Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alberto Palazzuoli | -- | 2702 | 2023-01-18 08:45:30 | | | |
| 2 | Rita Xu | Meta information modification | 2702 | 2023-01-18 09:00:00 | | | | |
| 3 | Rita Xu | Meta information modification | 2702 | 2023-01-18 09:00:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Palazzuoli, A.; Tramonte, F.; Beltrami, M. Heart Failure with Preserved Ejection Fraction. Encyclopedia. Available online: https://encyclopedia.pub/entry/40323 (accessed on 06 March 2026).
Palazzuoli A, Tramonte F, Beltrami M. Heart Failure with Preserved Ejection Fraction. Encyclopedia. Available at: https://encyclopedia.pub/entry/40323. Accessed March 06, 2026.
Palazzuoli, Alberto, Francesco Tramonte, Matteo Beltrami. "Heart Failure with Preserved Ejection Fraction" Encyclopedia, https://encyclopedia.pub/entry/40323 (accessed March 06, 2026).
Palazzuoli, A., Tramonte, F., & Beltrami, M. (2023, January 18). Heart Failure with Preserved Ejection Fraction. In Encyclopedia. https://encyclopedia.pub/entry/40323
Palazzuoli, Alberto, et al. "Heart Failure with Preserved Ejection Fraction." Encyclopedia. Web. 18 January, 2023.
Copy Citation
Heart failure with preserved ejection fraction (HFpEF) remains a poorly characterized syndrome with many unknown aspects related to different patient profiles, various associated risk factors and a wide range of aetiologies. It comprises several pathophysiological pathways, such as endothelial dysfunction, myocardial fibrosis, extracellular matrix deposition and intense inflammatory system activation.
biomarkers
metabolomic
microRNA
HFpEF
1. Introduction
Heart failure with preserved ejection fraction (HFpEF) is a heterogenous syndrome with specific molecular, genetic and metabolomic features, all of which reflect on vascular and myocardial cell adaptations [1]. HFpEF encompasses different pathophysiological pathways and cardiac structural profiles compared to heart failure with reduced ejection fraction (HFrEF) [2]. About half of individuals with heart failure (HF) are considered to be affected by HFpEF showing a peculiar clinical profile and cardiac structural and functional alterations. Therefore, the selection criteria are often elusive and mainly based upon ejection fraction cut-offs rather than distinct clinical and laboratory phenotypes [3]. Most inclusion criteria comprise the concomitant presence of left ventricular hypertrophy (LVH), altered diastolic dysfunction and elevation of serum natriuretic peptide (NP) levels associated with exertional dyspnea or reduced exercise tolerance. Indeed, recent clinical trials have adopted wide inclusion criteria and patient features creating inhomogeneous patterns with various morphologies and comorbidities [4][5]. In this framework, advanced analytic research, investigating specific biomarkers in a well-phenotyped population, could lead to better understanding about molecular pathways and biological mechanisms responsible for HFpEF syndrome. The interaction between clinical variables, imaging features and biomarkers could become the model for future research and a combined network analysis may change the current approach based on traditional knockdown/knockout study [6]. In HFrEF syndrome, myocyte loss, cellular death and consequent cardiac chamber enlargement are the main features causing the disease progression; conversely, HFpEF is characterized by collagen overexpression, myocardial fibrosis, extracellular matrix deposition and high inflammatory response [7]. All of these mechanisms occur differently according to specific risk factors, comorbidities and vascular and cardiac remodeling [8].
2. Different HFpEF Phenogroups
Despite recent improvements in treatment and diagnosis, HFpEF remains a poorly characterized syndrome with many unknown aspects related to different patient profiles, associated risk factors and pathophysiological pathways [9]. Vast trials have shown a wide prevalence of LVH left atrial dilatation, diastolic dysfunction and post-capillary pulmonary hypertension. Pulmonary hypertension (PH) is common in both patients with HFpEF and HFrEF and is associated with higher hospitalizations and mortality. Observational studies suggest an estimated prevalence of PH of 40–72% in patients with HFrEF and 36–83% in those with HFpEF [10]. Moreover, HFpEF patients have presented various extracardiac comorbidities such as diabetes, chronic kidney disease (CKD), anemia, chronic lung diseases, obesity and metabolic dysfunction [5][11][12]. Because the interventional trials did not distinguish between different risk factors and underlying diseases, the one-size-fits-all approach might explain the lack of efficacy and benefit of current treatments. Based on the different pathophysiological drivers, some authors have suggested that different HFpEF subtypes are linked to cardiometabolic alterations, body structural conformation and peripheral maladaptation. These assessments may be related to the presence of systemic disorders leading to skeletal muscle metabolism alterations and vascular rarefaction [13]. Since all of these features are widely expressed in HFpEF, the diagnosis based only on cardiac morphology and dysfunction remains difficult to interpret and is often misleading. Current pictures may configure a wide range of HFpEF phenotypes which differ in cardiac structure and cardiovascular remodeling, both related to the underlying biological process and pathophysiological contributor, despite having a similar EF.
Notably, recent machine learning analysis has attempted to cluster specific phenotypes by latent class study. In a post hoc analysis of TOPCAT, patients were classified into three categories according to vascular and cardiac remodeling: patients with mild LV hypertrophy and chronic pulmonary disease with normal vascular stiffness characterized by increased expression of metalloproteinase; older patients with multiple comorbidities, LV hypertrophy and reduced vascular compliance, characterized by an elevated tissue calcification biomarker; and obese subgroup with several metabolic alterations, increased renin–angiotensin system activity, lipidic profile derangement and increased inflammatory pattern [14]. Similarly, another study identified a group including young individuals with increased body mass index (BMI), typical abnormalities in cardiac structure and function and low serum levels of natriuretic peptides (NP); a cluster with high prevalence of diabetes and obesity characterized by severe diastolic dysfunction and elevated NP circulating levels; and a cluster characterized by RV dysfunction and combined pre- and post-capillary pulmonary hypertension and renal dysfunction, experiencing the worst outcomes [15].
Finally, an analysis of SwedeHF and CHECK HF registries differentiated between five distinct phenotypes according to a combined approach including risk factors and associated comorbidities. The study confirmed that the cluster with CKD, coronary artery disease (CAD) and high diuretic amount revealed the worst outcomes [16] (Figure 1).
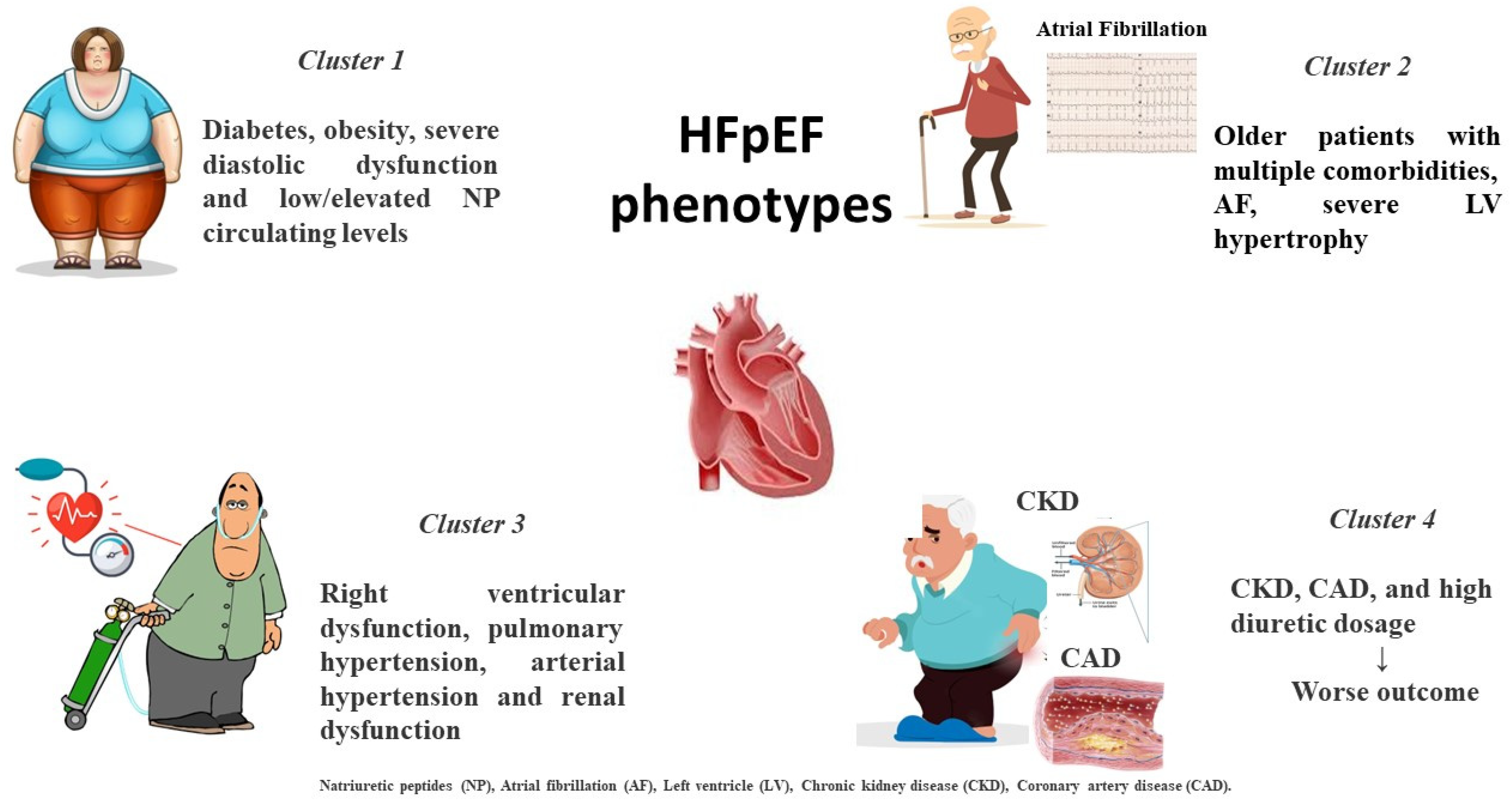
Figure 1. Distinct HFpEF clinical phenotypes based on clinical-presentation-associated metabolic disorders and comorbidities.
3. Current Biomarkers in HFpEF
Several HF risk prediction scores include biomarkers, mainly natriuretic peptides (NP), but important gaps exist regarding the knowledge of the underlying pathophysiological mechanisms, biological process and disease progression. Circulating biomarkers should reflect cardiac and extra cardiac disorders responsible for the HFpEF development and the related pathological pathways [17][18].
Myocardial Injury—High sensitivity troponin (HsTn) is universally considered a marker of myocardial damage in acute coronary syndrome (ACS). However, it has prognostic significance in HF and it implies myocardial damage apoptosis and progressive fiber loss independent of coronary vessel diseases. It could be the final outcome of microvascular dysfunction and subendocardial layer damage due to systemic oxygen reduction and therefore an altered supply–demand mismatch. Other features, such as increased wall tension, high left ventricle (LV) filling pressure and right ventricular dysfunction, are related with increased HsTn levels [19]. In patients with HFpEF, increased HsTn serum levels correlate with a more severe diastolic degree and a higher pulmonary pressure. Moreover, high HsTn serum levels are also associated with an increase in wall stress, a higher degree of LV hypertrophy and an increase in cardiac workload [20]. Many reports have shown that HsTn predicts poor outcomes in HFpEF, especially in men rather than women. In hospitalized patients with acute HfpEF, the persistence of high HsTn serum levels at both admission and discharge is related with increased rates of rehospitalization and death [21][22]. Similarly, in the TOPCAT trial, elevation of HsTn was independently associated with a higher risk of hospitalization and cardiovascular events [23]. These findings were confirmed in the PARAGON study in which even a mild HsTn elevation was associated with a worse outcome during a follow-up of about three years; moreover, patients taking sacubitril/valsartan treatment showed a significant reduction compared to the placebo [24].
Natriuretic peptides (NPs) are the hallmark biomarkers in HF and their measurement is accounted for in HF guidelines across the spectrum of the whole EF [25]. The biologically active NP form and its amino-terminal portion precursor (pro B type natriuretic peptide) are cleaved into NT-proBNP and BNP and released in response to enhanced cardiac wall tension and increased filling pressure; moreover, their levels increase proportionally to the degree of systolic dysfunction. The two peptides are released in response to sympathetic activity, in particular to systemic vasoconstriction and fluid retention, as an opposite response to the increased neurohormonal overdrive [26]. NP activity counteracts sympathetic activity promoting cardiac afterload reduction and myocardial relaxation by directly eliciting vasodilatation and myocardial relaxation effects. The main mechanism of action is related to diuresis and natriuresis that lead to congestion reduction and euvolemia [27]. Serum levels of NPs are directly related to intracardiac pressure, including LV end diastolic pressure (LVEDP), wedge pressure and pulmonary systolic pressure. Both peptides are largely analyzed in patients with reduced systolic function as valuable diagnostic and prognostic features. In HFpEF serum, NP levels are generally less increased but they keep their diagnostic relevance [28]. Some authors believe that this feature is due to the reduced wall stress in this setting together with extracardiac conditions such as metabolic syndrome, chronic lung disease and, in particular, obesity in which adipocyte cells favor a reduced NP receptor expression [29]. These comorbidities are often associated with one another in HFpEF causing a wide range of NP levels. Although some studies have revealed that some HFpEF clusters experience low NPs below 100 pg/mL, a recent meta-analysis has shown an optimal diagnostic accuracy in this setting (AUC 0.80 CI 0.73-0.87) [30]. Moreover, a combined analysis of NPs and HsTn has shown that patients with higher serum levels have an increased risk of death and hospitalization [31]. Finally, in acute settings, NP assays reveal similar prognostic information in HFpEF as in HFrEF and the related changes during hospitalization confer equal risk assessment adjusted for potential confounding factors [32].
Adrenomedullin (ADM) is a regulatory peptide produced by endothelial and smooth muscle cells with antiproliferative, vasodilatatory and antiapoptotic effects. It is synthesized mainly by adrenal medulla but its receptors are expressed in many tissues such as lungs, heart and kidneys [33]. It is considered an important biomarker of pulmonary and systemic congestion and it is produced in relation to increased sympathetic activity [34]. It counteracts systemic vasoconstriction induced by renin angiotensin system activation facilitating vascular permeability and elastance. Due to its serum instability, mainly caused by interactions with plasma proteins, and short half-life, a reliable quantification of ADM is difficult to achieve and its precursor ‘mid regional pro-hormone’ (MRpro-ADM) is usually measured [35]. A large study confirmed the close relationship between ADM and congestion in patients with worsening heart failure; therefore, a high plasma level appears to be related to increased risk and recurrent hospitalization for HF [36]. MRpro-ADM measured at admission is also related to all causes of cardiovascular mortality, sudden death and cardiac arrest [37]. In patients with acute coronary syndrome (ACS), elevated MRpro-ADM levels predict the risk of HF occurrence. Finally, in the PROTECT trial, the MRpro-ADM was related to longer hospitalization, increased congestion signs and elevated NP levels; moreover, its assessment before discharge conferred relevant prognostic information related to incomplete decongestion status and therefore early rehospitalization risk [38].
Extracellular Fibrosis—Collagen deposition and increased myocardial fibrosis are two relevant features in HFpEF. The more extensively analyzed biomarkers of this process are galectin-3 and soluble ST2. Galectin-3 is a glycoprotein involved in many inflammatory and profibrotic processes as a galactosidase family member, and it is synthetized by macrophage [39]. It directly increases fibroblast proliferation and fibrogenesis in animal models, inducing myocardial and vascular stiffness. It is also associated with renal dysfunction and LV remodeling [40]. Galectin-3 inhibition mitigates myocardial fibrosis and elicits a reverse remodeling through a reduction in systemic overload [41][42]. High galectin-3 serum levels are associated with poor outcome in both patients with HFrEF and HFpEF. In patients with elevated levels, galectin-3 is associated with other comorbidities, such as hypertension and CKD, and it is a useful marker for target therapy and risk stratification [43]. Moreover, changes in galectin-3 levels over a period of follow-up provide prognostic insights in patients with HFpEF [44].
Soluble ST2 is another marker reflecting myocardial fibrosis and it is overexpressed in HFrEF and HFpEF patients. It is primarily produced by myocardial cells, but also smooth muscle and endothelium cells, in relation to congestion or profibrotic stimuli [45]. In HFpEF patients, the addition of ST2 to NPs provides more complete prognostic information; therefore, a higher ST2 phenotype could indicate a more compromised diastolic dysfunction [46][47]. Notably, a meta-analysis demonstrated that ST2 could predict outcomes independently of EF values [48].
Matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinases (TIMP) are two endopeptidases which induce extracellular collagen deposition; therefore, they are reasonably considered as two biomarkers of fibrosis in HFpEF [49]. Collagenase is an enzyme family with different characteristics and may be considered in the context between collagen synthesis and collagen degradation. Elevated levels of MMP2 and MMP9 are related to an increased risk in HFpEF but also high levels are found in HFrEF after myocardial infarction [50]. In the PARAGON trial, a high level of TIMP, a marker of impaired collagen degradation, is associated with increased event rate [51].
Inflammation—Systemic inflammation is a typical feature of HFpEF. It reflects the immune response to cardiac remodeling, systemic vascular injury and underlying triggers often associated with diseases, such as metabolic syndrome, diabetes, chronic lung disease and anemia [52]. Inflammation can occur differently in every HFpEF phenotype and it can be analyzed using several biomarkers. C-reactive protein (CRP) is the wider analyzed marker and it is associated with an increased risk in ACS and HF. A comparison study differentiating CRP from HFrEF and HFpEF has demonstrated that in the latter it has a better prognostic meaning, adding new information rather than just that of NPs alone [53]. CRP and pentraxin are significantly higher in acute HFpEF patients compared to non-acute patients and they correlate with diastolic dysfunction degree [54]. CRP has a direct role in inducing complement cascade activation and cytokine stimulation causing myocyte loss and endothelial dysfunction by decreasing nitric oxide (NO) production. A CRP increase is also related to immune response mediated by lymphocyte T and monocyte cells. Inflammatory status may also trigger microvascular dysfunction by inducing endothelial permeability and adhesion molecule production and increasing reactive oxygen species bioavailability [55].
Grow differentiation factor 15 (GDF-15) is a member of the cytokines family and it belongs to the transforming growth factor beta (TGFβ) family. It is highly expressed in inflammatory chronic diseases, and pulmonary, kidney, and cardiovascular diseases [56]. Since it integrates information from cardiac and systemic diseases, it could reflect the interplay among different apparatuses, but it is not specific to CV diseases or HF [57]. A recent meta-analysis demonstrated that in patients with a high risk burden it is related to an increased incidence of HF providing additional information on LV remodeling and function [58]. In HFpEF, it is similarly elevated as in HFrEF but it has a more prognostic value compared to NTproBNP. Indeed, in subjects with low NT-proBNP and high GDF-15, the risk of cardiovascular death is comparable to those with high NPs [57]. This finding confirms the role of GDF-15 as an intermediate marker of inflammatory and multi-organ injury.
Endothelial dysfunction—Microcirculation and endothelial cells are two important features for HFpEF occurrence and microvascular dysfunction is one of the most common therapeutic targets. Dysfunctional endothelium increases the expression of adhesion molecules such as vascular cell adhesion molecules (VCAM), induced cell adhesion molecules (ICAM) and E-selectin that activates von Willebrand and other prothrombotic factors. Therefore, tissue growth factors (TGFs) and insulin growth factors (IGFs) are two other items of vascular alteration and increased proliferation [59]. The prothrombotic cascade is also emphasized by several coagulation alterations involving factor V and VII, tissue plasminogen activator (TPA), inducing endothelial damage and loss of vascular integrity. Vascular, coagulative and thrombotic alterations may lead to a progressive microvascular obstruction, capillary obliteration and loss of capillary integrity [60]. These processes induce increased vascular resistance and enhanced cardiac workload at both systemic and pulmonary districts. Therefore, vascular damage is characterized by intima and media hyperplasia, disarray of smooth muscle cells and intimal fibrosis, ultimately leading to progressive capillary reduction and narrowing. All of these features reduce nitric oxide (NO) production and its mediator ‘guanosine monophosphate cyclase’ (GMPc), causing vasoconstriction, reduction in viscoelastic properties and altered oxygen consumption and utilization with increased oxidative stress [61][62]. Unfortunately, no reliable blood biomarker exists to measure these processes and only in vitro studies can document these endothelial alterations. Nevertheless, a direct GMPc activator ‘Vericiguat’ is capable of improving vascular tone and of reducing cardiac stiffness.
Plasminogen activator inhibitor (PAI-1) is the main inhibitor of tissue plasminogen activator and the intrinsic fibrinolytic system. It is increased in patients with HFpEF in association with D-dimer levels, suggesting an association with prothrombotic and procoagulant states in this setting [63]. In the LURIC study, it is a prognostic index of mortality and CV events, although a longitudinal study confirmed only an association with markers of renal damage and NPs [64].
Insulin growth factor binding protein (IGFBP) is associated with inflammation, cell adhesion and senescence. It is increased according to left atrial dysfunction and dilatation reflecting diastolic dysfunction in HFpEF [65]. In a machine learning study, in subjects with a high inflammatory phenotype, elevated comorbidity burden and renal dysfunction, it is elevated and associated with increased hospitalization risk [66]. In the I-PRESERVE trial, IGFBP was associated with an increased risk of CV events and HF severity [67]. Finally, in asymptomatic patients with LV hypertrophy, IGFBP identifies subjects with altered diastolic function suggesting a role in early identification and screening of HFpEF [68] (Figure 2, graphical abstract).
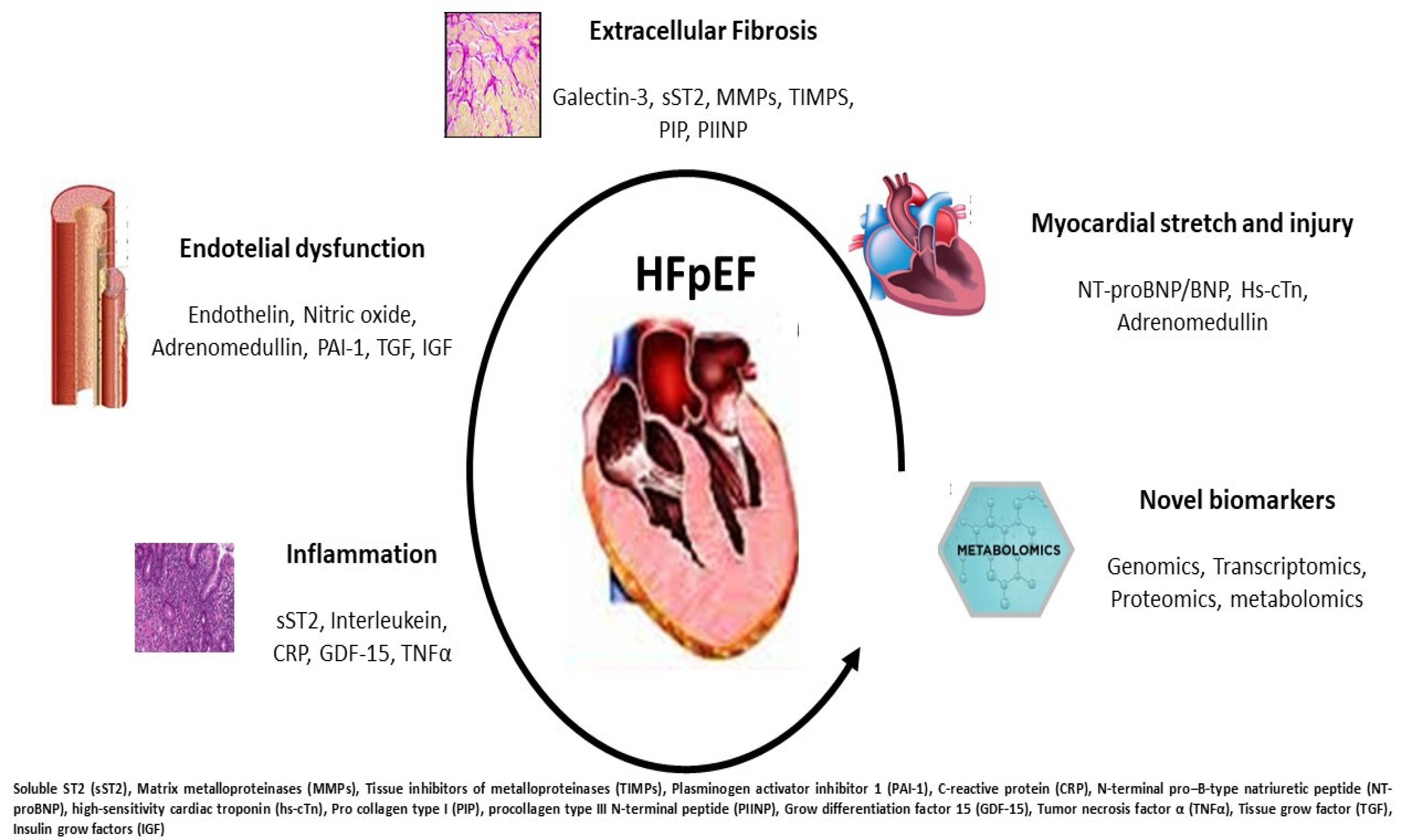
Figure 2. Potential pathophysiological mechanisms occurring in HFpEF: each disorder can be recognized by specific biomarker increase and overexpression. The partnership between clinical and laboratory information may better target the HFpEF profile.
References
- Lewis, G.A.; Schelbert, E.B.; Williams, S.G.; Cunnington, C.; Ahmed, F.; McDonagh, T.A.; Miller, C.A. Biological Phenotypes of Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 2186–2200.
- Borlaug, B.A.; Paulus, W.J. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur. Heart J. 2011, 32, 670–679.
- Luo, H.; Xu, Y.; Yue, F.; Zhang, C.; Chen, C. Quality of inclusion criteria in the registered clinical trials of heart failure with preserved ejection fraction: Is it time for a change? Int. J. Cardiol. 2018, 254, 210–214.
- Shah, A.M.; Shah, S.J.; Anand, I.S.; Sweitzer, N.K.; O’Meara, E.; Heitner, J.F.; Sopko, G.; Li, G.; Assmann, S.F.; McKinlay, S.M.; et al. Cardiac structure and function in heart failure with preserved ejection fraction: Baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ. Heart Fail. 2014, 7, 104–115.
- Shah, A.M.; Cikes, M.; Prasad, N.; Li, G.; Getchevski, S.; Claggett, B.; Rizkala, A.; Lukashevich, I.; O’Meara, E.; Ryan, J.J.; et al. Echocardiographic Features of Patients with Heart Failure and Preserved Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2019, 74, 2858–2873.
- Shah, S.J.; Katz, D.H.; Deo, R.C. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail. Clin. 2014, 10, 407–418.
- Kelly, J.P.; Mentz, R.J.; Mebazaa, A.; Voors, A.A.; Butler, J.; Roessig, L.; Fiuzat, M.; Zannad, F.; Pitt, B.; O’Connor, C.M.; et al. Patient selection in heart failure with preserved ejection fraction clinical trials. J. Am. Coll. Cardiol. 2015, 65, 1668–1682.
- Vaduganathan, M.; Michel, A.; Hall, K.; Mulligan, C.; Nodari, S.; Shah, S.J.; Senni, M.; Triggiani, M.; Butler, J.; Gheorghiade, M. Spectrum of epidemiological and clinical findings in patients with heart failure with preserved ejection fraction stratified by study design: A systematic review. Eur. J. Heart Fail. 2016, 18, 54–65.
- Palazzuoli, A.; Caravita, S.; Paolillo, S.; Ghio, S.; Tocchetti, C.G.; Ruocco, G.; Correale, M.; Ambrosio, G.; Perrone Filardi, P.; Senni, M.; et al. Current gaps in HFpEF trials: Time to reconsider patients’ selection and to target phenotypes. Prog. Cardiovasc. Dis. 2021, 67, 89–97.
- Shah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; O’Meara, E.; Desai, A.S.; Heitner, J.F.; Li, G.; Fang, J.; et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: Findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ. Heart Fail. 2014, 7, 740–751.
- Zile, M.R.; Gottdiener, J.S.; Hetzel, S.J.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Baicu, C.F.; Massie, B.M.; Carson, P.E.; I-PRESERVE Investigator. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011, 124, 2491–2501.
- Campbell, R.T.; Jhund, P.S.; Castagno, D.; Hawkins, N.M.; Petrie, M.C.; McMurray, J.J. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-preserved, and I-PRESERVE? J. Am. Coll. Cardiol. 2012, 60, 2349–2356.
- Iorio, A.; Senni, M.; Barbati, G.; Greene, S.J.; Poli, S.; Zambon, E.; Di Nora, C.; Cioffi, G.; Tarantini, L.; Gavazzi, A.; et al. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: A community-based study. Eur. J. Heart Fail. 2018, 20, 1257–1266.
- Cohen, J.B.; Schrauben, S.J.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Yarde, M.; Wang, Z.; Bhattacharya, P.T.; Chirinos, D.A.; et al. Clinical Phenogroups in Heart Failure with Preserved Ejection Fraction: Detailed Phenotypes, Prognosis, and Response to Spironolactone. JACC Heart Fail. 2020, 8, 172–184.
- Shah, S.J.; Katz, D.H.; Selvaraj, S.; Burke, M.A.; Yancy, C.W.; Gheorghiade, M.; Bonow, R.O.; Huang, C.C.; Deo, R.C. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015, 131, 269–279.
- Uijl, A.; Savarese, G.; Vaartjes, I.; Dahlström, U.; Brugts, J.J.; Linssen, G.C.M.; van Empel, V.; Brunner-La Rocca, H.P.; Asselbergs, F.W.; Lund, L.H.; et al. Identification of distinct phenotypic clusters in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2021, 23, 973–982.
- Palazzuoli, A.; Beltrami, M. Are HFpEF and HFmrEF So Different? The Need to Understand Distinct Phenotypes. Front Cardiovasc Med. 2021, 8, 676658.
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; de Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L., Jr.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1054–e1091, Erratum in Circulation 2017, 136, e345.
- Kociol, R.D.; Pang, P.S.; Gheorghiade, M.; Fonarow, G.C.; O’Connor, C.M.; Felker, G.M. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J. Am. Coll. Cardiol. 2010, 56, 1071–1078.
- Greenberg, B. Heart failure preserved ejection fraction with coronary artery disease: Time for a new classification? J. Am. Coll. Cardiol. 2014, 63 Pt A, 2828–2830.
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Kane, G.C.; Olson, T.P.; Jarolim, P.; Borlaug, B.A. Myocardial Injury and Cardiac Reserve in Patients with Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2018, 72, 29–40.
- Gohar, A.; Chong, J.P.C.; Liew, O.W.; den Ruijter, H.; de Kleijn, D.P.V.; Sim, D.; Yeo, D.P.S.; Ong, H.Y.; Jaufeerally, F.; Leong, G.K.T.; et al. The prognostic value of highly sensitive cardiac troponin assays for adverse events in men and women with stable heart failure and a preserved vs. reduced ejection fraction. Eur. J. Heart Fail. 2017, 19, 1638–1647.
- Myhre, P.L.; O’Meara, E.; Claggett, B.L.; de Denus, S.; Jarolim, P.; Anand, I.S.; Beldhuis, I.E.; Fleg, J.L.; Lewis, E.; Pitt, B.; et al. Cardiac Troponin I and Risk of Cardiac Events in Patients with Heart Failure and Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005312.
- Gori, M.; Senni, M.; Claggett, B.; Liu, J.; Maggioni, A.P.; Zile, M.; Prescott, M.F.; Van Veldhuisen, D.J.; Zannad, F.; Pieske, B.; et al. Integrating High-Sensitivity Troponin T and Sacubitril/Valsartan Treatment in HFpEF: The PARAGON-HF Trial. JACC Heart Fail. 2021, 9, 627–635.
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.F.; Coats, A.J.S.; Januzzi, J.L.; Maisel, A.S.; McDonald, K.; Mueller, T.; et al. Circulating heart failure biomarkers beyond natriuretic peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632.
- Maisel, A.; Mueller, C.; Nowak, R.; Peacock, W.F.; Landsberg, J.W.; Ponikowski, P.; Mockel, M.; Hogan, C.; Wu, A.H.; Richards, M.; et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: Results from the BACH (Biomarkers in Acute Heart Failure) trial. J. Am. Coll. Cardiol. 2010, 55, 2062–2076.
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717.
- Tromp, J.; Khan, M.A.; Klip, I.T.; Meyer, S.; de Boer, R.A.; Jaarsma, T.; Hillege, H.; van Veldhuisen, D.J.; van der Meer, P.; Voors, A.A. Biomarker Profiles in Heart Failure Patients with Preserved and Reduced Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e003989.
- Sakane, K.; Kanzaki, Y.; Tsuda, K.; Maeda, D.; Sohmiya, K.; Hoshiga, M. Disproportionately low BNP levels in patients of acute heart failure with preserved vs. reduced ejection fraction. Int. J. Cardiol. 2021, 327, 105–110.
- Remmelzwaal, S.; van Ballegooijen, A.J.; Schoonmade, L.J.; Dal Canto, E.; Handoko, M.L.; Henkens, M.T.H.M.; van Empel, V.; Heymans, S.R.B.; Beulens, J.W.J. Natriuretic peptides for the detection of diastolic dysfunction and heart failure with preserved ejection fraction-a systematic review and meta-analysis. BMC Med. 2020, 18, 290.
- Lopuszynski, J.B.; Downing, A.J.; Finley, C.M.; Zahid, M. Prognosticators of All-Cause Mortality in Patients with Heart Failure With Preserved Ejection Fraction. Am. J. Cardiol. 2021, 158, 66–73.
- Kociol, R.D.; Horton, J.R.; Fonarow, G.C.; Reyes, E.M.; Shaw, L.K.; O’Connor, C.M.; Felker, G.M.; Hernandez, A.F. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: Data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ. Heart Fail. 2011, 4, 628–636.
- Nishikimi, T.; Nakagawa, Y. Adrenomedullin as a Biomarker of Heart Failure. Heart Fail. Clin. 2018, 14, 49–55.
- Voors, A.A.; Kremer, D.; Geven, C.; Ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H.D.; et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur. J. Heart Fail. 2019, 21, 163–171.
- Kremer, D.; Ter Maaten, J.M.; Voors, A.A. Bio-adrenomedullin as a potential quick, reliable, and objective marker of congestion in heart failure. Eur. J. Heart Fail. 2018, 20, 1363–1365.
- Ter Maaten, J.M.; Kremer, D.; Demissei, B.G.; Struck, J.; Bergmann, A.; Anker, S.D.; Ng, L.L.; Dickstein, K.; Metra, M.; Samani, N.J.; et al. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. Eur. J. Heart Fail. 2019, 21, 732–743.
- Kozhuharov, N.; Ng, L.; Wussler, D.; Strebel, I.; Sabti, Z.; Hartmann, O.; Eltayeb, M.; Squire, I.; Nowak, A.; Rieger, M.; et al. Activity of the adrenomedullin system to personalise post-discharge diuretic treatment in acute heart failure. Clin Res Cardiol. 2022, 111, 627–637.
- Pandhi, P.; Ter Maaten, J.M.; Emmens, J.E.; Struck, J.; Bergmann, A.; Cleland, J.G.; Givertz, M.M.; Metra, M.; O’Connor, C.M.; Teerlink, J.R.; et al. Clinical value of pre-discharge bio-adrenomedullin as a marker of residual congestion and high risk of heart failure hospital readmission. Eur. J. Heart Fail. 2020, 22, 683–691.
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; André, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128.
- de Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817.
- Beltrami, M.; Ruocco, G.; Dastidar, A.G.; Franci, B.; Lucani, B.; Aloia, E.; Nuti, R.; Palazzuoli, A. Additional value of Galectin-3 to BNP in acute heart failure patients with preserved ejection fraction. Clin Chim Acta 2016, 457, 99–105.
- de Boer, R.A.; Lok, D.J.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011, 43, 60–68.
- Edelmann, F.; Holzendorf, V.; Wachter, R.; Nolte, K.; Schmidt, A.G.; Kraigher-Krainer, E.; Duvinage, A.; Unkelbach, I.; Düngen, H.D.; Tschöpe, C.; et al. Galectin-3 in patients with heart failure with preserved ejection fraction: Results from the Aldo-DHF trial. Eur. J. Heart Fail. 2015, 17, 214–223.
- Ghorbani, A.; Bhambhani, V.; Christenson, R.H.; Meijers, W.C.; de Boer, R.A.; Levy, D.; Larson, M.G.; Ho, J.E. Longitudinal Change in Galectin-3 and Incident Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2018, 72, 3246–3254.
- Weinberg, E.O.; Shimpo, M.; De Keulenaer, G.W.; MacGillivray, C.; Tominaga, S.; Solomon, S.D.; Rouleau, J.L.; Lee, R.T. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002, 106, 2961–2966.
- Ky, B.; French, B.; McCloskey, K.; Rame, J.E.; McIntosh, E.; Shahi, P.; Dries, D.L.; Tang, W.H.; Wu, A.H.; Fang, J.C.; et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ. Heart Fail. 2011, 4, 180–187.
- Wang, Y.C.; Yu, C.C.; Chiu, F.C.; Tsai, C.T.; Lai, L.P.; Hwang, J.J.; Lin, J.L. Soluble ST2 as a biomarker for detecting stable heart failure with a normal ejection fraction in hypertensive patients. J. Card. Fail. 2013, 19, 163–168.
- Aimo, A.; Vergaro, G.; Ripoli, A.; Bayes-Genis, A.; Pascual Figal, D.A.; de Boer, R.A.; Lassus, J.; Mebazaa, A.; Gayat, E.; Breidthardt, T.; et al. Meta-Analysis of Soluble Suppression of Tumorigenicity-2 and Prognosis in Acute Heart Failure. JACC Heart Fail. 2017, 5, 287–296.
- Krebber, M.M.; van Dijk, C.G.M.; Vernooij, R.W.M.; Brandt, M.M.; Emter, C.A.; Rau, C.D.; Fledderus, J.O.; Duncker, D.J.; Verhaar, M.C.; Cheng, C.; et al. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Extracellular Matrix Remodeling during Left Ventricular Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 6742.
- Ferreira, J.M.; Ferreira, S.M.; Ferreira, M.J.; Falcão-Pires, I. Circulating Biomarkers of Collagen Metabolism and Prognosis of Heart Failure with Reduced or Mid-Range Ejection Fraction. Curr. Pharm. Des. 2017, 23, 3217–3223.
- Cunningham, J.W.; Claggett, B.L.; O’Meara, E.; Prescott, M.F.; Pfeffer, M.A.; Shah, S.J.; Redfield, M.M.; Zannad, F.; Chiang, L.M.; Rizkala, A.R.; et al. Effect of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients with HFpEF. J. Am. Coll. Cardiol. 2020, 76, 503–514.
- Pasceri, V.; Willerson, J.T.; Yeh, E.T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 2000, 102, 2165–2168.
- Koller, L.; Kleber, M.; Goliasch, G.; Sulzgruber, P.; Scharnagl, H.; Silbernagel, G.; Grammer, T.; Delgado, G.; Tomaschitz, A.; Pilz, S.; et al. C-reactive protein predicts mortality in patients referred for coronary angiography and symptoms of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 758–766.
- Abernethy, A.; Raza, S.; Sun, J.L.; Anstrom, K.J.; Tracy, R.; Steiner, J.; VanBuren, P.; LeWinter, M.M. Pro-Inflammatory Biomarkers in Stable Versus Acutely Decompensated Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e007385.
- DuBrock, H.M.; AbouEzzeddine, O.F.; Redfield, M.M. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS ONE 2018, 13, e0201836.
- Kempf, T.; von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060.
- Chan, M.M.; Santhanakrishnan, R.; Chong, J.P.; Chen, Z.; Tai, B.C.; Liew, O.W.; Ng, T.P.; Ling, L.H.; Sim, D.; Leong, K.T.G.; et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur. J. Heart Fail. 2016, 18, 81–88.
- Kato, E.T.; Morrow, D.A.; Guo, J.; Berg, D.D.; Blazing, M.A.; Bohula, E.A.; Bonaca, M.P.; Cannon, C.P.; de Lemos, J.A.; Giugliano, R.P.; et al. Growth differentiation factor 15 and cardiovascular risk: Individual patient meta-analysis. Eur. Heart J. 2022, ehac577.
- Shantsila, E.; Wrigley, B.J.; Blann, A.D.; Gill, P.S.; Lip, G.Y. A contemporary view on endothelial function in heart failure. Eur. J. Heart Fail. 2012, 14, 873–881.
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxén, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450, Erratum in Eur. Heart J. 2019, 40, 541..
- Rush, C.J.; Berry, C.; Oldroyd, K.G.; Rocchiccioli, J.P.; Lindsay, M.M.; Touyz, R.M.; Murphy, C.L.; Ford, T.J.; Sidik, N.; McEntegart, M.B.; et al. Prevalence of Coronary Artery Disease and Coronary Microvascular Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2021, 6, 1130–1143.
- Emdin, M.; Aimo, A.; Castiglione, V.; Vergaro, G.; Georgiopoulos, G.; Saccaro, L.F.; Lombardi, C.M.; Passino, C.; Cerbai, E.; Metra, M.; et al. Targeting Cyclic Guanosine Monophosphate to Treat Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1795–1807.
- Winter, M.P.; Kleber, M.E.; Koller, L.; Sulzgruber, P.; Scharnagl, H.; Delgado, G.; Goliasch, G.; März, W.; Niessner, A. Prognostic significance of tPA/PAI-1 complex in patients with heart failure and preserved ejection fraction. Thromb. Haemost. 2017, 117, 471–478.
- Jug, B.; Vene, N.; Salobir, B.G.; Sebestjen, M.; Sabovic, M.; Keber, I. Procoagulant state in heart failure with preserved left ventricular ejection fraction. Int. Heart J. 2009, 50, 591–600.
- Hage, C.; Bjerre, M.; Frystyk, J.; Gu, H.F.; Brismar, K.; Donal, E.; Daubert, J.C.; Linde, C.; Lund, L.H. Comparison of Prognostic Usefulness of Serum Insulin-Like Growth Factor-Binding Protein 7 in Patients with Heart Failure and Preserved versus Reduced Left Ventricular Ejection Fraction. Am. J. Cardiol. 2018, 121, 1558–1566.
- Sabbah, M.S.; Fayyaz, A.U.; de Denus, S.; Felker, G.M.; Borlaug, B.A.; Dasari, S.; Carter, R.E.; Redfield, M.M. Obese-Inflammatory Phenotypes in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2020, 13, e006414.
- Gandhi, P.U.; Chow, S.L.; Rector, T.S.; Krum, H.; Gaggin, H.K.; McMurray, J.J.; Zile, M.R.; Komajda, M.; McKelvie, R.S.; Carson, P.E.; et al. Prognostic Value of Insulin-Like Growth Factor-Binding Protein 7 in Patients with Heart Failure and Preserved Ejection Fraction. J. Card. Fail. 2017, 23, 20–28.
- Gandhi, P.U.; Gaggin, H.K.; Redfield, M.M.; Chen, H.H.; Stevens, S.R.; Anstrom, K.J.; Semigran, M.J.; Liu, P.; Januzzi, J.L., Jr. Insulin-Like Growth Factor-Binding Protein-7 as a Biomarker of Diastolic Dysfunction and Functional Capacity in Heart Failure with Preserved Ejection Fraction: Results from the RELAX Trial. JACC Heart Fail. 2016, 4, 860–869.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
3 times
(View History)
Update Date:
18 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





