathimani, T. Extraction and Characterization of Polyhydroxyalkanoates from Marine Green Alga and Cyanobacteria. Biocatal. Agric. Biotechnol. 2019, 22, 101358.
- Faidi, A.; Lassoued, M.A.; Becheikh, M.E.H.; Touati, M.; Stumbé, J.F.; Farhat, F. Application of Sodium Alginate Extracted from a Tunisian Brown Algae Padina Pavonica for Essential Oil Encapsulation: Microspheres Preparation, Characterization and in Vitro Release Study. Int. J. Biol. Macromol. 2019, 136, 386–394.
- Morales-Jiménez, M.; Gouveia, L.; Yáñez-Fernández, J.; Castro-Muñoz, R.; Barragán-Huerta, B.E. Production, Preparation and Characterization of Microalgae-Based Biopolymer as a Potential Bioactive Film. Coatings 2020, 10, 120.
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, Antiradical, and Antimicrobial Activities of Polysaccharides Obtained by Microwave-Assisted Extraction Method: A Review. Carbohydr. Polym. 2020, 229, 115421.
- Ponthier, E.; Domínguez, H.; Torres, M.D. The Microwave Assisted Extraction Sway on the Features of Antioxidant Compounds and Gelling Biopolymers from Mastocarpus Stellatus. Algal Res. 2020, 51, 102081.
- Flórez-Fernández, N.; López-García, M.; González-Muñoz, M.J.; Vilariño, J.M.L.; Domínguez, H. Ultrasound-Assisted Extraction of Fucoidan from Sargassum Muticum. J. Appl. Phycol. 2017, 29, 1553–1561.
- Hmelkov, A.B.; Zvyagintseva, T.N.; Shevchenko, N.M.; Rasin, A.B.; Ermakova, S.P. Ultrasound-Assisted Extraction of Polysaccharides from Brown Alga Fucus Evanescens. Structure and Biological Activity of the New Fucoidan Fractions. J. Appl. Phycol. 2018, 30, 2039–2046.
- Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. A Green Approach for Alginate Extraction from Sargassum Muticum Brown Seaweed Using Ultrasound-Assisted Technique. Int. J. Biol. Macromol. 2019, 124, 451–459.
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.S. Recovery of Carrageenan from Solomon Islands Red Seaweed Using Ionic Liquid-Assisted Subcritical Water Extraction. Sep. Purif. Technol. 2018, 196, 309–317.
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S.G.; Mariatti, F.; Cravotto, G. Subcritical Water Extraction as an Efficient Technique to Isolate Biologically-Active Fucoidans from Nizamuddinia Zanardinii. Int. J. Biol. Macromol. 2019, 128, 244–253.
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Kim, G.D.; Woo, H.C.; Chun, B.S. Subcritical Water Extraction of Fucoidan from Saccharina Japonica: Optimization, Characterization and Biological Studies. J. Appl. Phycol. 2018, 30, 579–590.
- Saravana, P.S.; Cho, Y.N.; Woo, H.C.; Chun, B.S. Green and Efficient Extraction of Polysaccharides from Brown Seaweed by Adding Deep Eutectic Solvent in Subcritical Water Hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484.
- Cassuriaga, A.P.A.; Freitas, B.C.B.; Morais, M.G.; Costa, J.A.V. Innovative Polyhydroxybutyrate Production by Chlorella Fusca Grown with Pentoses. Bioresour. Technol. 2018, 265, 456–463.
- Costa, S.S.; Miranda, A.L.; Andrade, B.B.; de Jesus Assis, D.; Souza, C.O.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Influence of Nitrogen on Growth, Biomass Composition, Production, and Properties of Polyhydroxyalkanoates (PHAs) by Microalgae. Int. J. Biol. Macromol. 2018, 116, 552–562.
- Ramos, F.D.; Delpino, C.A.; Villar, M.A.; Diaz, M.S. Design and Optimization of Poly(Hydroxyalkanoate)s Production Plants Using Alternative Substrates. Bioresour. Technol. 2019, 289, 121699.
- Rueda, E.; García-Galán, M.J.; Ortiz, A.; Uggetti, E.; Carretero, J.; García, J.; Díez-Montero, R. Bioremediation of Agricultural Runoff and Biopolymers Production from Cyanobacteria Cultured in Demonstrative Full-Scale Photobioreactors. Process Saf. Environ. Prot. 2020, 139, 241–250.
- Sirohi, R.; Prakash Pandey, J.; Kumar Gaur, V.; Gnansounou, E.; Sindhu, R. Critical Overview of Biomass Feedstocks as Sustainable Substrates for the Production of Polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536.
- Abdo, S.M.; Ali, G.H. Analysis of Polyhydroxybutrate and Bioplastic Production from Microalgae. Bull. Natl. Res. Cent. 2019, 43, 97.
- Kavitha, G.; Kurinjimalar, C.; Sivakumar, K.; Palani, P.; Rengasamy, R. Biosynthesis, Purification and Characterization of Polyhydroxybutyrate from Botryococcus Braunii Kütz. Int. J. Biol. Macromol. 2016, 89, 700–706.
- Ghosh, S.; Gnaim, R.; Greiserman, S.; Fadeev, L.; Gozin, M.; Golberg, A. Macroalgal Biomass Subcritical Hydrolysates for the Production of Polyhydroxyalkanoate (PHA) by Haloferax Mediterranei. Bioresour. Technol. 2019, 271, 166–173.
- Akdoğan, M.; Çelik, E. Enhanced Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Biopolymer by Recombinant Bacillus Megaterium in Fed-Batch Bioreactors. Bioprocess Biosyst. Eng. 2021, 44, 403–416.
- Sayin, S.; Kohlhaas, T.; Veziroglu, S.; Okudan, E.; Naz, M.; Schröder, S.; Saygili, E.I.; Açil, Y.; Faupel, F.; Wiltfang, J.; et al. Marine Algae-PLA Composites as de Novo Alternative to Porcine Derived Collagen Membranes. Mater. Today Chem. 2020, 17, 100276.
- Tran, D.T.; Lee, H.R.; Jung, S.; Park, M.S.; Yang, J.W. Lipid-Extracted Algal Biomass Based Biocomposites Fabrication with Poly(Vinyl Alcohol). Algal Res. 2018, 31, 525–533.
- Kumar, L.; Brice, J.; Toberer, L.; Klein-Seetharaman, J.; Knauss, D.; Sarkar, S.K. Antimicrobial Biopolymer Formation from Sodium Alginate and Algae Extract Using Aminoglycosides. PLoS ONE 2019, 14, e0214411.
- Zhang, C.; Show, P.L.; Ho, S.H. Progress and Perspective on Algal Plastics—A Critical Review. Bioresour. Technol. 2019, 289, 121700.
- Yuan, Y.; Macquarrie, D.J. Microwave Assisted Step-by-Step Process for the Production of Fucoidan, Alginate Sodium, Sugars and Biochar from Ascophyllum Nodosum through a Biorefinery Concept. Bioresour. Technol. 2015, 198, 819–827.
- Abraham, R.E.; Su, P.; Puri, M.; Raston, C.L.; Zhang, W. Optimisation of Biorefinery Production of Alginate, Fucoidan and Laminarin from Brown Seaweed Durvillaea Potatorum. Algal Res. 2019, 38, 101389.
- Charoensiddhi, S.; Lorbeer, A.J.; Lahnstein, J.; Bulone, V.; Franco, C.M.M.; Zhang, W. Enzyme-Assisted Extraction of Carbohydrates from the Brown Alga Ecklonia Radiata: Effect of Enzyme Type, PH and Buffer on Sugar Yield and Molecular Weight Profiles. Process Biochem. 2016, 51, 1503–1510.
- Etman, S.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Fucoidan, a Natural Biopolymer in Cancer Combating: From Edible Algae to Nanocarrier Tailoring. Int. J. Biol. Macromol. 2020, 147, 799–808.
- Bittkau, K.S.; Neupane, S.; Alban, S. Initial Evaluation of Six Different Brown Algae Species as Source for Crude Bioactive Fucoidans. Algal Res. 2020, 45, 101759.
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Molecular Characteristics and Antioxidant Activity of Laminarin Extracted from the Seaweed Species Laminaria Hyperborea, Using Hydrothermal-Assisted Extraction and a Multi-Step Purification Procedure. Food Hydrocoll. 2021, 112, 106332.
- Becker, S.; Scheffel, A.; Polz, M.F.; Hehemann, J.H. Accurate Quantification of Laminarin in Marine Organic Matter with Enzymes from Marine Microbes. Appl. Environ. Microbiol. 2017, 83, e03389-16.
- Qureshi, D.; Nayak, S.K.; Maji, S.; Kim, D.; Banerjee, I.; Pal, K. Carrageenan: A Wonder Polymer from Marine Algae for Potential Drug Delivery Applications. Curr. Pharm. Des. 2019, 25, 1172–1186.
- Bouanati, T.; Colson, E.; Moins, S.; Cabrera, J.C.; Eeckhaut, I.; Raquez, J.M.; Gerbaux, P. Microwave-Assisted Depolymerization of Carrageenans from Kappaphycus Alvarezii and Eucheuma Spinosum: Controlled and Green Production of Oligosaccharides from the Algae Biomass. Algal Res. 2020, 51, 102054.
- Bui, V.T.N.T.; Nguyen, B.T.; Renou, F.; Nicolai, T. Structure and Rheological Properties of Carrageenans Extracted from Different Red Algae Species Cultivated in Cam Ranh Bay, Vietnam. J. Appl. Phycol. 2019, 31, 1947–1953.
- Cebrián-Lloret, V.; Metz, M.; Martínez-Abad, A.; Knutsen, S.H.; Ballance, S.; López-Rubio, A.; Martínez-Sanz, M. Valorization of Alginate-Extracted Seaweed Biomass for the Development of Cellulose-Based Packaging Films. Algal Res. 2022, 61, 102576.
- Steinbruch, E.; Drabik, D.; Epstein, M.; Ghosh, S.; Prabhu, M.S.; Gozin, M.; Kribus, A.; Golberg, A. Hydrothermal Processing of a Green Seaweed Ulva Sp. for the Production of Monosaccharides, Polyhydroxyalkanoates, and Hydrochar. Bioresour. Technol. 2020, 318, 124263.
- Biswas, M.C.; Jony, B.; Nandy, P.K.; Chowdhury, R.A.; Halder, S.; Kumar, D.; Ramakrishna, S.; Hassan, M.; Ahsan, M.A.; Hoque, M.E.; et al. Recent Advancement of Biopolymers and Their Potential Biomedical Applications. J. Polym. Environ. 2022, 30, 51–74.
- Lu, Y.; Biswas, M.C.; Guo, Z.; Jeon, J.W.; Wujcik, E.K. Recent Developments in Bio-Monitoring via Advanced Polymer Nanocomposite-Based Wearable Strain Sensors. Biosens. Bioelectron. 2019, 123, 167–177.
- Chang, J.; Xiao, W.; Liu, P.; Liao, X.; Wen, Y.; Bai, L.; Li, L.; Li, M. Carboxymethyl Cellulose Assisted Preparation of Water-Processable Halloysite Nanotubular Composites with Carboxyl-Functionalized Multi-Carbon Nanotubes for Simultaneous Voltammetric Detection of Uric Acid, Guanine and Adenine in Biological Samples. J. Electroanal. Chem. 2016, 780, 103–113.
- Zhang, Q.; Hu, J.; Lee, D.J. Microbial Fuel Cells as Pollutant Treatment Units: Research Updates. Bioresour. Technol. 2016, 217, 121–128.
- Upadhyay, A.K.; Singh, R.; Vijay, D.; Singh, L.; Singh, D.P. Environmental Technology & Innovation Microalgal Consortia Technology: A Novel and Sustainable Approach of Resource Reutilization, Waste Management and Lipid Production. Environ. Technol. Innov. 2021, 23, 101600.
- Del Mar Orta, M.; Martín, J.; Santos, J.L.; Aparicio, I.; Medina-Carrasco, S.; Alonso, E. Biopolymer-Clay Nanocomposites as Novel and Ecofriendly Adsorbents for Environmental Remediation. Appl. Clay Sci. 2020, 198, 105838.
- Xia, L.; Tan, J.; Wu, P.; He, Q.; Song, S.; Li, Y. Biopolymers Extracted from Klebsiella Sp. and Bacillus Sp. in Wastewater Sludge as Superb Adsorbents for Aqueous Hg(II) Removal from Water. Chem. Phys. Lett. 2020, 754, 137689.
- Silva, M.S.; Silva, L.S.; Ferreira, F.J.L.; Bezerra, R.D.S.; Marques, T.M.F.; Meneguin, A.B.; Barud, H.S.; Osajima, J.A.; Silva Filho, E.C. Study of Interactions between Organic Contaminants and a New Phosphated Biopolymer Derived from Cellulose. Int. J. Biol. Macromol. 2020, 146, 668–677.
- Sathiyavimal, S.; Vasantharaj, S.; LewisOscar, F.; Selvaraj, R.; Brindhadevi, K.; Pugazhendhi, A. Natural Organic and Inorganic–Hydroxyapatite Biopolymer Composite for Biomedical Applications. Prog. Org. Coat. 2020, 147, 105858.
- Bahmani, A.; Comeau, P.A.; Montesano, J.; Willett, T.L. Extrudable Hydroxyapatite/Plant Oil-Based Biopolymer Nanocomposites for Biomedical Applications: Mechanical Testing and Modeling. Mater. Des. 2019, 174, 107790.
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current Advances and Future Perspectives of 3D Printing Natural-Derived Biopolymers. Carbohydr. Polym. 2019, 207, 297–316.
- Sangiorgi, A.; Gonzalez, Z.; Ferrandez-Montero, A.; Yus, J.; Sanchez-Herencia, A.J.; Galassi, C.; Sanson, A.; Ferrari, B. 3D Printing of Photocatalytic Filters Using a Biopolymer to Immobilize TiO2 Nanoparticles. J. Electrochem. Soc. 2019, 166, H3239–H3248.
- Shishkovsky, I.; Sherbakov, V.; Ibatullin, I.; Volchkov, V.; Volova, L. Nano-Size Ceramic Reinforced 3D Biopolymer Scaffolds: Tribomechanical Testing and Stem Cell Activity. Compos. Struct. 2018, 202, 651–659.
- Gu, L.; Peng, N.; Chang, C.; McClements, D.J.; Su, Y.; Yang, Y. Fabrication of Surface-Active Antioxidant Food Biopolymers: Conjugation of Catechin Polymers to Egg White Proteins. Food Biophys. 2017, 12, 198–210.
- Pérez Córdoba, L.J.; Sobral, P.J.A. Physical and Antioxidant Properties of Films Based on Gelatin, Gelatin-Chitosan or Gelatin-Sodium Caseinate Blends Loaded with Nanoemulsified Active Compounds. J. Food Eng. 2017, 213, 47–53.
- Zanutto-Elgui, M.R.; Vieira, J.C.S.; do Prado, D.Z.; Buzalaf, M.A.R.; de Magalhães Padilha, P.; Elgui de Oliveira, D.; Fleuri, L.F. Production of Milk Peptides with Antimicrobial and Antioxidant Properties through Fungal Proteases. Food Chem. 2019, 278, 823–831.
- Gopu, M.; Selvam, K. Polysaccharides from Marine Red Algae Amphiroa Rigida and Their Biomedical Potential: An in-Vitro Study. Biocatal. Agric. Biotechnol. 2020, 29, 101769.
- Aswathi Mohan, A.; Robert Antony, A.; Greeshma, K.; Yun, J.H.; Ramanan, R.; Kim, H.S. Algal Biopolymers as Sustainable Resources for a Net-Zero Carbon Bioeconomy. Bioresour. Technol. 2022, 344, 126397.
- Chiellini, E.; Cinelli, P.; Ilieva, V.I.; Martera, M. Biodegradable Thermoplastic Composites Based on Polyvinyl Alcohol and Algae. Biomacromolecules 2008, 9, 1007–1013.
- Zini, E.; Scandola, M. Green Composites: An Overview. Polym. Compos. 2011, 32, 1905–1915.
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641.
- Zenkiewicz, M.; Richert, J.; Rytlewski, P.; Moraczewski, K.; Stepczyńska, M.; Karasiewicz, T. Characterisation of Multi-Extruded Poly(Lactic Acid). Polym. Test. 2009, 28, 412–418.
- Ariffin, H.; Nishida, H.; Hassan, M.A.; Shirai, Y. Chemical Recycling of Polyhydroxyalkanoates as a Method towards Sustainable Development. Biotechnol. J. 2010, 5, 484–492.




