Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vishnu D. Rajput | -- | 2302 | 2023-01-18 10:44:05 | | | |
| 2 | Amina Yu | + 9 word(s) | 2311 | 2023-01-19 02:11:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Singh, A.; Tyagi, P.; Ranjan, R.; Sushkova, S.N.; Minkina, T.; Burachevskaya, M.; Rajput, V.D. Green Synthesis of Nanoparticles and Hazardous Wastes. Encyclopedia. Available online: https://encyclopedia.pub/entry/40335 (accessed on 08 February 2026).
Singh A, Tyagi P, Ranjan R, Sushkova SN, Minkina T, Burachevskaya M, et al. Green Synthesis of Nanoparticles and Hazardous Wastes. Encyclopedia. Available at: https://encyclopedia.pub/entry/40335. Accessed February 08, 2026.
Singh, Ayushi, Parul Tyagi, Rajiv Ranjan, Svetlana N. Sushkova, Tatiana Minkina, Marina Burachevskaya, Vishnu D. Rajput. "Green Synthesis of Nanoparticles and Hazardous Wastes" Encyclopedia, https://encyclopedia.pub/entry/40335 (accessed February 08, 2026).
Singh, A., Tyagi, P., Ranjan, R., Sushkova, S.N., Minkina, T., Burachevskaya, M., & Rajput, V.D. (2023, January 18). Green Synthesis of Nanoparticles and Hazardous Wastes. In Encyclopedia. https://encyclopedia.pub/entry/40335
Singh, Ayushi, et al. "Green Synthesis of Nanoparticles and Hazardous Wastes." Encyclopedia. Web. 18 January, 2023.
Copy Citation
The combination of two sciences, i.e., nanotechnology and biotechnology, is gradually expanding its roots in almost all the sectors involving biology, engineering, cosmetics, remediation, biomedical, agriculture, food and so on. Numerous nanoscale (below 100 nm) materials show remarkable features in contrast to their bulk elements and components. With progressive studies, researchers have developed nano-based composites and materials, and found their effective applicatin in almost every field including waste remediation, solar applications, and nano-sensors.
green technology
metal-based nanoparticles
pollutant
toxic dyes
1. Nanoparticles and Approaches to Green Synthesis
1.1. Top-Down Approach
The base material is exploited in bulk to reduce the size to nanoscale in top-down approaches, which can be achieved by both physical and chemical techniques [1]. These methods include photochemical and chemical reduction as well as electrochemical changes using various techniques such as laser and thermal ablation and mechanical milling, which yield stabilised NPs. These are easier to implement but might induce changes in properties and surface chemistry of the prepared nanoparticles (NPs) [2].
Laser ablation involves the application of high-powered lasers onto metal plates, yielding ablated NPs in the liquid medium. This method is suitable for NP fabrication and is affected by ablation time, wavelength and energy of the laser, and absorption by the liquid medium. This technique has been used for the synthesis of Cu, Au, and Ag NPs [3]. Mechanical milling involves the reduction of coarse particles into the desired smaller size. It is achieved by using an agitator at the speed of 75–500 rpm as per the requirements. The size of the vial in which the operation is carried out, the speed, time, temperature, and environment (dry or wet) of milling, and the usage of inert or reactive gases affect the particle size and homogeneity of the prepared NPs [4].
1.2. Bottom-Up Approach
On the other hand, bottom-up approaches involve the assembly of atoms or molecules to form nanostructured building units which eventually form the desired NPs. It can be achieved via both chemical and biological techniques. Studies have clearly shown that biological methods for NP synthesis have the fewest drawbacks compared with the other methods. Physical methods have high expenditures in time, energy and cost, as well as low production rate. Chemical synthesis is attained by involvement of toxic chemicals and solvents and results in noxious derivatives and byproducts [1].
In contrast with various alternatives, the solid-state method of NP synthesis is cost effective and convenient to use. The stages involved are milling, calcination, and sintering [5]. Particles produced using this method are unlikely to aggregate and are highly stable. Secondly, the preparation process involves the removal of solvents, which facilitates simpler handling and large-scale synthesis of the NPs, for example, AgNPs [6]. One of the liquid-state methods, i.e., synthesis via sol-gel technique, provides economic production of metal-oxide NPs with various other advantages. In addition to being a simple and fast technique, the method is suitable for the synthesis of high quality and complex nanocomposites with low processing temperature and higher purity. Various metal-oxide NPs such as zinc oxide (ZnO), tungsten oxide (WO3), tin oxide (SnO2), and titanium dioxide (TiO2) have been prepared using this method [7]. This chemical technique involves the formation of a gel-like phase which comprises both a solid and liquid phase. The organic solvents used in the procedure might be toxic for human beings, which is a major drawback of this process [8].
The gas-phase method of synthesis involves magnetron-sputtering using gas-phase condensation and inert-gas cooling which provides high cluster yield under the controlling parameters including temperature, pressure, and vapor concentration [9]. The technique offers an advantage in yielding NPs of desired size and is controlled by the flow rate of the inert gas, which could be argon or helium. Another approach called biological synthesis or green synthesis is subsequently gaining attention as it involves exploitation of microorganisms for the synthesis of NPs [10], which will be specifically discussed in detail in the following sections.
2. Green Synthesis of Nanoparticles
Formation of NPs using green synthesis is currently growing as an emerging approach combining both biotechnology and nanotechnology. This technique involves production of NPs using biological resources, which effectively overcomes the drawbacks imposed by physical and chemical methods [11]. A few examples include Cu NPs from seeds of Illicium vercium (star anise) and Mysristica fragrans (nutmeg) [12], Ag NPs from agricultural wastes [13] and extracts of red currant and bilberry wastes [14]. Being eco-friendly, this approach does not require the involvement of noxious chemicals and extreme conditions. It is cost effective and could be useful for large-scale production [11][15]. The green synthesis approach serves various advantages of being simple, reproducible, biocompatible, eco-friendly, and economic. Different biological agents have their own mechanisms to acknowledge metal ions for the synthesis of respective NPs. Major advantages of using biological resources over non-biological means is obtained via biomolecules present in the biological system which maintains the properties of NPs and therefore stabilizing or capping agents are not required [16].
Extraction from plants includes drying and downsizing of the plant component, leading to increased surface area [17]. Green synthesis involves heating and boiling for preparation of plant extracts. Powdered peels of Punica granatum (pomegranate) for copper oxide (CuO) NPs [18], Cucurbita pepo (pumpkin) seeds for TiO2 NPs [19], Chromolaena odorata roots for smart nanocomposites [20], and Basella alba leaves for Ag NPs [21] were boiled in distilled water for 30 min at 55 °C, 2 h at 90 °C, 2 h at 85 °C, 20 min at 60 °C, respectively. Nepeta leucophylla roots were boiled in methanol for 8 h to synthesize Ag NPs [22]. Alternatively, various other methods such as sonication, maceration, autoclaving and so on., have also been employed. Ground kernels of Caesalpina bonducella were sonicated for 30 min [23], fruits of Solanum mammosum [24] and Crateagus pentagyna [25] were macerated, roots of Scutellaria biacalensis [26] were autoclaved at 100 °C for 30 min for green synthesis of CuO, Ag, (Fe/Si/Cu-Ag), and ZnO NPs, respectively. Another technique called co-precipitation has been used to synthesize ferric sulfate (Fe2SO3) NPs from marine alga (Turbinaria ornate) [27]. Cube-shaped ferroso ferric oxide (Fe3SO4) NPs were synthesised using Fenton process from extracts of Rhamnidium elaeocarpum and therefore could be regarded as a green chemical approach [28]. In another study, the biogenic deposition precipitation approach has been used for biogenic preparation of Ag-ZnO nanocomposites via extracts of fennel seeds, which were found to degrade chlorpyrifos pesticide and rhodamine dye [29].
Cyanobacteria, regarded as ‘cell factories’, are the most appropriate biological resource for synthesis of metal-based NPs. In one study [30], they employed Haloleptolyngbya alcalis KR2005/106 cyanobacterial extract as a reducing agent acting upon silver ions to yield Ag NPs of size < 50 nm when exposed to photosynthetically active radiation (PAR). The synthesised Ag NPs were shown to possess ammonia-sensing properties which could be used to monitor water quality. In another study, Ag NPs were biologically synthesised using Bacillus brevis (NCIM 2533). The prepared NPs showed potential antibacterial activity against various pathogenic bacteria such as Staphylococcus aureus and Salmonella typhi which could further be used in disease management [31]. Another advantage of green synthesised NPs includes generation of no by-products in the process. Additionally, various phytochemicals and natural compounds present in biological extracts stabilize and enhance various physico-chemical properties of the NPs without needing any other external agent [32].
3. Mechanisms Involved in Green Synthesis
The mechanism in biosynthesis simply involves the reduction of metal ions into the respective NPs. Ref. [33] synthesised Ag/TiO2 nanocomposites (NCs) of sizes ranging from 25–50 nm using leaf extract of Origanum majorana, serving as reducing agent, under ultrasound irradiation. Ref. [34] employed Cleistocalyx operculatus extract as reductant to synthesize Ag/TiO2 NCs of size ranging from 20–40 nm, which was found to be 91.4% efficient in photocatalytic degradation of Rhodamine B dye. The basic procedure makes use of biological resources which might be carried out both intra- and extracellularly as depicted in Figure 1. This can be done using cell free extracts, supernatants of bacterial cultures, and bacterial biomass. The complex down-streaming process in intra-cellular synthesis is the reason that an extracellular process is preferred [16].
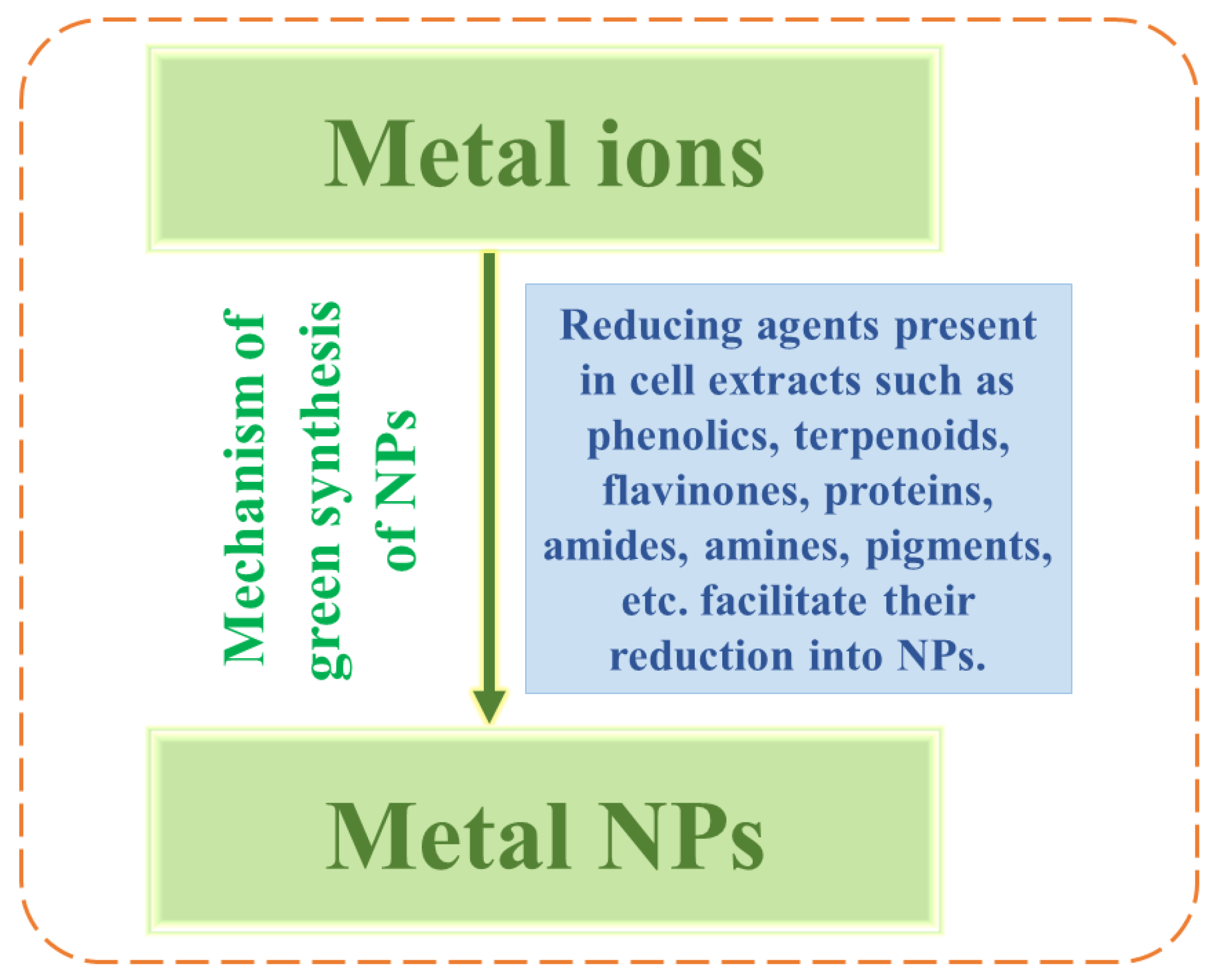
Figure 1. Mechanism of nanoparticle synthesis via reduction of metal ions.
Besides enzymes, various cofactors such as NADH, compounds such as quinones and glutathione [16] and biomolecules such as vitamins, tannins, steroids, flavonoids, amino acids and peptides, and carboxylic acids are also responsible for the reduction of the metal ions [35]. Other compounds such as phytochemicals and secondary metabolites found in medicinal plants such as saponins, alkaloids, terpenes, phenols, alcohols and extracellular enzymes and metabolites such as hemicellulose, acetyl xylem asterase, glucosidase, paracelsin, and cell wall lytic enzymes are excreted by several fungal species and aid in the reduction of metal ions [36]. Comprehensively, the overall mechanism and the biomolecules used in NP synthesis are summarized in Figure 2.
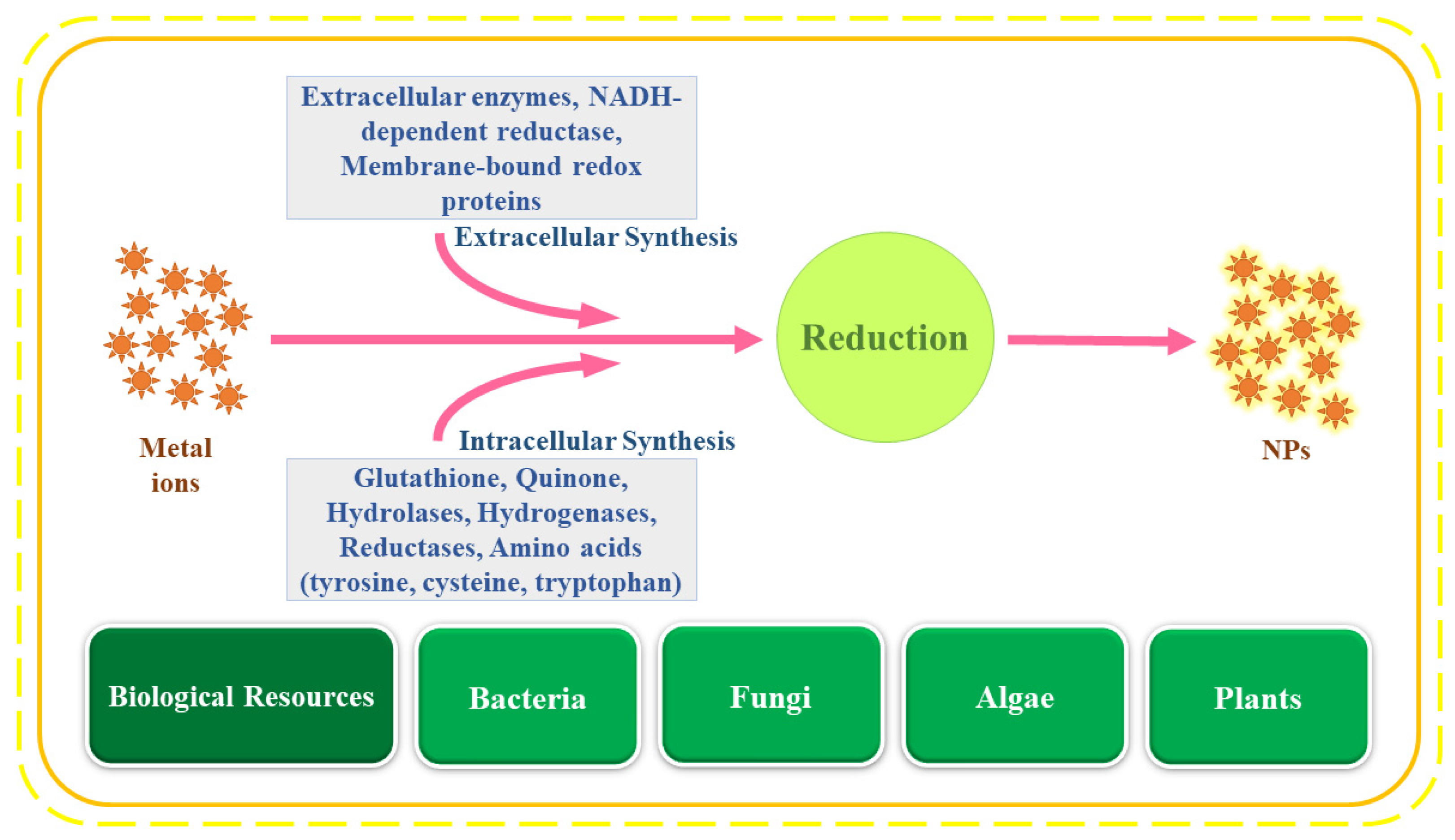
Figure 2. Brief mechanisms of nanoparticle synthesis.
4. Green Synthesised Nanoparticles in Remediation for Degrading Toxic Substances
Remediation processes involving the exploitation of biological resources, or their components and extracts, are referred to as ‘bioremediation’, which is achieved by the breakdown of toxic components into less toxic ones [37] as shown in Figure 3.
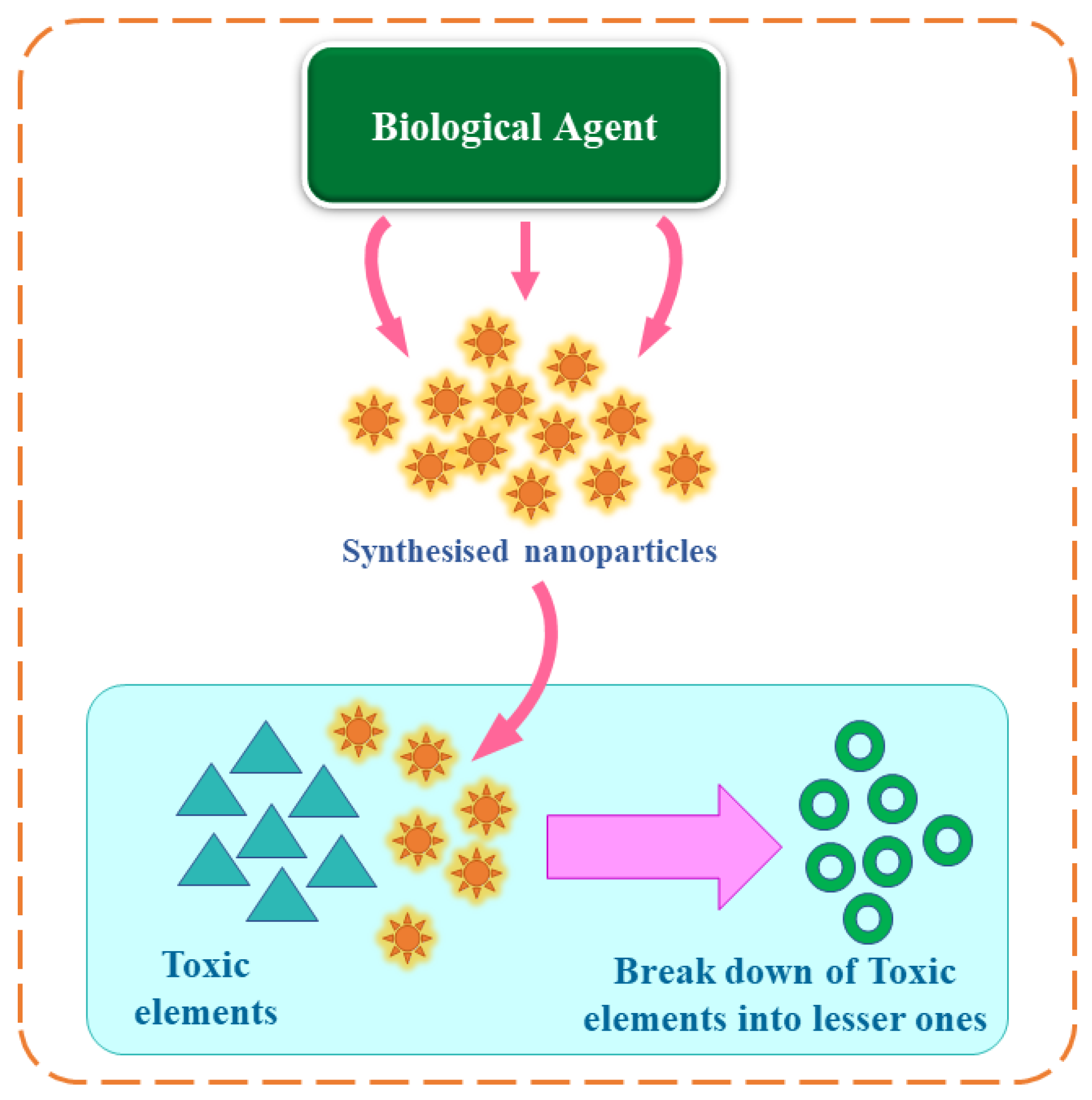
Figure 3. Concept of bioremediation using green nanoparticles.
Nanoformulations are great alternatives to get rid of contaminants present in the environment. They are actively used in remediation processes for treating and eliminating hazardous wastes. Heavy metals and dye contaminants cause serious issues for both land and aquatic biota by restraining uptake and consumption of dissolved oxygen and diminishing photosynthetic capability. Fe-based NPs possess properties required not only to disinfect water but also for removal of heavy metals from soil [38].
4.1. Bacterial Nanoparticles in Remediation
Bacterial cells are suitable options for the production of NPs as they contain biomolecules required for the reduction of metal ions, and NPs can be synthesised both intra- and extracellularly in the medium and display a large number of applications [39] such as green synthesised ZnO nanoflower from Bacillus licheniformis MTCC 9555 [40], which possesses the capability of photocatalytic degradation of pollutant dye methylene blue (C16H18N3SCl). Bacterial cells secrete stabilising enzymes that prevent the agglomeration of NPs. Photocatalysis is a helpful technology, regarded as an advanced oxidation process, which requires direct application of solar energy for elimination of numerous organic pollutants [41]. ZnO NPs are most extensively studied as photocatalysts in remediating aquatic wastewater [42]. V
4.2. Fungal Nanoparticles in Remediation
Mycosynthesised NPs have gained tremendous importance as they are cost effective and their yield is relatively good. Among other biological agents, fungi are considered to be the most suitable because they possess a large number of mycelia and fruiting bodies [43], and contain an ample amount of biomolecules required for NP synthesis [44][45]. Therefore, the amount of mycosynthesised NPs are sufficient and quick as compared with other biological agents [46]. Numerous fungal species have been exploited for biosynthesis of NPs employed for remediation. In one study, the waste substrate of Lentinula edodes was used to synthesise ferroferric oxide NPs which were found to be effective in the reduction of pollutants such as Cr, NH4-N, Pb, Ni, and Cu [47].
4.3. Algal Nanoparticles in Remediation
Algae have been considered as ‘bio-nano factories’ as they actively absorb metal ions from their surroundings, resulting in their reduction and the synthesis of respective NPs in both living and dry dead form [39]. The controlled growth rate and energy of forming NPs are conventionally achieved by using suitable capping agents or surfactants. Due to being non-biodegradable in nature, these chemicals are present as remnants which are difficult to remove completely. In order to circumvent this issue, naturally occurring biomolecules found in variety of algae are employed for the stabilisation and synthesis of NPs [48] as they have a huge capacity for metal-binding. Ag NPs are the most studied and prevalent among the others. In one study, AgNO3 was exposed to extracts of seaweed Enteromorpha flexusa [49] and Chaetomorpha linum. Reduction of metal ions was facilitated by the water-soluble components found in the extract, such as terpenoids, flavonoids, amines, and peptides, which resulted in the formation of Ag NPs [50]. Prasiola crispa, a freshwater alga, was employed for green synthesis of Au NPs carried out by reduction of aqueous solution of chloroauric acid [51]. Toxicity issues are important to consider as they must not harm living biota. Algal synthesis serves to provide no or negligible toxicity hence it is a safer and green approach. Additionally, they are considered as ‘nano-reserves’ and could be cultured conveniently with less effort [52].
Many algal species have been employed in remediation processes for the degradation of hazardous dyes and chemicals as earlier methods such as redox treatment, UV degradation, activated carbon sorption and so on, were inefficient [48]. Ag NPs were green synthesised using seaweed Ulva lactuca [53] and Hypnea musciformis [54], which have been very efficient in degrading methyl orange dye. Green synthesised iron oxide NPs via Spirulina were found to be very effective in adsorbing crystal violet. NPs when treated with water containing dyes results in decolorisation of the solution, which was reaffirmed via analytical techniques hence could be used in treating wastewater [55].
4.4. Plant Nanoparticles in Remediation
Synthesis of NPs using plant extracts have been used for a long time. Cinnamomum camphora sun-dried leaves were employed for the biosynthesis of Ag and Au NPs of sizes ranging from 55 nm to 80 nm [56]. Utilising plant extracts is beneficial compared to microbes because they are non-pathogenic and this is a one-step process [39]. Synthetic dyes are majorly found in wastewaters, mainly via industrial effluents, inflict a serious threat to the environment, causing severe health issues and imbalances in nature [57]. CoO NPs were biosynthesised via Vitis rotundifolia, commonly named Jumbo Mascadine, using co-precipitation, which was found effective in degrading Acid Blue-74 (AB-74) [58].
Water treatment tools and techniques are now more refined, eco-friendly, and inexpensive. Salvia rosmarinus extract-mediated TiO2 NPs were found effective in degrading Rhodamine B, Methyl orange, and Methylene blue [59]. FeO NPs were firstly biosynthesised using Ruellia tuberosa leaf extract and were shown to possess antimicrobial activity against various pathogenic bacteria such as Klebsiella pneumonia, Staphylococcus aureus and so on, and have the ability to degrade toxic dyes [60]. In one study, the synthesis of iron oxide (FeO, Fe3O4, and Fe2O3) particles was mediated by plant extracts of Petlophorum pterocarpum, which were found to be effective in elimination of rhodamine from wastewater [61].
References
- Pathak, J.; Ahmed, H.; Singh, D.K.; Pandey, A.; Singh, S.P.; Sinha, R.P. Recent developments in green synthesis of metal nanoparticles utilizing cyanobacterial cell factories. In Nanomaterials in Plants, Algae, and Microorganisms; Academic Press: Cambridge, MA, USA, 2019; pp. 237–265.
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174.
- Sadrolhosseini, A.R.; Mahdi, M.A.; Rashid, F.A. Laser Ablation Technique for Synthesis of Metal Nanoparticle in Liquid. In Laser Technology and its Applications; Ma, Y., Ed.; IntechOpen: London, UK, 2018.
- El-Eskandarany, M.S.; Al-Hazza, A.; Al-Hajji, L.A.; Ali, N.; Al-Duweesh, A.A.; Banyan, M.; Al-Ajmi, F. Mechanical Milling: A Superior Nanotechnological Tool for Fabrication of Nanocrystalline and Nanocomposite Materials. Nanomaterials 2021, 11, 2484.
- Jalalian-Khakshour, A.; Phillips, C.O.; Jackson, L. Solid-state synthesis of NASICON (Na3Zr2Si2PO12) using nanoparticle precursors for optimisation of ionic conductivity. J. Mater. Sci. 2020, 55, 2291–2302.
- Abdelgawad, A.M.; El-Naggar, M.E.; Eisa, W.H.; Rojas, O.J. Clean and high-throughput production of silver nanoparticles mediated by soy protein via solid state synthesis. J. Clean. Prod. 2017, 144, 501–510.
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749.
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662.
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity, and regulations. Beilstein J. Nanotechnol. 2018, 3, 1050–1074.
- Rane, A.V.; Kanny, K.; Thomas, A.S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials: Advances and Key Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139.
- Grammatikopoulos, P.; Stephan, S.; Jerome, V.; Singh, V.; Sowwan, M. Nanoparticle design by gas-phase synthesis. Adv. Phys. X 2016, 1, 81–100.
- Vijayakumar, G.; Kesavan, H.; Kannan, A.; Arulanandam, D.; Kim, J.H.; Kim, K.J.; Song, H.J.; Kim, H.J.; Rangarajulu, S.K. Phytosynthesis of Copper Nanoparticles Using Extracts of Spices and Their Antibacterial Properties. Processes 2021, 9, 1341.
- Wolny-Koładka, K.; Malina, D.; Suder, A.; Pluta, K.; Wzorek, Z. Bio-Based Synthesis of Silver Nanoparticles from Waste Agricultural Biomass and Its Antimicrobial Activity. Processes 2022, 10, 389.
- Zuorro, A.; Iannone, A.; Natali, S.; Lavecchia, R. Green Synthesis of Silver Nanoparticles Using Bilberry and Red Currant Waste Extracts. Processes 2019, 7, 193.
- Patel, M. Green Synthesis of Nanoparticles: A Solution to Environmental Pollution. In Handbook of Solid Waste Management; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1965–1993.
- Ovais, M.; Khalil, A.; Ayaz, M.; Ahmad, I.; Nethi, S.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100.
- Vincent, J.; Lau, K.S.; Evyan, Y.C.-Y.; Chin, S.X.; Sillanpää, M.; Chia, C.H. Biogenic Synthesis of Copper-Based Nanomaterials Using Plant Extracts and Their Applications: Current and Future Directions. Nanomaterials 2022, 12, 3312.
- Siddiqui, V.U.; Ansari, A.; Chauhan, R.; Siddiqi, W.A. Green Synthesis of Copper Oxide (CuO) Nanoparticles by Punica Granatum Peel Extract. Mater. Today Proc. 2019, 36, 751–755.
- Abisharani, J.M.; Devikala, S.; Dinesh Kumar, R.; Arthanareeswari, M.; Kamaraj, P. Green Synthesis of TiO2 Nanoparticles Using Cucurbita pepo Seeds Extract. Mater. Today Proc. 2019, 14, 302–307.
- Nnadozie, E.C.; Ajibade, P.A. Green Synthesis and Characterization of Magnetite (Fe3O4) Nanoparticles Using Chromolaena odorata Root Extract for Smart Nanocomposite. Mater. Lett. 2020, 263, 127145.
- Mani, M.; Pavithra, S.; Mohanraj, K.; Kumaresan, S.; Alotaibi, S.S.; Eraqi, M.M.; Gandhi, A.D.; Babujanarthanam, R.; Maaza, M.; Kaviyarasu, K. Studies on the Spectrometric Analysis of Metallic Silver Nanoparticles (Ag NPs) Using Basella alba Leaf for the Antibacterial Activities. Environ. Res. 2021, 199, 111274.
- Singh, J.; Dhaliwal, A.S. Novel Green Synthesis and Characterization of the Antioxidant Activity of Silver Nanoparticles Prepared from Nepeta leucophylla Root Extract. Anal. Lett. 2019, 52, 213–230.
- Sukumar, S.; Rudrasenan, A.; Padmanabhan Nambiar, D. Green-Synthesized Rice-Shaped Copper Oxide Nanoparticles Using Caesalpinia bonducella Seed Extract and Their Applications. ACS Omega 2020, 5, 1040–1051.
- Pilaquinga, F.; Morejón, B.; Ganchala, D.; Morey, J.; Piña, N.; Debut, A.; Neira, M. Green Synthesis of Silver Nanoparticles Using Solanum mammosum L. (Solanaceae) Fruit Extract and Their Larvicidal Activity against Aedes aegypti L. (Diptera: Culicidae). PLoS ONE 2019, 14, e0224109.
- Ebrahimzadeh, M.A.; Mortazavi-Derazkola, S.; Zazouli, M.A. Eco-Friendly Green Synthesis and Characterization of Novel Fe3O4/SiO2/Cu2O–Ag Nanocomposites Using Crataegus pentagyna Fruit Extract for Photocatalytic Degradation of Organic Contaminants. J. Mater. Sci. Mater. Electron. 2019, 30, 10994–11004.
- Chen, L.; Batjikh, I.; Hurh, J.; Han, Y.; Huo, Y.; Ali, H.; Li, J.F.; Rupa, E.J.; Ahn, J.C.; Mathiyalagan, R.; et al. Green Synthesis of Zinc Oxide Nanoparticles from Root Extract of Scutellaria baicalensis and Its Photocatalytic Degradation Activity Using Methylene Blue. Optik 2019, 184, 324–329.
- Prerna, D.I.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Vasantharaja, R.; Chaturvedi, S.; Shkolnik, D. Influence of nanoscale micro-nutrient α-Fe2O3 on seed germination, seedling growth, translocation, physiological effects and yield of rice (Oryza sativa) and maize (Zea mays). Plant Physiol. Biochem. 2021, 162, 564–580.
- Jacinto, M.J.; Souto, R.S.; Silva, V.C.P. Biosynthesis of Cube-Shaped Fe3O4 Nanoparticles for Removal of Dyes Using Fenton Process. Water Air Soil Pollut. 2021, 232, 270.
- Choudhary, M.K.; Kataria, J.; Bhardwaj, V.K.; Sharma, S. Green biomimetic preparation of efficient Ag–ZnO heterojunctions with excellent photocatalytic performance under solar light irradiation: A novel biogenic-deposition-precipitation approach. Nanoscale Adv. 2019, 1, 1035–1044.
- Tomer, A.K.; Rahi, T.; Neelam, D.K. Cyanobacterial extract-mediated synthesis of silver nanoparticles and their application in ammonia sensing. Int. Microbiol. 2019, 22, 49–58.
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226.
- Kurhade, P.; Kodape, S.; Choudhury, R. Overview on green synthesis of metallic nanoparticles. Chem. Pap. 2022, 75, 5187–5222.
- Bhardwaj, D.; Singh, R. Green biomimetic synthesis of Ag–TiO2 nanocomposite using Origanum majorana leaf extract under sonication and their biological activities. Bioresour. Bioprocess 2021, 8, 1.
- Nguyen, T.H.; Hoang, N.H.; Van Tran, C.; Nguyen, P.T.M.; Dang, T.D.; Chung, W.J.; La, D.D. Green synthesis of a photocatalyst Ag/TiO2 nanocomposite using Cleistocalyx operculatus leaf extract for degradation of organic dyes. Chemosphere 2022, 306, 135474.
- Elsakhawy, T.; Omara, A.D.; Abowaly, M.; El-Ramady, H.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Prokisch, J. Green Synthesis of Nanoparticles by Mushrooms: A Crucial Dimension for Sustainable Soil Management. Sustainability 2022, 14, 4328.
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814.
- Jayaprakash, K.; Govarthanan, M.; Mythili, R.; Selvankumar, T.; Chang, Y.C. Bioaugmentation and Biostimulation Remediation Technologies for Heavy Metal Lead Contaminant. In Microbial Biodegradation of Xenobiotic Compounds; CRC Press: Boca Raton, FL, USA, 2019; pp. 24–36.
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459.
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emra, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ.-Sci. 2022, 34, 101869.
- Tripathi, R.M.; Singh, A.B.; Gupta, R.K.; Singh, P.; Shrivastava, A.; Shrivastava, B.R. ZnO nanoflowers: Novel biogenic synthesis and enhanced photocatalytic activity. J. Photochem. Photobiol. B Biol. 2014, 141, 288–295.
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214.
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; Biao, Y. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640.
- Abdul-Hadi, S.Y.; Owaid, M.N.; Rabeea, M.A.; Aziz, A.A.; Jameel, M.S. Rapid mycosynthesis and characterization of phenols-capped crystal gold nanoparticles from Ganoderma applanatum, Ganoderma taceae. Biocatal. Agric. Biotechnol. 2020, 27, 101683.
- Owaid, M.N.; Al-Saeedi, S.S.; Abed, I.A. Biosynthesis of gold nanoparticles using yellow oyster mushroom Pleurotus cornucopiae var. citrinopileatus. Environ. Nanotechnol. Monit. Manag. 2017, 8, 157–162.
- Sudheer, S.; Bai, R.G.; Muthoosamy, K.; Tuvikene, R.; Gupta, V.K.; Manickam, S. Biosustainable production of nanoparticles via mycogenesis for biotechnological applications: A critical review. Environ. Res. 2022, 204, 111963.
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: Status and future challenges. J. Nanostruct. Chem. 2018, 8, 369–391.
- Wang, C.; Tan, H.; Liu, H.; Wu, B.; Xu, F.; Xu, H. A nanoscale ferroferric oxide coated biochar derived from mushroom waste to rapidly remove Cr (VI) and mechanism study. Bioresour. Technol. Rep. 2019, 7, 100253.
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600.
- Yousefzadi, M.; Rahimi, Z.; Ghafori, V. The green synthesis, characterization and antimicrobial activities of silver nanoparticles synthesized from green alga Enteromorpha flexuosa (wulfen). J. Agardh Mater. Lett. 2014, 137, 1–4.
- Kannan, R.R.; Arumugam, R.; Ramya, D.; Manivannan, K.; Anantharaman, P. Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Appl. Nanosci. 2013, 3, 229–233.
- Sharma, B.; Purkayastha, D.D.; Hazra, S.; Gogoi, L.; Bhattacharjee, C.R.; Ghosh, N.N.; Rout, J. Biosynthesis of gold nanoparticles using a freshwater green alga, Prasiola crispa. Mater. Lett. 2014, 116, 94–97.
- Baker, S.; Harini, B.P.; Rakshith, D.; Satish, S. Marine microbes: Invisible nanofactories. J. Pharm. Res. 2013, 6, 383–388.
- Kumar, P.; Govindaraju, M.; Senthamilselvi, S.; Premkumar, K. Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Colloids Surf. B Biointerfaces 2013, 103, 658–661.
- Selvam, G.G.; Sivakumar, K. Phycosynthesis of silver nanoparticles and photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Hypnea musciformis (Wulfen) J.V. Lamouroux. Appl. Nanosci. 2015, 5, 617–622.
- Bhukal, S.; Sharma, A.; Kumar, S.; Deepak, B.; Pal, K.; Mona, S. Spirulina Based Iron Oxide Nanoparticles for Adsorptive Removal of Crystal Violet Dye. Top. Catal. 2022, 65, 1675–1685.
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104.
- Wahab, A.; Imran, M.; Ikram, M. Dye degradation property of cobalt and manganese doped iron oxide nanoparticles. Appl Nanosci. 2019, 9, 1823–1832.
- Samuel, M.S.; Selvarajan, E.; Mathimani, T.; Santhanam, N.; Phuong, T.N.; Brindhadevi, K.; Pugazhendhi, A. Green synthesis of cobalt-oxide nanoparticle using jumbo Muscadine (Vitis rotundifolia): Characterization and photo-catalytic activity of acid Blue-74. J. Photochem. Photobiol. B Biol. 2020, 211, 112011.
- Silva-Osuna, E.R.; Vilchis-Nestor, A.R.; Villarreal-Sanchez, R.C.; Castro-Beltran, A.; Luque, P.A. Study of the optical properties of TiO2 semiconductor nanoparticles synthesized using Salvia rosmarinus and its effect on photocatalytic activity. Opt. Mater. 2022, 124, 112039.
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; LewisOscar, F.; Pugazhendhi, A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J. Photochem. Photobiol. B Biol. 2019, 192, 74–82.
- Shah, Y.; Maharana, M.; Sen, S. Peltophorum pterocarpum leaf extract mediated green synthesis of novel iron oxide particles for application in photocatalytic and catalytic removal of organic pollutants. Biomass Conv. Bioref 2022, 1–14.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
808
Revisions:
2 times
(View History)
Update Date:
19 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




