Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wojciech Łabuś | -- | 1821 | 2023-01-18 11:17:36 | | | |
| 2 | Catherine Yang | + 1 word(s) | 1822 | 2023-01-28 04:37:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gierek, M.; Łabuś, W.; Kitala, D.; Lorek, A.; Ochała-Gierek, G.; Zagórska, K.M.; Waniczek, D.; Szyluk, K.; Niemiec, P. Human Acellular Dermal Matrix in Reconstructive Surgery. Encyclopedia. Available online: https://encyclopedia.pub/entry/40343 (accessed on 08 February 2026).
Gierek M, Łabuś W, Kitala D, Lorek A, Ochała-Gierek G, Zagórska KM, et al. Human Acellular Dermal Matrix in Reconstructive Surgery. Encyclopedia. Available at: https://encyclopedia.pub/entry/40343. Accessed February 08, 2026.
Gierek, Marcin, Wojciech Łabuś, Diana Kitala, Andrzej Lorek, Gabriela Ochała-Gierek, Karolina Mikuś Zagórska, Dariusz Waniczek, Karol Szyluk, Paweł Niemiec. "Human Acellular Dermal Matrix in Reconstructive Surgery" Encyclopedia, https://encyclopedia.pub/entry/40343 (accessed February 08, 2026).
Gierek, M., Łabuś, W., Kitala, D., Lorek, A., Ochała-Gierek, G., Zagórska, K.M., Waniczek, D., Szyluk, K., & Niemiec, P. (2023, January 18). Human Acellular Dermal Matrix in Reconstructive Surgery. In Encyclopedia. https://encyclopedia.pub/entry/40343
Gierek, Marcin, et al. "Human Acellular Dermal Matrix in Reconstructive Surgery." Encyclopedia. Web. 18 January, 2023.
Copy Citation
Reconstructive surgery often confronts large tissue defects. This creates a need to look for materials that are immunogenic but offer the possibility of tissue filling. ADM—acellular dermal matrix—is a biological collagen matrix without immunogenicity, which is more commonly used in surgical treatment. Reconstructive surgery is still searching for various biocompatible materials that can be widely used in surgery. The available materials have their advantages and disadvantages.
acellular dermal matrix
bioengineering
allograft
1. Decellularization of Human Allogeneic Skin
Human acellular dermal matrix (ADM) is made by taking a full-thickness section of skin from a donor source. In most cases, this is a human cadaver. However, porcine or bovine dermis is used as well. The process of cell removal from tissues and organs has been described extensively and in detail in previous literature [1][2][3][4][5][6][7][8][9]. The material for ADM preparation is allogeneic human skin procured during multi-organ/multi-tissue donations from the deceased donor. In further stages of preparation, allogeneic human skin is subjected to the action of selected cell-removing factors (e.g., proteolytic enzymes, detergents, etc.). Removal of cells from allogeneic human dermis yields an acellular mesh made of collagen which is non-immunogenic. [1][2][3][4][5][6][7][8][9]. The biological source and the manufacturing process of ADM is shown in Figure 1.
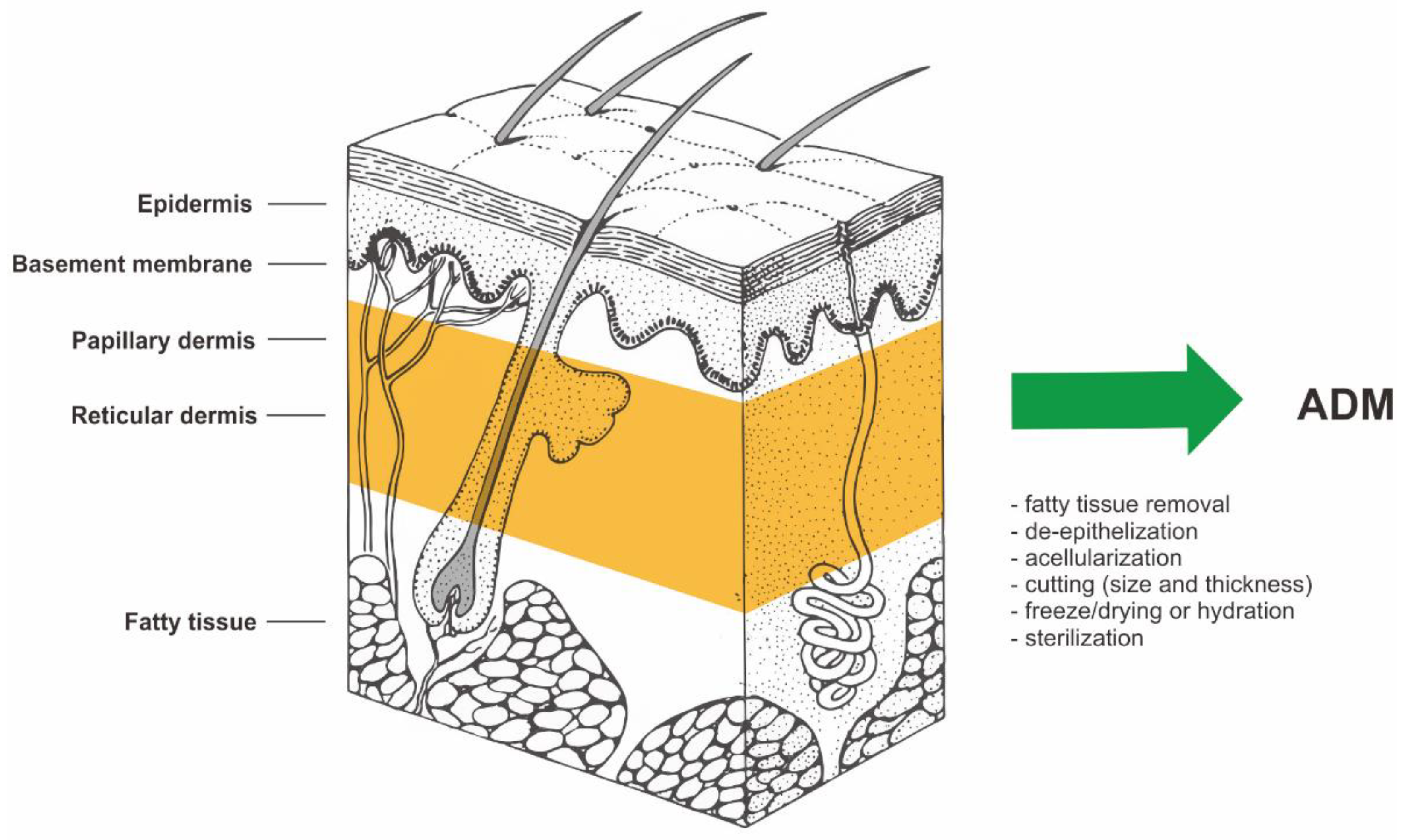
Figure 1. The biological source and the manufacturing process of ADM (acellular dermal matrix). Structure of human skin based on https://visualsonline.cancer.gov/details.cfm?imageid=2496 (access on 1 January 2001) [10].
2. Burns and Wound Management
The organ with the largest surface area in the human body is the skin. That makes the skin very important. Reconstruction of skin continuity after a very severe burn is crucial for healing. Reconstruction after a severe burn injury also involves healing of the skin’s natural elasticity, texture, and contour. Complete destruction of the skin requires the use of a skin substitute. The method of choice is a graft consisting of the epidermis and a superthin layer of dermis. It is important to rebuild and replace this tissue to achieve good epithelial coverage [1][2][3][4][5][6][7][8].
The optimal method of treating extensive full-thickness (III) skin burns is split-thickness skin grafts (STSGs). STSGs are collected from an undamaged site for wound closure [1][2][3][4][5][6][7][8]. The main limitation of this method is the lack of healthy, undamaged skin that could be used as a graft. Moreover, the donor fields constitute an additional wound that weighs down the organism [9]. The sites from which the skin was removed for transplantation cause pain, therefore requiring the use of analgesic therapy, and leave scars while healing [6][9]. The limitations related to the use of autologous slow split-thickness skin grafts necessitate the search for new methods of treating severely burned patients. An alternative clinical standard that has been available for over three decades in the treatment of extensive full-thickness burns of the skin in patients with a deficit of donor sites is the use of in vitro cultured autologous keratinocytes (CEA) [5][6]. The effect of this method is the acceleration of the coating regeneration process, while long-term follow-up may develop functionally unacceptable and cosmetically hypertrophic scars [5][6][10][11]. Another alternative treatment for burns is the use of allogeneic skin grafts (allografts). This method has been used for over 70 years. Clinical indications for allogeneic skin grafts include protection against the drying out of the wound. Another indication is protection against infection and a mechanical barrier that prevents the loss of heat, proteins, and electrolytes. Although the use of allogeneic skin grafts is a temporary solution, it remains widely used in the treatment of burns. This kind of transplant is a temporary biological dressing. Almost always, this allograft is rejected by the human body (the recipient’s) after some time [7][8][12].
In the beginning, acellular dermal grafts were invented for use in the treatment of full-thickness burns (third-degree burns). The management of badly burned patients is complicated when donor skin is not available. When ADM is applied to a full-thickness skin defect with an overlying very thin split skin graft, migration and ingrowth of cells into the ADM are observed from both the skin graft and the underlying tissue and peripheral skin margins [13]. It all provides laminin for adhesion and type IV collagen, which is needed for epithelialization. The processing of allogeneic grafts results in scaffolding, which preserves normal collagen structure. Acellular vascular channels are preserved in this way as well [14]. In optimal conditions, vascular channels are repopulated by the host within a week, and the endothelium is being rebuilt [14]. ADMs eliminate the need for graft harvesting, which reduces scarring at the donor site that is seen with skin grafts [15].
In Figure 2, the researchers attached a histological image of ADM.
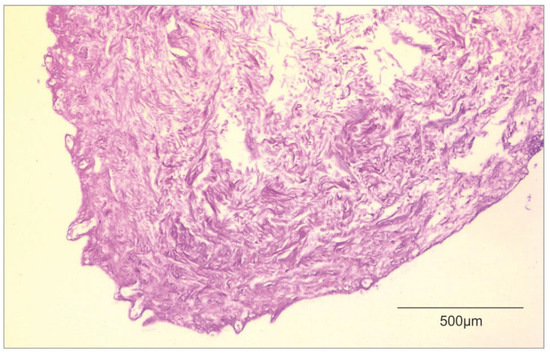
Figure 2. Histological image of ADM (own material).
This kind of biological dressing has become more popular in burn treatment indications over the past nearly 30 years. This skin substitute is used for open soft tissue defects like burns. Skin grafting is probably a less expensive technique but is often associated with very painful donor site. Application of ADM can undoubtedly improve patients’ quality of life after surgery. AlloDerm has been reported in the literature as a dermal substitute in full-thickness burn wounds [15][16].
3. Reconstructive Breast Procedures
Breast cancer is the most common form of cancer in the population and is the second most common cause of death in women. There are many literature reports on the use of ADM in breast reconstructive surgery [17]. Reconstructive breast surgery after a mastectomy is very limited. Women are more likely to choose reconstructions with implants rather than reconstructions based on autologous tissue transfer. This is due to the occurrence of side effects (i.e. a very painful donor site) [18]. Acellular dermal matrices are well-known biological scaffolds of human, bovine, or porcine origin that are increasingly used in the fields of plastic surgery and surgical oncology, mainly in breast reconstruction after conservative mastectomy in order to support implants [19].
In 2001, Duncan et al. reported the first use of ADM in breast surgery. Duncan et al. showed that visible implant undulation was decreased in a revision of primary augmentation with capsulectomy and ADM application [20]. The first published report mentioning the use of ADM in breast reconstruction came in 2005 (Breuing et al.). Breuing documented ten cases of patients who had undergone mastectomies with single-stage reconstruction and AlloDerm application [21]. This led to an increase in the use of ADM in breast reconstruction, making it one of the most popular applications. Reconstruction with tissue expanders and implants is a multi-step process that can be very difficult for patients. A main limitation of immediate single-stage reconstruction has always been the inability to completely cover the implant with enough soft tissue to cause tension-free closure and avoid implant extrusion [22]. This is where ADM is used; instead of lifting the local serratus fascia and rectus fascia defined by the lateral range of the pocket, ADM allows to actively shape the lower pole, including the ability to establish a critically important subcutaneous fold in an ideal location. ADMs are also used when there are viable but weakened or thin skin flaps after a mastectomy that are at risk of wound spread or necrosis, as well as when the subcutaneous and lateral nipple folds have been leveraged during the mastectomy. According to some authors, ADMs also have the added benefit of reducing the capsular contracture rate and improving surface roughness [23][24]. Postoperative pain and expansion rates remain without significant changes [25][26]. The first results of a recent prospective randomized controlled trial on breast reconstruction with ADM indicate a complication rate of 36%, of which about half are minor. Half of the complications require surgical intervention [27]. The analysis showed that inadequate integration of the ADM increased the incidence of infection. Poor integration has been noted and linked to an increase in breast size, expander size, initial tissue-expander fill volume, and obesity [27]. In addition to breast reconstruction, ADMs have also gained greater acceptance for breast revision surgery. Allergan (Allergan Inc., USA) presented a reoperation rate of 30% for patients after primary augmentation and a rate of 41% for patients after revision augmentation [28]. In some studies, ADM combined with oxidized, regenerated cellulose was also used as an aid to obtain better cosmetic results in partial breast reconstruction [29]. Gwak et al. reported the first improvemed aesthetic outcomes using human ADM as a filler in the BCS (breast-conserving surgery) of 117 breast cancer patients treated at the Division of Breast and Thyroid Surgical Oncology of St. Vincent’s Hospital (Seoul, Republic of Korea) [30]. Broyles et al. compare two commercial ADMs in breast reconstruction in a multicenter, prospective, randomized, control trial in 2021. The researchers presented results supporting the use of human acellular dermal matrices in implant-based breast reconstruction and demonstrated no significant difference in matrix-related complication rates between FlexHD Pliable (acellular hydrated dermis) and AlloDerm RTU (ready to use) [31]. There is still a field in research for aesthetic outcomes in surgical oncology with acellular dermal matrix. This biomaterial seems to be starting to become a standard in breast reconstructive surgery.
4. Hernia Repair
Abdominal surgery is often associated with major abdominal wall closure problems. Leaving aside anatomical differences, it is always a challenge in general surgery. Incisional hernias often occur after laparotomies [32]. Surgeons often operate in the abdominal cavity, where the fascia is weak to close the wound, combined with debris from the intestines. As an implant, mesh has always been vulnerable to infection. Mesh made of non-absorbable material is inapplicable in these cases, and temporary artificial mesh often does not provide optimal tensile strength to avoid the occurrence of hernias. Studies have shown that ADM is effective in reducing the rate of hernia recurrence in infected fields compared to primary closure without mesh [33][34]. The use of mesh materials in infected wounds results in a high incidence of mesh infection, fibrosis, fistulas, and adhesion formation. To avoid such complications, biomaterials obtained from animal or human sources are now being used [35]. Acellular dermal matrices are advertised as an alternative to man-made mesh material. The main aim of this application is to decrease the rates of infection, disintegration, extrusion, and rejection compared to artificial materials [35][36].
Lung hernia following minimally invasive cardiac surgery is rare, with few reported cases in the literature. Surgical repair is debated, and several methods have been described, including a variety of synthetic and biological materials. Stanizzi et al. presented successful reconstruction with six year follow-up on a patient with a lung hernia after a minithoracotomy. Stanizzi et al. suggest that ADMs are a safe and reliable surgical technique for lung hernia repair due to their biological and mechanical properties, even in those secondary hernias to minithoracotomy where complete muscle coverage of the matrix could not be provided [37]. Lightfoot et al.’s performed a retrospective cohort study comparing 40 consecutive patients who underwent open component separation (CS/VHR—Component Separation/Ventral Hernia Repair) with porcine ADM reinforcement to 39 consecutive patients who underwent open CS/VHR with bovine ADM [38]. Lightfoot et al. study showed that recurrences and complications requiring reoperation were fewer, which trended toward but did not reach statistical significance [38]. There is still room for further research in the field of ADM in hernioplasty.
References
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233.
- Gierek, M.; Kawecki, M.; Mikuś, K.; Klama-Baryła, A.; Nowak, M. Biological dressings as a substitutes of the skin in the treatment of burn wounds. Pol. Przegląd Chir. 2013, 85, 354–359.
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25.
- Brown, B.N.; Freund, J.M.; Han, L.; Rubin, J.P.; Reing, J.E.; Jeffries, E.M.; Wolf, M.T.; Tottey, S.; Barnes, C.A.; Ratner, B.D.; et al. Comparison of Three Methods for the Derivation of a Biologic Scaffold Composed of Adipose Tissue Extracellular Matrix. Tissue Eng. Part C Methods 2011, 17, 411.
- Brown, B.N.; Badylak, S.B. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268.
- Łabuś, W.; Kawecki, M.; Glik, J.; Maj, M.; Kitala, D.; Misiuga, M.; Klama-Baryła, A.; Kraut, M.; Nowak, M. Own experience from the use of a substitute of an allogeneic acellular dermal matrix revitalized with in vitro cultured skin cells in clinical practice. Pol. Przegląd Chir. 2015, 10, 929.
- Vorotnikova, E.; McIntosh, D.; Dewilde, A.; Zhang, J.; Reing, J.E.; Zhang, L. Extracellular matrix derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010, 29, 690–700.
- Barkan, D.; Green, J.E.; Chambers, A.F. Extracellular matrix: A gatekeeper in the transition from dormancy to metastatic growth. Eur. J. Cancer 2010, 46, 1181–1188.
- Łabuś, W.; Kawecki, M.; Nowak, M. The role of tissue engineering in the treatment of burn wounds. Pol. Przegląd Chir. 2012, 3, 167–171.
- Available online: https://visualsonline.cancer.gov/details.cfm?imageid=2496 (accessed on 1 January 2001).
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I. Perfusiondecellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221.
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.L.; Guzzardi, M.A.; Shulman, C. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16, 814–820.
- Livesey, S.A.; Herndon, D.N.; Hollyoak, M.A.; Atkinson, Y.H.; Nag, A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation 1995, 60, 1–9.
- Clark, J.M.; Saffold, S.H.; Israel, J.M. Decellularized dermal grafting in cleft palate repair. Arch. Facial Plast. Surg. 2003, 5, 40–44.
- Wainwright, D.; Madden, M.; Luterman, A. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J. Burn Care Rehabil. 1996, 17, 124–136.
- Wainwright, D.J. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995, 21, 243–248.
- Freitas-Silva, R.; Conde, D.M.; de Freitas-Junior, R.; Martinez, E.Z. Comparison of quality of life, satisfaction with surgery and shoulder-arm morbidity in breast cancer survivors submitted to breast-conserving therapy or mastectomy followed by immediate breast reconstruction. Clinics 2010, 65, 781–787.
- Rawlani, V.; Buck, D.W., 2nd; Johnson, S.A.; Heyer, K.S.; Kim, J.Y. Tissue expander breast reconstruction using prehydrated human acellular dermis. Ann. Plast. Surg. 2011, 66, 593–597.
- Cuomo, R. Submuscular and pre-pectoral ADM assisted immediate breast reconstruction: A literature review. Medicina 2020, 56, 256.
- Duncan, D.I. Correction of implant rippling using allograft dermis. Aesthetic Surg. J. 2001, 21, 81–84.
- Breuing, K.H.; Warren, S.M. Immediate bilateral breast reconstruction with implants and infero- lateral AlloDerm slings. Ann. Plast. Surg. 2005, 55, 232–239.
- Fosnot, J.; Kovach, S.J.; Scarlatti, J.M. Acellular Dermal Matrix: General Principles for the Plastic Surgeon. Aesthetic Surg. J. 2011, 31 (Suppl. 7), 5S–12S.
- Tevlin, R.; Borrelli, M.R.; Irizarry, D.; Nguyen, D.; Wan, D.C.; Momeni, A. Acellular Dermal Matrix Reduces Myofibroblast Presence in the Breast Capsule. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2213.
- Hartzell, T.L.; Taghinia, A.H.; Chang, J.; Lin, S.J.; Slavin, S.A. The use of human acellular dermal matrix for the correction of secondary deformities after breast augmentation: Results and costs. Plast. Reconstr. Surg. 2010, 126, 1711–1720.
- McCarthy, C.M.; Lee, C.N.; Halvorson, E.G. The use of acellular dermal matrices in two-stage expander/implant reconstruction: A multicenter, blinded, randomized controlled trial. Plast. Reconstr. Surg. 2012, 130 (Suppl. 2), 57S–66S.
- Davila, A.A.; Seth, A.K.; Wang, E. Human acellular dermis versus submuscular tissue expander breast reconstruction: A multivariate analysis of short-term complications. Arch. Plast. Surg. 2013, 40, 19–27.
- Mendenhall, S.D.; Anderson, L.A.; Ying, J. The BREASTrial: Stage I. Outcomes from the time of tissue expander and acellular dermal matrix placement to definitive reconstruction. Plast. Reconstr. Surg. 2015, 135, 29e–42e.v.
- Allergan. Company Document. Important Information for Women About Breast Augmenta-Tion with Natrelle® Gel-Filled Breast Implants. 2015. Available online: http://www.allergan.com/assets/pdf/ca_natrelle_gel_aug_info_en.pdf (accessed on 1 January 2015).
- Lee, J.; Yang, J.D.; Lee, J.W.; Li, J.; Jung, J.H.; Kim, W.W.; Park, C.S.; Lee, J.S.; Park, H.Y. Acellular dermal matrix combined with oxidized regenerated cellulose for partial breast reconstruction: Two case reports. Medicine 2020, 99, e21217.
- Gwak, H.; Jeon, Y.W.; Lim, S.T.; Park, S.Y.; Suh, Y.J. Volume replacement with diced acellular dermal matrix in oncoplastic breast-conserving surgery: A prospective single-center experience. World J. Surg. Oncol. 2020, 18, 60.
- Broyles, J.M.; Liao, E.C.; Kim, J.; Heistein, J.; Sisco, M.; Karp, N.; Lau, F.H.; Chun, Y.S. Acellular Dermal Matrix-Associated Complications in Implant-Based Breast Reconstruction: A Multicenter, Prospective, Randomized Controlled Clinical Trial Comparing Two Human Tissues. Plast. Reconstr. Surg. 2021, 148, 493–500.
- Zhu, K.Q.; Carrougher, G.J.; Gibran, N.S.; Isik, F.F.; Engrav, L.H. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair Regen. 2007, 15, S32–S39.
- Diaz, J.J., Jr.; Conquest, A.M.; Ferzoco, S.J. Multi-institutional experience using human acellular dermal matrix for ventral hernia repair in a compromised surgical field. Arch. Surg. 2009, 144, 209–215.
- Candage, R.; Jones, K.; Luchette, F.A.; Sinacore, J.M.; Vandevender, D.; Reed, R.L. Use of human acellular dermal matrix for hernia repair: Friend or foe? Surgery 2008, 144, 703–709.
- Garvey, P.B.; Martinez, R.A.; Baumann, D.P.; Liu, J.; Butler, C.E. Outcomes of abdominal wall recon- struction with acellular dermal matrix are not affected by wound contamination. J. Am. Coll. Surg. 2014, 219, 853–864.
- Guerra, O. Noncrosslinked porcine-derived acellular dermal matrix for single-stage com- plex abdominal wall herniorrhaphy after removal of infected synthetic mesh: A retrospective review. Am. Surg. 2014, 80, 489–495.
- Stanizzi, A.; Torresetti, M.; Salati, M.; Benedetto, G.D. Use of porcine acellular dermal matrix to repair lung Hernia after minithoracotomy: A case report with 6-Year follow-up. JPRAS Open 2021, 28, 56–60.
- Lightfoot, R.W.; Thrash, C.; Thompson, S.; Richmond, B.K. A Comparison of Component Separation with Porcine Acellular Dermal Reinforcement to Bovine Acellular Dermal Matrix in the Repair of Significant Midline Ventral Hernia Defects. Am. Surg. 2021, 00031348211050844.
More
Information
Subjects:
Surgery
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
970
Revisions:
2 times
(View History)
Update Date:
28 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




