Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Junming Sun | -- | 2887 | 2023-01-18 09:15:26 | | | |
| 2 | Jessie Wu | Meta information modification | 2887 | 2023-01-19 02:24:10 | | | | |
| 3 | Jessie Wu | -4 word(s) | 2883 | 2023-01-19 02:30:24 | | | | |
| 4 | Jessie Wu | Meta information modification | 2883 | 2023-01-19 02:33:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Agyenim-Boateng, K.G.; Zhang, S.; Shohag, M.J.I.; Shaibu, A.S.; Li, J.; Li, B.; Sun, J. Strategies for Folate Biofortification in Soybean. Encyclopedia. Available online: https://encyclopedia.pub/entry/40328 (accessed on 07 February 2026).
Agyenim-Boateng KG, Zhang S, Shohag MJI, Shaibu AS, Li J, Li B, et al. Strategies for Folate Biofortification in Soybean. Encyclopedia. Available at: https://encyclopedia.pub/entry/40328. Accessed February 07, 2026.
Agyenim-Boateng, Kwadwo Gyapong, Shengrui Zhang, Md. Jahidul Islam Shohag, Abdulwahab S. Shaibu, Jing Li, Bin Li, Junming Sun. "Strategies for Folate Biofortification in Soybean" Encyclopedia, https://encyclopedia.pub/entry/40328 (accessed February 07, 2026).
Agyenim-Boateng, K.G., Zhang, S., Shohag, M.J.I., Shaibu, A.S., Li, J., Li, B., & Sun, J. (2023, January 18). Strategies for Folate Biofortification in Soybean. In Encyclopedia. https://encyclopedia.pub/entry/40328
Agyenim-Boateng, Kwadwo Gyapong, et al. "Strategies for Folate Biofortification in Soybean." Encyclopedia. Web. 18 January, 2023.
Copy Citation
Folate (vitamin B9) is an essential water-soluble vitamin in plants and microorganisms that plays a role in one-carbon metabolism. It functions as a cofactor in the synthesis of nucleic acids, metabolism of amino acids, and methylation of hormones, lipids, proteins, and chlorophyll. Folate is particularly important for cell division in pregnant and lactating women. However, humans cannot synthesise folate de novo and must obtain it from dietary sources, such as crops, animal-based foods, or nutritional supplements.
soybean
breeding
folate
strategy
biofortification
1. Structure, Distribution, Content and Composition of Folate in Soybean
1.1. Structure and Distribution of Folate Vitamers in Soybean
Folate comprises a pterin moiety attached by a methylene bridge to para-aminobenzoic acid coupled to one or more glutamyl residues. In vivo, folates exist as tetrahydrofolate (H4folate) and its derivatives, which vary in the state of oxidation of the pteridine ring, single carbon substituents linked at the 5 and 10 positions, and with a variable number of glutamyl residues [1]. In theory, over 150 folate derivatives exist, but a few exist in plants and humans.
Recent chromatographic methods have indicated the folate vitamers present in soybean seeds include H4folate (THF), 5-CH3-H4folate (5MTHF), 5-CHO-H4folate (5FTHF), H2folate (DHF), 10-CHO-H4folate, PteGlu (FA), 5,10-CH=H4folate (5,10-MTHF), 10-CHO-PteGlu (10FFA), and MeFox (Figure 1). However, the distribution of these vitamers in soybean seeds has not been frequently reported. Soybean vitamer distribution can be influenced by factors such as storage, plant developmental stage, cultivar, accession type, environment and analytical method. Some studies have reported THF as the most abundant folate vitamer in soybean [2][3], while other studies have identified 5FTHF as the most abundant [4][5]. 5FTHF accounted for more than 60% of the total folate content of soybean accessions grown in southern China [6]. Climate conditions, particularly temperature and humidity, may influence folate synthesis and accumulation. For example, lower temperatures have been shown to increase 5MTHF accumulation in sweet corn seedlings [7], while higher temperatures can lead to a significant decrease in the folate content of lettuce [8].
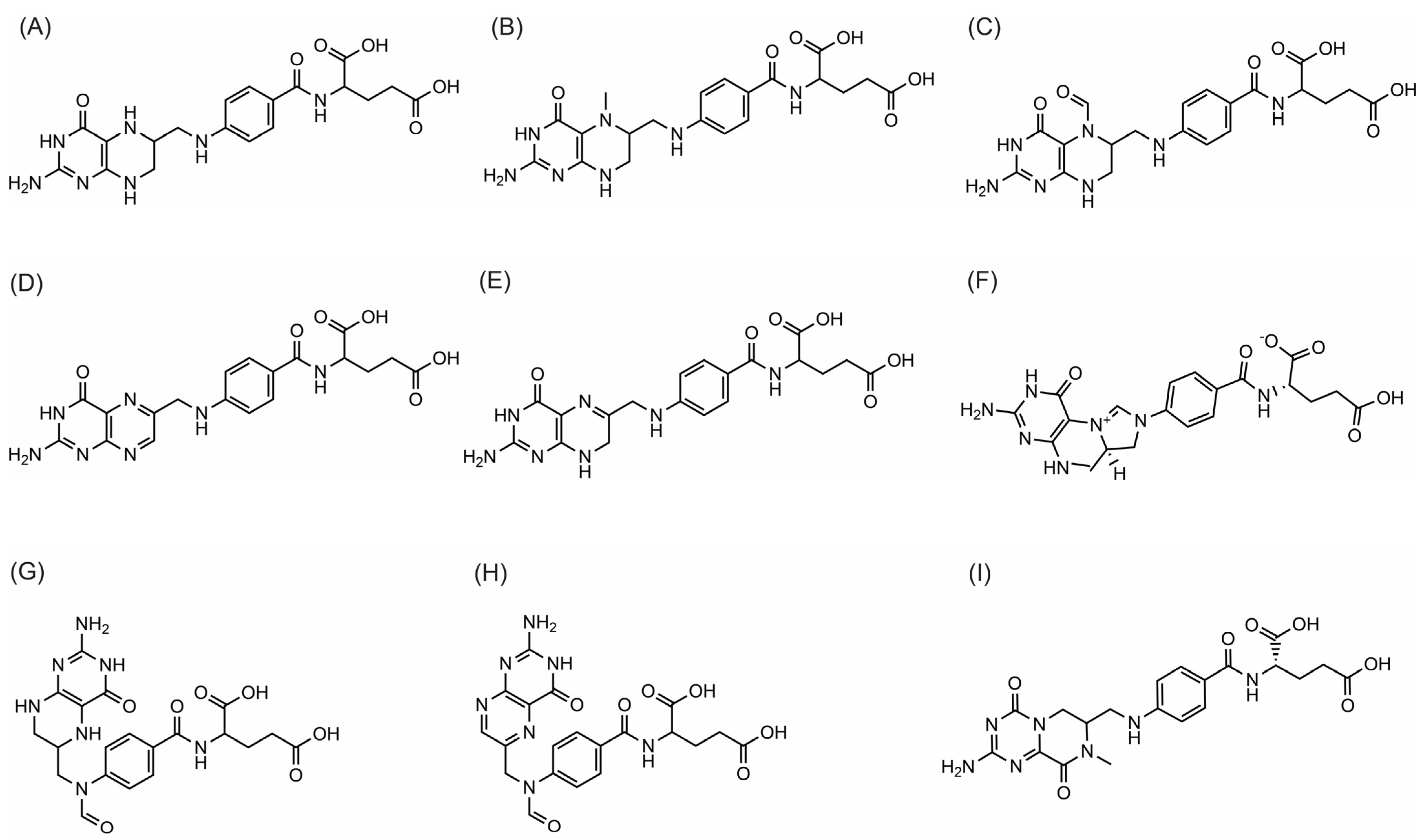
Figure 1. Chemical structures of folate vitamers in soybean: (A) H4folate (THF); (B) 5-CH3-H4folate (5MTHF); (C) 5-CHO-H4folate (5FTHF); (D) PteGlu (FA); (E) H2folate (DHF); (F) 5,10-CH=H4folate (5,10-MTHF); (G) 10-CHO-H4folate; (H) 10-CHO-PteGlu (10FFA); (I) MeFox.
Whereas a few studies have reported THF as the most dominant vitamer in soybean [2][3], that was not observed in researcher's recent studies [6]. THF is one of the most labile vitamers that can easily degrade or convert right from harvest. Researchers assume that THF might have been converted into FA or degraded right after harvest and during post-harvest storage. Studies on the homeostasis and dynamics of plant folates during storage have been limited. Biosynthesis may occur during post-harvest storage in some organisms, while variations in the folate pool in other organisms may be due to spontaneous or enzymatic reactions [9]. Further studies will be necessary to confirm vitamer distribution during post-harvest storage in soybean seeds.
1.2. The Folate Content and Composition of Soybean and Soy-Based Products
Soybean can be processed into various forms through germination, soaking, boiling, and fermentation [10][11]. However, folates are sensitive to light, air, heat and pH; therefore, their content and composition may be indirectly affected during processing. Mature unprocessed soybean seeds contain total folate levels ranging from 64.51–691.24 µg/100 g FW and 199 to 464 µg/100 g DW (Table 1). The differences in folate contents can be attributed to cultivar type, environment, and analytical methods used. In researcher's recent study, researchers identified a 10-fold variation in the total folate content of over 1000 germplasm consisting of landraces and cultivars [6]. However, most studies have been limited to a few cultivars. A larger sample size is needed to evaluate the variation among soybean folates and, most importantly, different accession types (wild-type, landraces and cultivars).
Cooked soybean seeds contained lower levels of folate (44.70–77.90 µg/100 g) at a retention rate of 24–45%, depending on the cooking treatment [12]. Similarly, significant losses were observed during the preparation of soymilk and tofu [11]. The significant loss of folates during tofu and soymilk processing was mainly caused by soaking and boiling, as most folates were recovered in the cooking or soaking media. Thus, the major cause of folate loss is leaching and, to a certain extent, oxidation. To avoid such losses, shorter boiling times and possible consumption of the cooking media are recommended.
Folate in tempeh ranged from 149.30 to 416.40 µg/100 g. Fermentation contributes to the increase in folate during tempeh preparation. During fermentation, folate compounds may be liberated by the actions of enzymes produced by the microorganism, leading to increased folate concentrations. The de novo formation of folate compounds during fermentation may also increase folate content [5]. However, the extent of the increase of folates will depend on the microorganism involved [10].
Seed germination is an age-old practice used to improve the nutritional value of crops, especially legumes [13]. Germination affects folate profiles and alters the distribution of individual vitamers [14][15]. In soybean, folate content increased 3.5 to 3.7-fold from 230.50 to 815.20 μg/100 g in Bangladesh soybean-4 and from 202.90–759.50 μg/100 g in Heinong 48 [2]. In germinated soybean, approximately 80% of the total folate content is 5MTHF, the most active folate vitamer.
In researcher's recent study, the total folate content of immature soybean seeds at the R6–R7 stage, a typical stage for edamame or maodou, ranged between 344.06–685.81 μg/100 g FW among 12 soybean cultivars, with 5MTHF contributing approximately 70% of the total folate content and 5FTHF contributing approximately 15% [16].
Table 1. The folate content of soybean seeds and soy-based products.
| Sample | THF | 5MTHF | 5,10-MTHF | 10FFA | 5FTHF | DHF | FA | Total Folate | References |
|---|---|---|---|---|---|---|---|---|---|
| Soybean seed | 16.90 | 53.80 | 121.00 | 199.00–464.00 | [4][5][11][17][18] | ||||
| Soybean seed | 20.00–75.00 * | 28.00–205.74 * | 5.00–28.60 | 11.00–71.06 | 160.00–590.56 | 2.90–29.44 | 28.50–34.40 * | 64.51–691.24 * | [2][3][6][12] |
| Soybean seed (cooked) | 44.70–77.90 * | [12] | |||||||
| Vegetable soybean | 12.55 * | 356.18 * | 10.17 * | 4.33 * | 75.07 * | 2.98 * | 1.00 * | 344.06–685.81 | [16] |
| Soymilk | 34.00–276.00 | [17][19] | |||||||
| Tofu | 15.00–127.30 | [10][17][19] | |||||||
| Tempeh | 231.80 | 149.30–416.40 | [10][17] | ||||||
| Soybean sprouts | 759.50–815.20 * | [2] |
The folate content of soybean seeds and soy-based products μg/100 g on dry weight basis. Values with * indicate folate content on fresh weight basis.
2. Prospects for Biofortification of Folates in Legumes
Biofortification can be classified based on a variety of approaches, including agronomic and genetic methods and the consumption of functional foods, such as fermented foods and sprouts [20][21]. Agronomic biofortification involves using agricultural techniques to increase the nutrient content in crops. This can include applying fertilizers, such as zinc or iron, or soil microbes to the soil in which the crops are grown [22][23][24][25]. The soil is the primary source of nutrients, and the abundance of nutrients in the soil and their availability to plants determine the synthesis of plant metabolites [26]. Agronomic biofortification is relatively simple and straightforward but can be expensive and time-consuming [27]. This is because this approach can be limited by the inherent variability in the soil nutrient availability, which is affected by factors such as soil type, climate and crop requirements. As a result, the effectiveness of agronomic biofortification may vary and may require extensive maintenance and input. To date, agronomic fortification has not been studied for folates in soybean. However, research has shown that the folate content of plants can increase when they have access to nutrients, such as phosphorus, nitrogen, and boron, suggesting that agronomic fortification of folates in soybean may be worth investigating in the future [28].
Genetic biofortification is the most well-known form of biofortification and has been widely studied and researched by scientists and plant breeders. It involves using genetic techniques to increase the nutrients in crops and can be achieved through several approaches, including conventional breeding, genomics-assisted breeding, and metabolic engineering.
Conventional breeding involves using traditional breeding techniques, such as crossbreeding and selection, to develop new varieties of crops that are rich in nutrients such as folate. Conventional breeding typically involves several steps, including identifying genetic variation in a germplasm pool, selecting elite-folate accessions and crossing these accessions with local varieties to create new varieties with improved nutrient content. One of the main advantages of conventional breeding is that it is a relatively low-cost and low-risk approach to biofortification. However, it can be a slow process, as it can take several years to develop and test new varieties of crops. Additionally, crops with low genetic variation for folate [9] may not be suitable for conventional breeding as there may not be sufficient genetic diversity to create new varieties with improved folate content.
Overall, conventional breeding can be an effective approach to folate biofortification, particularly in crops that have a high level of genetic variation, such as rice, potato and soybean [6][29][30]. Additionally, wild and landrace accessions of crops are often adapted to local growing conditions and may contain important genes that can be used to improve the nutrient content of cultivated species [31][32]. For example, wild lentils and soybean landraces have been found to contain higher folate content than cultivated accessions and introgressing these traits into cultivated varieties could help to increase the overall folate content of these crops [6][33].
Genomics-assisted breeding involves the use of genomic tools and technologies such as marker-assisted breeding (MAB), marker-assisted selection (MAS), marker-assisted backcrossing (MABC) and marker-assisted recurrent selection (MARC) to improve crop breeding programs [34] (Figure 2). These techniques can be used in conjunction with multi-omics data, databases, and genes generated by genomics to better understand the genetic basis of traits such as folate and to develop new varieties with improved folate profiles. High-throughput genotyping methods, such as genotyping by sequencing (GBS), can be used to genotype a large number of accessions [35], while phenotyping methods can be used to measure the folate content of these accessions. Linkage mapping, genome-wide association studies (GWAS) [36], and bulked segregant analysis-sequencing (BSA-Seq) [37] can then be used to identify quantitative trait loci (QTL) [38] or quantitative trait nucleotides (QTN) [39] that are associated with folate content. RNA-Seq can be combined with identified QTL or QTN and analysed using weighted gene co-expression network analysis (WGCNA) to group genes with similar expression patterns and identify candidate genes [40]. After the identification of candidate genes, functional analysis and metabolic pathway analysis can be conducted to further understand their roles in folate biosynthesis. MAB can then be utilised to develop new hybrids with improved folate content.
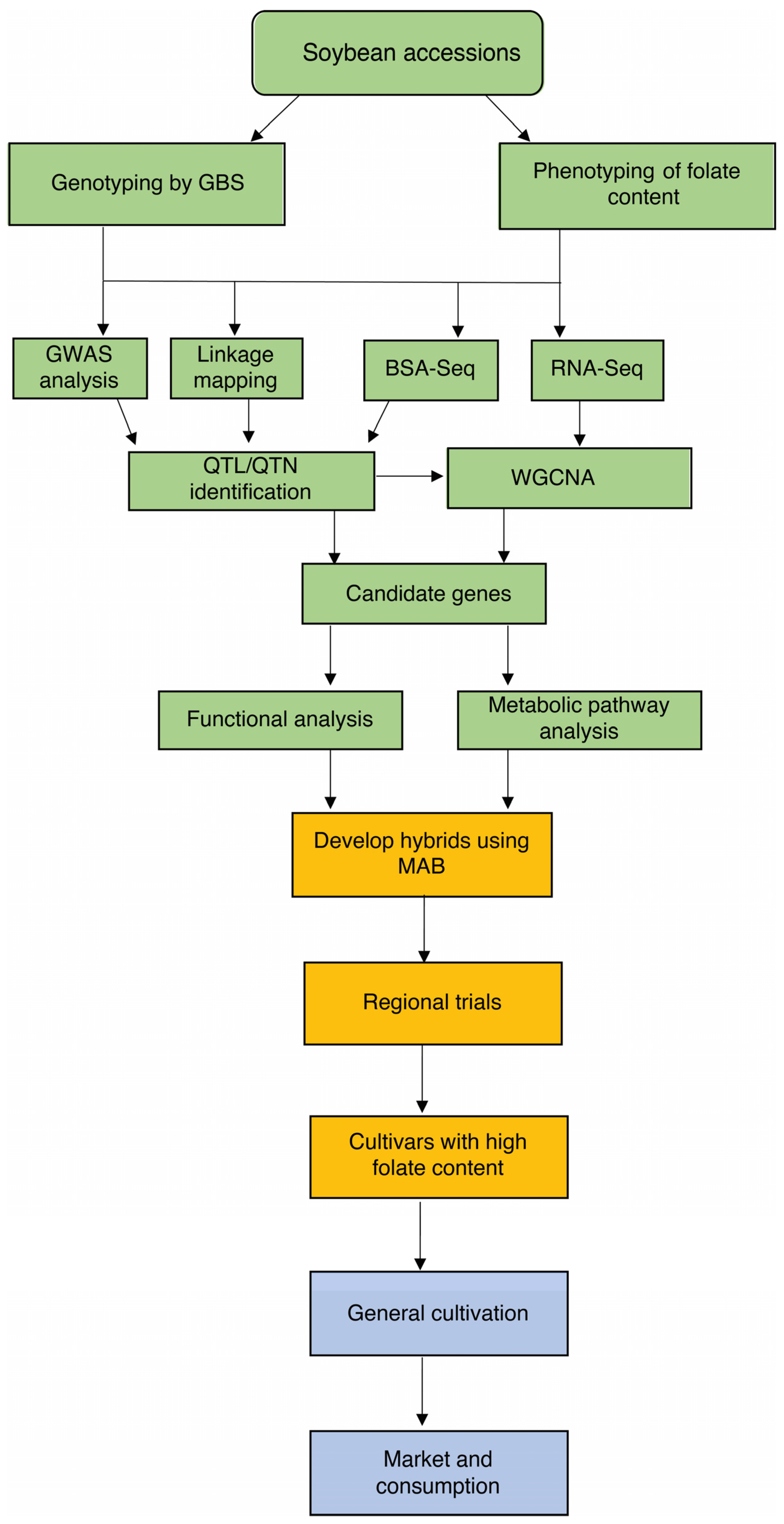
Figure 2. Framework for the genetic biofortification of folates in soybean. To improve the folate content of soybean, accessions can be genotyped using sequencing techniques, such as genotyping by sequencing (GBS) and phenotyped for their folate content. Linkage mapping, genome-wide association study (GWAS), and bulked-segregant analysis-sequencing (BSA-Seq) can be used to identify quantitative trait loci or quantitative trait nucleotides (QTL/QTN). The results from RNA-Seq can then be combined with QTL/QTN using weighted gene co-expression analysis (WGCNA) to identify candidate genes. These candidate genes can be further analysed for their functions and roles in the metabolic pathway. Finally, marker-assisted breeding (MAB) can be used to develop hybrids with improved folate content.
Many QTL and genes associated with folate content have been identified in various crops using these techniques, including pea [41], common bean [42][43], rice [29], maize [44], potato [45] and sweet corn [46]. For example, a genome-wide association study in 85 pea accessions revealed 9 SNPs significantly associated with 5MTHF, 5FTHF, THF and total folate, with two SNPs linked to higher levels of 5MTHF and total folate content. Linkage mapping has also been used to identify 4 QTL for 5MTHF in common bean, while a GWAS in 96 common bean genotypes identified 6 QTL for folate accumulation, including QTL on chromosome 11 that occurred in genomic regions syntenic to already reported QTL in maize, rice and common bean [42] (Table 2). However, to date, there have been no studies on QTL/QTN related to folate components in soybean.
Table 2. QTL identified for folate content in legumes.
| Crop | Population Type | Population Size | Total Number of QTL Identified | PVE (%) | Model of Analysis | Reference |
|---|---|---|---|---|---|---|
| Pea | Natural population | 85 | 9 | - | MLM | [41] |
| Common bean | Bi-parental | 6 | 4 | 8–19 | SMA | [43] |
| Common bean | Natural population | 96 | 6 | - | Fast-LMM/EMMA | [42] |
PVE—phenotypic variation explained; MLM—mixed linear model; SMA—single marker analysis; Fast-LMM—factored spectrally transformed linear mixed model; EMMA—efficient mixed model analysis.
One way to improve the folate content of crops is through metabolic engineering. This involves modifying the genetic makeup of crops to increase the production of specific nutrients or other bioactive compounds. Conventional breeding techniques may not always be sufficient to improve the folate content of crops in which case metabolic engineering can be used [21]. The metabolic engineering of folates has focused on enhancing folate synthesis [20]. Most of the enzymes that partake in the biosynthesis of folates have been characterised and cloned. However, transcriptomic and gene expression studies have revealed that the key enzymes in folate synthesis include GTPCHI, ADCS, HPPK/DHPS, FPGS and GGS. GTPCHI, a homolog of the folE gene in E. coli, is the first enzyme of the de novo biosynthesis pathway of folates in bacteria, fungi, and plants. Hence, it has been mostly targeted for metabolic engineering because it is thought to be the first rate-determining step controlling flux into the folate pathway [47]. Similarly, ADCS catalyses the first step of pABA synthesis in plants.
Regarding the metabolic engineering of folates in legumes, a single study has been conducted in the common bean with no reported investigation in soybean. The Mexican common bean (Phaseolus vulgaris L.) was metabolically engineered by overexpressing GTPCHI, which enhanced folate levels in the seeds by 3-fold and pteridine levels by 150-fold [48]. In other studies, two key soybean folate biosynthesis genes, GmGCHI (GTPCHI) and GmADCS (ADCS) were cloned and co-overexpressed in maize and wheat [49]. Transgenic maize and wheat grains had folate content increased by 4.2-fold and 2.3-fold, respectively. A subsequent co-expression of GCHI from soybean and ADCS from tomato significantly increased folate levels in wheat (Gm8gGCHI+/LeADCS+).
Studies in other species and crops have shown that the metabolic engineering of folate biosynthesis genes enhances folate levels. The overexpression of the folE gene in microorganisms resulted in increased folate levels [50]. The ectopic expression of GTPCHI increased folate levels in rice by 3.3 to 6.1-fold [51], increased the folate content of tomato by an average of 2-fold and pteridine levels by 140-fold and increased lettuce folates from 2.1 to 8.5-fold [52]. A 1250-fold increase in pterins and a 2- to 4-fold enhancement of folates in E. coli folE overexpressed Arabidopsis plants have been reported [47]. Folate levels were also doubled by the overexpression of E. coli folE in a simultaneous biofortification study with vitamins β-carotenoid and vitamin C [53]. A 1.5–1.8-fold increase was reported in transgenic AtADCS lines [51].
Increasing the pteridine levels of tomato by the overexpression of GTPCHI resulted in the depletion of pABA, increased pteridine levels, and a 2-fold increase in folates [54]. The depleted pABA levels indicated that the pABA supply limits further accumulation. Further exogenous application of pABA in pABA-depleted crops increased the folate levels [54][55]. Thus, a combined engineering of p-ABA and pteridine production in tomatoes achieved 25-fold higher folates than controls and increased pABA and pteridine levels [56]. Similarly, the two-gene strategy for metabolic engineering of pterin and pABA in rice resulted in a 100-fold increase in folates [57]. However, co-overexpressing GTPCHI with ADCS could not increase folate content in potato and Arabidopsis [58], suggesting the need to engineer other pathways.
HPPK/DHPS is bifunctional and combines activities catalysing two consecutive steps to form dihydropteroate from HMDHP. HPPK/DHPS performs the condensation reaction of pterin and pABA in the mitochondria, while in E. coli, HPPK and DHPS are monofunctional enzymes, with their encoding genes being folk and folP, respectively. However, these enzymes are coupled as one protein in plants, protozoa, and fungi. Wheat HPPK/DHPS has been singly overexpressed in rice, which increased folate content 2-fold [59]. However, overexpressing AtHPPK/DHPS in rice resulted in no difference in folate levels [51].
FGPS is the enzyme that catalyses the attachment of the glutamate tail to the THF molecule. Polyglutamylation by FPGS is regarded as an essential regulatory point in folate metabolism [60][61]. This is because the overexpression of GGH in animal systems reduces polyglutamate abundance and intracellular folate levels, whereas increased FPGS enhances intracellular folate levels. For instance, over-expression of GGH in Arabidopsis and tomato resulted in reduced folate levels [61]. Thus, FGPS plays an important role in folate homeostasis. Overexpression of AtFPGS in rice increased seed folate content by 7.50 to 19.90% and 4.30–45.50% [51]. In a recent study, the overexpression of foxtail millet FPGS gene SiFPGS2 in Arabidopsis increased folate content [62]. Owing to the previous unsuccessful attempt to enhance folates in potatoes by the two-gene approach (GTPCHI and ADCS), the four-gene approach overexpressing GTPCH1, ADCS, HPPK/DHPS and FPGS was studied, resulting in a 12-fold increase in folate content [63].
Soybean is a promising candidate for folate biofortification due to the variation and diversity of its folate content, as revealed in recent studies. Additionally, the availability of the soybean reference genome, along with advanced next-generation sequencing technologies and developed omic databases, has facilitated a deeper understanding of the genetics underlying various agronomic traits in soybean [64][65]. This understanding can be leveraged to develop new varieties with enhanced folate content through approaches such as conventional breeding, genomics-assisted breeding and metabolic engineering.
Some functional foods can be used for biofortification, such as fermented foods and sprouts. For example, traditional fermented soy-based foods, such as tempeh, natto, miso, soy sauce, douchi, and fermented soymilk, can help to increase the bioavailability of nutrients, making them more easily absorbed and used by the body [66][67][68]. Fermentation can also help to increase the overall nutrient content of these foods, as many microorganisms used in fermentation can synthesise essential vitamins and minerals. Fermentation has been shown to increase the folate content of some fermented soy-based foods such as tempeh and soymilk [10][11][69]. Similarly, soybean sprouts, which are made by germinating soybean seeds, are rich source of folates and contain higher amounts of the active component, 5MTHF [2]. By including these functional foods in the diet, it is possible to increase the intake of folate and other essential nutrients, helping to improve overall nutrition and address nutrient deficiencies.
References
- Rébeillé, F.; Ravanel, S.; Jabrin, S.; Douce, R.; Storozhenko, S.; Van Der Straeten, D. Folates in plants: Biosynthesis, distribution, and enhancement. Physiol. Plant. 2006, 126, 330–342.
- Shohag, M.; Wei, Y.; Yang, X. Changes of folate and other potential health-promoting phytochemicals in legume seeds as affected by germination. J. Agric. Food Chem. 2012, 60, 9137–9143.
- Rychlik, M.; Englert, K.; Kapfer, S.; Kirchhoff, E. Folate contents of legumes determined by optimized enzyme treatment and stable isotope dilution assays. J. Food Compos. Anal. 2007, 20, 411–419.
- Shin, Y.; Kim, E.; Watson, J.; Stokstad, E. Studies of folic acid compounds in nature. IV. Folic acid compounds in soybeans and cow milk. Can. J. Biochem. 1975, 53, 338–343.
- Ginting, E.; Arcot, J. High-performance liquid chromatographic determination of naturally occurring folates during tempe preparation. J. Agric. Food Chem. 2004, 52, 7752–7758.
- Agyenim-Boateng, K.G.; Zhang, S.; Islam, M.S.; Gu, Y.; Li, B.; Azam, M.; Abdelghany, A.M.; Qi, J.; Ghosh, S.; Shaibu, A.S.; et al. Profiling of naturally occurring folates in a diverse soybean germplasm by HPLC-MS/MS. Food Chem. 2022, 132520.
- Xiang, N.; Hu, J.; Wen, T.; Brennan, M.A.; Brennan, C.S.; Guo, X. Effects of temperature stress on the accumulation of ascorbic acid and folates in sweet corn (Zea mays L.) seedlings. J. Sci. Food Agric. 2020, 100, 1694–1701.
- Okazaki, S.; Yamashita, T. A manipulation of air temperature and light quality and intensity can maximize growth and folate biosynthesis in leaf lettuce. Environ. Control. Biol. 2019, 57, 39–44.
- Diaz de la Garza, R.; Ramos-Parra, P.A.; Vidal-Limon, H.R. Biofortification of crops with folates: From plant metabolism to able. In Nutritional Quality Improvement in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 137–175.
- Mo, H.; Kariluoto, S.; Piironen, V.; Zhu, Y.; Sanders, M.G.; Vincken, J.; Wolkers-Rooijackers, J.; Nout, M.R. Effect of soybean processing on content and bioaccessibility of folate, vitamin B12 and isoflavones in tofu and tempe. Food Chem. 2013, 141, 2418–2425.
- Arcot, J.; Wong, S.; Shrestha, A.K. Comparison of folate losses in soybean during the preparation of tempeh and soymilk. J. Sci. Food Agric. 2002, 82, 1365–1368.
- Hoppner, K.; Lampi, B. Folate retention in dried legumes after different methods of meal preparation. Food Res. Int. 1993, 26, 45–48.
- Guo, S.; Ge, Y.; Jom, K.N. A review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts (Helianthus annuus L.). Chem. Cent. J. 2017, 11, 95.
- Hefni, M.; Witthöft, C.M. Folate content in processed legume foods commonly consumed in Egypt. LWT-Food Sci. Technol. 2014, 57, 337–343.
- Sallam, S.M.; Shawky, E.; Sohafy, S. Determination of the effect of germination on the folate content of the seeds of some legumes using HPTLC-mass spectrometry-multivariate image analysis. Food Chem. 2021, 362, 130206.
- Agyenim-Boateng, K.G.; Zhang, S.; Zhang, S.; Aimal, N.; Shaibu, A.S.; Abdelghany, A.M.; Jie, Q.; Azam, M.; Ma, C.; Feng, Y.; et al. The nutritional composition of the vegetable soybean (Maodou) and its potential in combatting malnutrition. Front. Nutr. 2023, 9, 1034115.
- Ginting, E.; Arcot, J.; Chox, J.M. Determination of folate retention during tofu preparation using trienzyme treatment and microbiological assay. Indones. J. Agric. Sci. 2013, 4, 12–17.
- Puwastien, P.; Pinprapai, N.; Judprasong, K.; Tamura, T. International inter-laboratory analyses of food folate. J. Food Compos. Anal. 2005, 18, 387–397.
- Yon, M.; Hyun, T.H. Folate content of foods commonly consumed in Korea measured after trienzyme extraction. Nutr. Res. 2003, 23, 735–746.
- Blancquaert, D.; De Steur, H.; Gellynck, X.; Van Der Straeten, D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 2014, 65, 895–906.
- Van Der Straeten, D.; Bhullar, N.K.; De Steur, H.; Gruissem, W.; MacKenzie, D.; Pfeiffer, W.; Qaim, M.; Slamet-Loedin, I.; Strobbe, S.; Tohme, J. Multiplying the efficiency and impact of biofortification through metabolic engineering. Nat. Commun. 2020, 11, 5203.
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic biofortification with Se, Zn, and Fe: An effective strategy to enhance crop nutritional quality and stress defense—A Review. J. Soil Sci. Plant Nutr. 2022, 22, 1129–1159.
- Adu, M.O.; Asare, P.A.; Yawson, D.O.; Nyarko, M.A.; Osei-Agyeman, K. Agronomic biofortification of selected underutilised solanaceae vegetables for improved dietary intake of potassium (K) in Ghana. Heliyon 2018, 4, e00750.
- Bhardwaj, A.K.; Chejara, S.; Malik, K.; Kumar, R.; Kumar, A.; Yadav, R.K. Agronomic biofortification of food crops: An emerging opportunity for global food and nutritional security. Front. Plant Sci. 2022, 13, 1055278.
- Marra, R.; Lombardi, N.; Piccolo, A.; Bazghaleh, N.; Prashar, P.; Vandenberg, A.; Woo, S. Mineral biofortification and growth stimulation of lentil plants inoculated with Trichoderma strains and metabolites. Microorganisms 2022, 10, 87.
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis regulation of folates and phenols in plants. Sci. Hortic. 2022, 291, 110561.
- Lal, M.K.; Kumar, A.; Kardile, H.B.; Raigond, P.; Singh, B. Biofortification of Vegetables. In Advances in Agri-Food Biotechnology; Springer: Singapore, 2020.
- Mozafar, A. Plant Vitamins: Agronomic, Physiological, and Nutritional Aspects; CRC press: Boca Raton, FL, USA, 1994.
- Dong, W.; Cheng, Z.; Xu, J.; Zheng, T.; Wang, X.; Zhang, H.; Jie, W.; Wan, J. Identification of QTLs underlying folate content in milled rice. J. Integr. Agric. 2014, 13, 1827–1834.
- Robinson, B.R.; Sathuvalli, V.; Bamberg, J.; Goyer, A. Exploring folate diversity in wild and primitive potatoes for modern crop improvement. Genes 2015, 6, 1300–1314.
- Shahzad, R.; Jamil, S.; Ahmad, S.; Nisar, A.; Khan, S.; Amina, Z.; Kanwal, S.; Aslam, H.M.U.; Gill, R.A.; Zhou, W. Biofortification of cereals and pulses using new breeding techniques: Current and future perspectives. Front. Nutr. 2021, 8, 721728.
- Huang, Y.; Wang, H.; Zhu, Y.; Huang, X.; Li, S.; Wu, X.; Zhao, Y.; Bao, Z.; Qin, L.; Jin, Y.; et al. THP9 enhances seed protein content and nitrogen-use efficiency in maize. Nature 2022, 612, 292–300.
- Zhang, H.; Jha, A.B.; De Silva, D.; Purves, R.W.; Warkentin, T.D.; Vandenberg, A. Improved folate monoglutamate extraction and application to folate quantification from wild lentil seeds by ultra-performance liquid chromatography-selective reaction monitoring mass spectrometry. J. Chromatogr. B 2019, 1121, 39–47.
- Ashokkumar, K.; Govindaraj, M.; Karthikeyan, A.; Shobhana, V.G.; Warkentin, T.D. Genomics-integrated breeding for carotenoids and folates in staple cereal grains to reduce malnutrition. Front. Genet. 2020, 11, 414.
- Li, Y.; Qin, C.; Wang, L.; Jiao, C.; Hong, H.; Tian, Y.; Li, Y.; Xing, G.; Wang, J.; Gu, Y.; et al. Genome-wide signatures of the geographic expansion and breeding of soybean. Sci. China Life Sci. 2022.
- Karikari, B.; Wang, Z.; Zhou, Y.; Yan, W.; Feng, J.; Zhao, T. Identification of quantitative trait nucleotides and candidate genes for soybean seed weight by multiple models of genome-wide association study. BMC Plant Biol. 2020, 20, 404.
- Ghosh, S.; Zhang, S.; Azam, M.; Agyenim-Boateng, K.G.; Qi, J.; Feng, Y.; Li, Y.; Li, J.; Li, B.; Sun, J. Identification of genomic loci and candidate genes related to seed tocopherol content in soybean. Plants 2022, 11, 1703.
- Zhong, Y.; Wen, K.; Li, X.; Wang, S.; Li, S.; Zeng, Y.; Cheng, Y.; Ma, Q.; Nian, H. Identification and mapping of QTLs for sulfur-containing amino acids in soybean (Glycine max L.). J. Agric. Food Chem. 2023, 71, 398–410.
- Kim, J.M.; Lyu, J.I.; Kim, D.; Hung, N.N.; Seo, J.S.; Ahn, J.; Lim, Y.J.; Eom, S.H.; Ha, B.; Kwon, S. Genome wide association study to detect genetic regions related to isoflavone content in a mutant soybean population derived from radiation breeding. Front. Plant Sci. 2022, 13, 968466.
- Li, M.; Li, H.; Sun, A.; Wang, L.; Ren, C.; Liu, J.; Gao, X. Transcriptome analysis reveals key drought-stress-responsive genes in soybean. Front. Genet. 2022, 13, 1060529.
- Jha, A.B.; Gali, K.K.; Zhang, H.; Purves, R.W.; Tar’an, B.; Vandenberg, A.; Warkentin, T.D. Folate profile diversity and associated SNPs using genome wide association study in pea. Euphytica 2020, 216, 18.
- Martin, C.J.; Torkamaneh, D.; Arif, M.; Pauls, K.P. Genome-wide association study of seed folate content in common bean. Front. Plant Sci. 2021, 12, 696423.
- Khanal, S.; Xue, J.; Khanal, R.; Xie, W.; Shi, J.; Pauls, K.; Navabi, A. Quantitative trait loci analysis of folate content in dry beans, Phaseolus vulgaris L. Int. J. Agron. 2013, 2013, 983641.
- Guo, W.; Lian, T.; Wang, B.; Guan, J.; Yuan, D.; Wang, H.; Safiul Azam, F.M.; Wan, X.; Wang, W.; Liang, Q. Genetic mapping of folate QTLs using a segregated population in maize. J. Integr. Plant Biol. 2019, 61, 675–690.
- Bali, S.; Robinson, B.R.; Sathuvalli, V.; Bamberg, J.; Goyer, A. Single Nucleotide Polymorphism (SNP) markers associated with high folate content in wild potato species. PLoS ONE 2018, 13, e0193415.
- Xiao, Y.; Yu, Y.; Xie, L.; Li, K.; Guo, X.; Li, G.; Liu, J.; Li, G.; Hu, J. A genome-wide association study of folates in sweet corn kernels. Front. Plant Sci. 2022, 13, 1004455.
- Hossain, T.; Rosenberg, I.; Selhub, J.; Kishore, G.; Beachy, R.; Schubert, K. Enhancement of folates in plants through metabolic engineering. Proc. Natl. Acad. Sci. USA 2004, 101, 5158–5163.
- Ramírez Rivera, N.G.; García-Salinas, C.; Aragao, F.J.; Díaz de la Garza, R.I. Metabolic engineering of folate and its precursors in Mexican common bean (Phaseolus vulgaris L.). Plant Biotechnol. J. 2016, 14, 2021–2032.
- Liang, Q.; Wang, K.; Liu, X.; Riaz, B.; Jiang, L.; Wan, X.; Ye, X.; Zhang, C. Improved folate accumulation in genetically modified maize and wheat. J. Exp. Bot. 2019, 70, 1539–1551.
- Sybesma, W.; Starrenburg, M.; Kleerebezem, M.; Mierau, I.; de Vos, W.M.; Hugenholtz, J. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 2003, 69, 3069–3076.
- Dong, W.; Cheng, Z.; Lei, C.; Wang, J.; Wang, J.; Wu, F.; Zhang, X.; Guo, X.; Zhai, H.; Wan, J. Overexpression of folate biosynthesis genes in rice (Oryza sativa L.) and evaluation of their impact on seed folate content. Plant Foods Hum. Nutr. 2014, 69, 379–385.
- Nunes, A.C.; Kalkmann, D.C.; Aragao, F.J. Folate biofortification of lettuce by expression of a codon optimized chicken GTP cyclohydrolase I gene. Transgenic Res. 2009, 18, 661.
- Naqvi, S.; Zhu, C.; Farre, G.; Ramessar, K.; Bassie, L.; Breitenbach, J.; Conesa, D.P.; Ros, G.; Sandmann, G.; Capell, T. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 7762–7767.
- Diaz de la Garza, R.; Quinlivan, E.P.; Klaus, S.M.; Basset, G.J.; Gregory, J.F., III; Hanson, A.D. Folate biofortification in tomatoes by engineering the pteridine branch of folate synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 13720–13725.
- Schubert, K. Metabolic engineering of folate biosynthesis in plants: Expression of bacterial GTP cyclohydrolase 1 in Arabidopsis thaliana results in increased pterin and folate levels in leaves and seeds. Pteridines 2005, 16, 79.
- Diaz de la Garza, R.; Gregory, J.F.; Hanson, A.D. Folate biofortification of tomato fruit. Proc. Natl. Acad. Sci. USA 2007, 104, 4218–4222.
- Storozhenko, S.; De Brouwer, V.; Volckaert, M.; Navarrete, O.; Blancquaert, D.; Zhang, G.; Lambert, W.; Van Der Straeten, D. Folate fortification of rice by metabolic engineering. Nat. Biotechnol. 2007, 25, 1277–1279.
- Blancquaert, D.; Storozhenko, S.; Van Daele, J.; Stove, C.; Visser, R.G.; Lambert, W.; Van Der Straeten, D. Enhancing pterin and para-aminobenzoate content is not sufficient to successfully biofortify potato tubers and Arabidopsis thaliana plants with folate. J. Exp. Bot. 2013, 64, 3899–3909.
- Gillies, S.A.; McIntosh, S.R.; Henry, R. A transgenic cereal crop with enhanced folate: Rice expressing wheat HPPK/DHPS. In Proceedings of the 11th International Wheat Genetics Symposium, Brisbane, Australia, 24–29 August 2008.
- Gorelova, V.; Ambach, L.; Rébeillé, F.; Stove, C.; Van Der Straeten, D. Folates in plants: Research advances and progress in crop biofortification. Front. Chem. 2017, 5, 21.
- Akhtar, T.A.; Orsomando, G.; Mehrshahi, P.; Lara-Núñez, A.; Bennett, M.J.; Gregory, J.F., III; Hanson, A.D. A central role for gamma-glutamyl hydrolases in plant folate homeostasis. Plant J. 2010, 64, 256–266.
- Zhang, Y.; Zhang, C.; Man, X.; Men, Y.; Ren, X.; Li, X.; Han, L.; Sun, Z.; Yang, Y.; Hou, S.; et al. Functional characterization of the SiFPGS2 gene of foxtail millet in folate accumulation and root development. Plant Growth Regul. 2022.
- De Lepeleire, J.; Strobbe, S.; Verstraete, J.; Blancquaert, D.; Ambach, L.; Visser, R.G.; Stove, C.; Van Der Straeten, D. Folate biofortification of potato by tuber-specific expression of four folate biosynthesis genes. Mol. Plant 2018, 11, 175–188.
- Zhang, M.; Liu, S.; Wang, Z.; Yuan, Y.; Zhang, Z.; Liang, Q.; Yang, X.; Duan, Z.; Liu, Y.; Kong, F.; et al. Progress in soybean functional genomics over the past decade. Plant Biotechnol. J. 2021, 20, 256–282.
- Feng, Y.; Zhang, S.; Li, J.; Pei, R.; Tian, L.; Qi, J.; Azam, M.; Agyenim-Boateng, K.G.; Shaibu, A.S.; Liu, Y.; et al. The dual-function C2H2-type zinc-finger transcription factor GmZFP7 contributes to isoflavone accumulation in soybean. New Phytol. 2022.
- Zhu, Y.; Thakur, K.; Feng, J.; Cai, J.; Zhang, J.; Hu, F.; Wei, Z. B-vitamin enriched fermented soymilk: A novel strategy for soy-based functional foods development. Trends Food Sci. Technol. 2020, 105, 43–55.
- Jang, C.H.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.S. Fermented soy products: Beneficial potential in neurodegenerative diseases. Foods 2021, 10, 636.
- Rekha, C.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010, 109, 1198–1208.
- Albuquerque, M.A.C.; Bedani, R.; LeBlanc, J.G.; Saad, S.M.I. Passion fruit by-product and fructooligosaccharides stimulate the growth and folate production by starter and probiotic cultures in fermented soymilk. Int. J. Food Microbiol. 2017, 261, 35–41.
More
Information
Subjects:
Agronomy; Food Science & Technology; Genetics & Heredity
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
954
Revisions:
4 times
(View History)
Update Date:
31 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




