Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sandrine Gerber | -- | 3333 | 2023-01-18 08:04:51 | | | |
| 2 | Peter Tang | Meta information modification | 3333 | 2023-01-18 11:37:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Robin, P.; Gerber-Lemaire, S. Sensing Surfaces for Capacitive Biodetection. Encyclopedia. Available online: https://encyclopedia.pub/entry/40321 (accessed on 08 February 2026).
Robin P, Gerber-Lemaire S. Sensing Surfaces for Capacitive Biodetection. Encyclopedia. Available at: https://encyclopedia.pub/entry/40321. Accessed February 08, 2026.
Robin, Perrine, Sandrine Gerber-Lemaire. "Sensing Surfaces for Capacitive Biodetection" Encyclopedia, https://encyclopedia.pub/entry/40321 (accessed February 08, 2026).
Robin, P., & Gerber-Lemaire, S. (2023, January 18). Sensing Surfaces for Capacitive Biodetection. In Encyclopedia. https://encyclopedia.pub/entry/40321
Robin, Perrine and Sandrine Gerber-Lemaire. "Sensing Surfaces for Capacitive Biodetection." Encyclopedia. Web. 18 January, 2023.
Copy Citation
Affinity-based biosensors operate by specifically capturing a biological target with biological or synthetic capture agents such as aptamers, DNAzymes, single stranded DNAs or antibodies. Despite their high sensitivity and their suitability for miniaturization, biosensors are still limited for clinical applications due to the lack of reproducibility and specificity of their detection performance. The design and preparation of sensing surfaces are suspected to be a cause of these limitations.
non-faradaic impedance
capacitive detection
biosensors
electrochemical impedance spectroscopy
surface functionalization
biomolecules immobilization
interdigitated electrodes
1. Introduction
Affinity-based biosensors operate by specifically capturing a biological target with biological or synthetic capture agents such as aptamers, DNAzymes, single stranded DNAs or antibodies. The binding event between the capture molecule and its target is later translated into a readable signal.
Labelling the target molecules with fluorophores, magnetic beads, quantum dots or enzymes, was reported to facilitate and amplify the readout signal. However, labelled-detection methods are expensive and necessitate multi-step processes, hence are limited for real-time detection. Label-free methods are therefore of interest for high throughput biomolecules screening, portable devices and suitable for large-scale production [1].
A variety of transducing methods have been described to translate the binding event between the targeted molecules and the probes (Figure 1). Conventional approaches include optical, electrical or mass-sensitive techniques [2]. Electrical biosensors are great candidates for miniaturized, portable and label-free detection, relying on potentiometric, voltammetric, amperometric or impedimetric readout signals. Impedance-based sensors are themselves divided in faradaic or non-faradaic detection. Faradaic biosensors refer to the detection of charge transfer across a membrane [3]. They often rely on electrochemical impedance spectroscopy (EIS) [4] which detects the binding events via the change of electron transfer resistance and double layer capacitance within a frequency range [5]. However, faradaic detection is complex as it necessitates a wide window of frequencies [4]. It also requires the addition of potentially hazardous redox couples, that can degrade biomolecules [4][6]. On the other hand, non-faradaic based sensors, also called capacitive sensors or third-generation biosensors [7], detect the changes of capacitance at the electrode surface caused by the molecular binding events. These sensors have a high-sensitivity potential [4], and do not require the addition of external reagent unlike other conventional methods, such as in situ hybridization or enzyme linked immunosorbent assays (ELISA) [4][8]. They offer a simple and rapid detection that can be inserted into portable devices [9]. Additionally, non-faradaic capacitive detection does not require trained laboratory personal or samples preparation, and is therefore interesting for point-of-care applications [4][10][11][12]. Unfortunately, it has been reported that capacitive biodetection suffers from poor to poor reproducibility [4][12][13][14][15] and large standard deviation [16][17], preventing their translation for clinical application. These limitations have been suspected to arise form sensing surface parameters such as its cleanliness [4][18], homogeneity [4] and insulation [10][19][20][21].
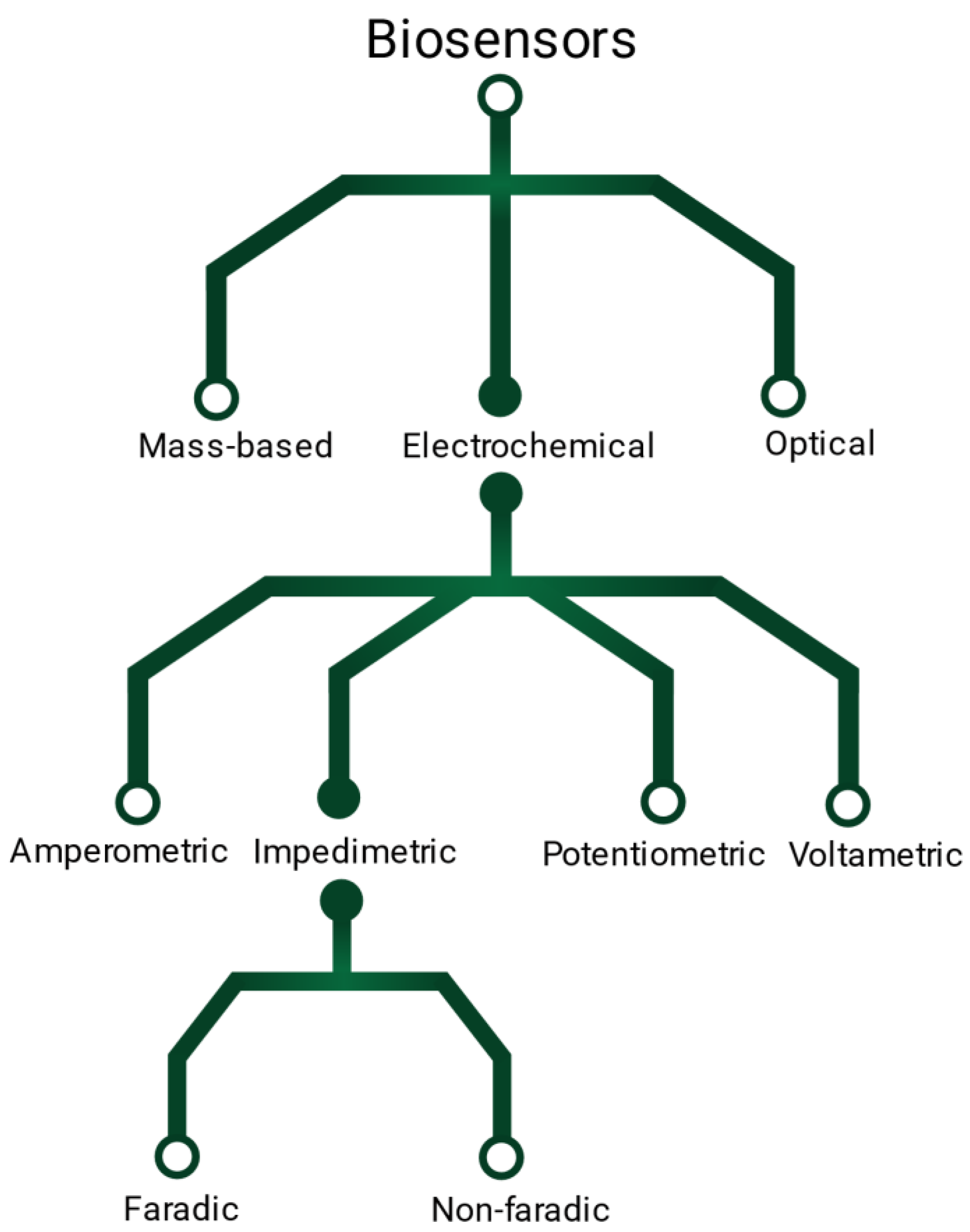
Figure 1. Illustration of the different types of electrical sensors.
2. Methodologies for Capacitive Biosensor Detection
Two main electrode geometries were reported for capacitive sensing, leading to two distinct capacitive methodologies for the detection of binding events between capture molecules and their targets. The most common is based on potentiostatic capacitance measured at the electrode/solution interface. In this case, the capture molecules are immobilized on the working electrodes. As an alternative, interdigitated electrodes have received growing attention over the last three decades for capacitive detection [3]. In this case, the recognition elements are immobilized on a substrate in between the electrodes, which undergo capacitance changes upon binding to the molecular targets [22].
3. Current State of Capacitive Biosensors Used in Clinical Applications
Due to the variety of capture probes which can be immobilized on the electrodes surface, capacitive biosensors can be designed for a wide range of medical applications. Viruses, unicellular and disease markers were detected in biological fluids, via non-faradaic measurements. The following sections focus on the most recent studies which enabled to significantly decrease the limits of detection (LoD) compared to previous systems and/or addressed capacitive detection in complex biological samples.
3.1. Infections
Immunosensors have been reported for the capacitive detection of viruses such as Influenza virus [23][24], foot and mouth disease [25], Hepatitis B [26], Norovirus [27], Zika virus [28] and SARS-CoV-2 [29]. However, genosensors were more widely described for viral capacitive detection, and generally displayed lower LoD than immunosensors. In 1999, Bergreen et al. could detect Cytomegalovirus with a limit of detection of 0.2 aM from DNA standard fragments [13]. More recently, commercial DNA sequence of West Nile virus could be detected with a limit of detection (LoD) of 1.5 aM [4].
While these studies investigated the specificity of the obtained sensors with non-targeted genes, none of them reported the detection of viral targets in complex biological samples. Matrix effect, coming from the interaction of non-targeted biomolecules with the sensors surface, can have deleterious effect on the sensing efficiency. For example, in 2017, Cheng et al. reported a genosensor for the detection of Herpes 1 virus [30]. While a LoD of 10.7 aM could be achieved in standard buffer, the limit increased to 0.21 fM in neat serum, likely due to unwanted interactions of non-targeted biomolecules with the sensor surface [30]. To the researchers' knowledge, no capacitive sensors displaying attomolar detection has been reported to detect viruses in real biological samples.
Diagnosing a viral infection can also be performed by recognizing antibodies, i.e., performing serological tests. In that regard, Zeng et al. recently reported an impedimetric biosensor that can detect SARS-CoV-2 antibodies (Abs). The authors studied two enhancement techniques to improve the LoD of the system, (1) by probing the targeted Abs with a gold nanoparticle (AuNP)-tagged secondary antibody and (2) by using dielectric electrophoresis (DEP). In the case of the AuNPs enhancement strategy, the presence of the nanoparticles enhances the measurable signal, that can reduce the LoD value. In the case of DEP enhancement, targeted biomolecules can be selectively moved and concentrated to the sensing surface due to the dielectric properties of each molecule. While a LoD of 2 μg/mL was obtained with the DEP enhancement technique, the authors could detect down to 200 ng/mL with AuNPs [31].
Non-faradaic impedimetric sensors were also reported for other type of pathogens, including protozoan, worms and bacteria. In 2022, Figueroa-Miranda et al. reported a aptasensor based on a graphene surface for the detection of a malaria marker, Plasmodium falciparum lactate dehydrogenase, with a limit of detection of 0.78 fM in diluted human serum [32], allowing the detection of low-density parasitemia, and surpassing the LoD previously achieved via Faradaic detection [33][34][35].
Worm’s antigens were also detected via non-faradaic impedance measurements. Zhou et al., reported the use of gold electrodes modified with Schistosoma japonicum antibodies to detect the worms’ antigens. A LoD of 0.1 ng/mL was reached in PBS., and the selectivity of the sensor was assessed by comparing the capacitance changes with other proteins [36].
The presence of bacteria can either be detected via the presence of toxins, or directly detecting the unicellular organisms. While capacitive detection of toxins was reported in food [37][38] or in water [39], detection of toxins has not been reported for diagnosis purposes. On the contrary, direct detection of bacteria has been reported and is described in the Unicellular detection section.
3.2. Cancer, Chronic and Inflammatory Diseases
Early detection and diagnosis of cancer, chronic or inflammatory diseases can drastically improve the chances of survival [40][41][42]. Capacitive detection of such biomarkers has been used in this sense for a variety of pathologies.
A variety of cancer biomarkers were selected as targeted molecules for early cancer detection. Recent studies pointed to the detection of markers with a sufficient LoD to enable cancer early diagnosis. For example, in 2018, Arya et al. [43] reported a LoD of 0.1 ng/mL in non-diluted serum. As patients suffering from breast cancer possess around 14 to 75 ng/mL of Her2 markers in their blood, this aptasensor could be used in the future for diagnosis [43].
The detection of marker for chronic and inflammatory diseases was also reported via capacitance measurements. For example, the capacitive detection of C-reactive protein (CRP), a biomarker for cardiovascular disease risks, sepsis and other tissue inflammation was extensively studied [11][12][44][45][46]. A decade later, Macwan et al. [45], could surpass the sensitivity of previous CRP electrochemical sensors [11][12] and reached a 10 fg/mL LoD in both PBS buffer and serum by switching to an interdigitated sensing device integrated sputtered with nanofibers to enhance the sensitivity and selectivity of the sensing surface. Human chitinase-3-like protein 1, a marker for tissue inflammation and cardiac disease was also detected by capacitive systems with a LoD of 0.07 μg/L. This is 300 times lower than ELISA method, currently used for this marker detection [47]. However, this study was performed in diluted serum to prevent the matrix effect and complementary studies should thus be performed to assess the LoD in real biological sample. Other biomarkers have been targeted for chronic-disease diagnosis, such as LDL-cholesterol [6], interleukin-3 [48] or transferrin [20][49]. Nampt, an obesity marker for which Park et al. improved the binding affinity with the surface compared to previously reported techniques, could be detected in the nanomolar range [50]. Finally, Kumar Sharam et al. recently reported the detection of amyloid beta within 5 s with a LoD of 0.1 fg/mL for the diagnostic of Alzheimer disease [51].
3.3. Unicellular Organisms
Capacitive sensors for whole cell detection and analysis were developed over the last decade. The first occurrence of unicellular organism detection by non-faradaic measurements dates back 2004, for E. Coli detection in food samples by using an immunosensor targeting antigens present at the bacteria surface [52]. Since then, the detection of Salmonella (bacteria), Cryptosporidium (protozoan) in food or water samples was disclosed. In 2013, Couniot et al. also reported a simulation for optimal design of capacitive sensors for bacteria detection [53]. Recently, Borsel-Oliu et al. reported the use of a 3D IDE platform that evaluates the response of bacteria to antibiotics [54]. This platform could in the future be used for a variety of toxicity evaluations.
Non-faradaic biosensors have also been developed for eukaryotic cell detection. In 2022, Zhang et al. [55], detailed the detection of peripheral blood mononuclear cell (PBMCs), that can indicate the immune function state of a patient. While PMBCs cells are normally found in concentrations ranging from 0.7 to 6.2·106 cells/mL, their sensor displayed a LoD of 104 PBMCs/mL, with the possibility to quantify the cells. However, further experiments would be needed to assess the LoD in real samples for potential clinical applications.
Finally, yeast detection has also been reported with a non-pathogenic strain, Saccharomyces cerevisiae. A LoD value of 0.1 ng/mL was achieved, with a detection range of 0.4 to 18 ng/mL [56].
4. Capacitive Sensing Surfaces Preparation, and Limitations
4.1. Capacitive Sensors Limitations
Capacitance biosensors, whether they are based on IDEs or potentiostatic capacitance measurements, necessitate the immobilization of biomolecules on a surface. In the case of IDEs, the molecules are immobilized on the interface between the electrodes. For potentiostatic capacitance measurements, probes are immobilized directly on the electrode, eventually covered with an insulating layer. The capture molecules can be antibodies, to further detect an antigen or a pathogen (immunosensor), single-strand DNAs, for the detection of RNA or DNA single strands (genosensor) or aptamers, for the fabrication of aptasensors.
Unfortunately, even after years of progress in capacitive biodetection, challenges remain. Poor reproducibility [4][12][12][13][14][15] and large standard-deviation [16][17] of capacitive biosensors have been reported and linked to non-optimal parameters of their sensing surfaces [4][13][19]. Critical parameters in the capacitive sensing surfaces preparation have been raised, such as the probe immobilization strategy [13][19][49], surface cleanliness [4][18], homogeneity [4] and insulation [10][19][20][21]. Obtaining a high specificity with capacitive sensors is also challenging as any adsorbed biomolecules at the sensing surface can prevent target binding or generate a false-positive signal [10][16][17][57]. After describing the most common strategies for the preparation of sensing surfaces, the surface parameters suspected to affect capacitive sensors behavior are presented, along with the solutions recently reported to overcome these limitations.
4.2. Biomolecules Immobilization Techniques
Various immobilization strategies have been reported for the immobilization of capture molecules on capacitive sensing surfaces. The functionalization procedures may depend on the type of electrodes used and the nature of capture molecules immobilized. Selection of the functionalization pathway for a capacitive sensor is crucial, as it can affect drastically its performance [58].
While electrodes are generally made from metals such as gold [4][8][11][12][14][19][21][23][24][25][26][30][36][37][43][44][45][47][50][52][55][57][59][60][61][62][63][64][65][66][67][68], platinum [38], graphene [32][69], glass carbon [7][49] or aluminum [30][70][71], electrodes made of titanium [9][72][73][74][75], nickel [76], or silicium [77][78][79][80] are less described even though they are great candidates for capacitive measurements. Indeed, they display high insulation properties, can present smooth surfaces with homogeneous functionalities for capture probe immobilization [58]. Finally, few more exotic materials have been reported as electrodes material. For example, tantalum was selected for antibodies [81] or bacteria detection [54]. Recently, polymers were reported for the production of electrodes for capacitive biodetection. Park et al. described the use of electrodes made of a conductive polymer layer of PEDOT:PSS for the detection of SARS-CoV-2 [29]. Frias et al. used polyvinyl alcohol, alginate and polyaniline to fabricate electrodes for Zika virus detection [28].
Gold electrodes are generally favored for capacitive measurements as they are frequently encountered in other detection transduction techniques such as surface plasmon resonance, quartz crystal microbalance or reflection absorption IR spectroscopy, thus allowing for dual-mode detection and direct comparison of readout signals [18].
Two main strategies are commonly described for the immobilization of capture molecules on sensing surfaces. A first method consists of the formation of a self-assembled monolayer (SAM) at the surface of metallic electrodes. This layer can be directly made of the capture molecule, or of a linker that is later used to conjugate the capture molecule [82]. A second methods relies on the deposition a thin insulating film at the surface of the electrodes, generally made of a polymer or silanes, followed by the conjugation of the capture molecule to this first layer [58].
4.3. Impact of Surface Cleanliness and Contamination
Significant variations in reported results among different studies may arise from the lack of homogeneity and reproducibility of the coating procedure, as a result of improper electrode cleaning before functionalization [4][83]. In the context of mercapto-alkyl SAM formation on gold electrodes, Love et al. discussed the importance of proper electrode cleaning procedures to achieve uniform coatings [18]. SAM formation is based on exchange process, suggesting that thiolated molecules can displace miscellaneous contaminants adsorbed at the electrode surface. However, the presence of contaminants greatly affects the kinetics of the reaction, and therefore its reproducibility. To achieve reproducible coatings, the electrodes can be cleaned with piranha solutions or oxygen plasma [18], or via electrochemical methods [14] in the case of metallic electrodes. In 2010, Bhalla et al. compared the efficacy of piranha, plasma, reductive and oxidative cleaning methods on micro-fabricated chips used for EIS detection [83]. By analyzing cyclic voltammetry scans, scanning electron microscope images and capacitance measurements, the authors demonstrated that the two electrochemical cleaning techniques could effectively remove contaminants from the chips without degradation. However, the reductive pathway may lead to the deposition of materials on the conducting surface. Therefore, oxidative electrochemical treatment was found to be the most suitable and reproducible method for cleaning gold electrodes [83].
4.4. Non-Specific Adsorption
Non-specific adsorption of molecules has a critical impact on biosensing measurements, especially when using capacitive detection [10][57]. Any molecule immobilized at the surface of a capacitive sensor through non-specific interactions may result in false-positive detection, and therefore greatly reduces its selectivity. Such matrix effect was described by Liao and Cui, in the context of capacitive detection of platelet-derived growth factor. The study demonstrated the beneficial effect of electrode potential sweeping in potentiostatic EIS for discriminating between specific target binding and non-specific adsorption of biomolecules at the surface of the sensors [77]. Although, despite this optimization, the ratio of the positive to negative control was still around 10:1. By increasing the background noise, the matrix effect can also decrease the sensitivity of the studied device. For example, the detection of Herpes virus 1 reached a LoD value of 0.21 fM in neat serum while the attomolar detection range was achieved in pure buffer [30].
A variety of anti-biofouling strategies—classified as active or passive techniques—were explored for many biomedical applications, such as bioelectronic devices, biosensors, nanoparticles, dental implants or polymeric materials [84][85][86][87][88]. Physical and chemical passive methods include the addition of adsorption blocking agents and the addition of a repelling chemical layer based on a polyethylene glycol (PEG) layer, alkanethiol SAM layer, or zwitterionic polymer. Controlling the extent of biomolecule adsorption may also be achieved by changing the surface topography. Active methods, on the other side, create shear forces that are stronger than the forces causing non-specific adsorption. They can be generated through acoustic waves generation, pressure-driven flow, or from electrical or mechanical transducers [84]. To the researchers' knowledge, only passive methods were reported to reduce non-specific adsorption of biomolecules on capacitive sensing surfaces.
Among physical methods, the addition of bovine serum albumin (BSA) as a blocking agent was reported for the detection of enzymes [32], Abs [31], disease protein markers [8][14][89], viruses [23][29] or cells [62]. The use of biotin [63] or glycine [50] was shown to minimize non-specific binding events. Additionally, the addition of concentrated solutions of KCl was found to greatly reduce non-specific adsorption by disrupting weak interactions. Dijskma et al. showed that the injection of 100 mM KCl solution completely remove interferon gamma from gold surface without damaging the SAM functionalized layer [64].
Among chemical methods, the addition of an anti-biofouling PEG layer was highlighted in DNA-hybridization and interleukin biosensors [54][65]. Miranda-Figueroa et al. demonstrated the beneficial effect of added PEG chains on malaria biosensors. Not only the matrix tolerance was improved, but also the LoD adynamic detection range were enhanced [27].
The design of suitable strategies against nonspecific binding highly depends on the nature of the targeted analytes, therefore requiring extensive trial iterations. Dykstra et al. developed a microfluidic platform that can measure protein adsorption on selected surfaces. This device offers the possibility to rapidly screen various materials toward their tendency to repel biomolecules, and could be of great interest for the design of capacitive biosensors in the future [90].
4.5. Surface Insulation and Coverage
The surface of capacitive sensors must be insulated and hole-free to avoid charges to move through the layer, leading to the apparition of a faradaic current between the conductors [3][19][20][60], that would result in a change of capacitance of the surface and therefore a decrease in sensitivity [10]. Common insulating strategies relies on SAM covering based on alkyl-thiols, polymeric layers and silanization [19].
In the context of gold-thiol SAM formation, alkylthiols are added to insulate the sensing layer. Mirsky et al. reported that long chains should be privileged as short chains were prone to desorption. Proper insulation of gold electrodes was achieved with 15-/16-mercaptohexadecanoic acid [62]. Later, dodecanethiol [21][36][37][65][66][67][91], hexadecanethiol [92], mercaptohexanol [93], and mercapto-undecanol [4] were extensively used to insulate gold electrodes.
The quality of surface insulation largely depends on the selection of the functionalization procedure. Rickert et al. studied the insulation of epitope-modified gold electrodes with hydroxyundecanethiol (HUT). Simultaneous adsorption of a mixture of HUT and peptide was compared to the sequential adsorption of both components. The adsorption of mixed solutions resulted in poorly reproducible functionalization. On the contrary, reproducible and highly resistive films were obtained when the HUT was adsorbed after the epitope was immobilized [25].
In addition to provide chemical functionalities for the post-conjugation of capture biomolecules, polymeric layers were reported for the insulation of conductive electrodes. The insulation of Abs-modified gold electrodes with a 50 nm polytyramine film led to the detection of HSA down to 1.6 ng/mL concentration and with high reproducibility [61]. The quality of the insulating layer was probed by cyclic voltammetry. Berney et al. developed a capacitive detector for transferrin and studied the effect of PEG, as a non-conductive polymer, to insulate the sensing surface. When transferrin Ab was immobilized on non-insulated surface, the capacitance measurements after exposition to the targeted antigen were not reproducible. The addition of a PEG overlay system indicated the possibility to develop differential capacitive biosensors. However, the lack of continuity and integrity of the PEG layer did not allow for quantitative measurement of transferrin [20].
In conclusion, several requirements must be followed when designing and preparing a sensing surface for capacitive biosensors. First, the surface must be free of contaminants and prepared in as clean conditions as possible. Then, an insulation layer must be present to avoid faradaic currents that would lead to a drastic decrease in sensitivity. In the case of deposition of an oxide or polymeric layer on top of the electrodes, this layer should be however as thin as possible to keep good sensitivity properties. Finally, non-specific adsorption should be avoided to reduce false-positive results. Toward this goal, the addition of BSA or PEG layers have been the most reported technique.
References
- Ertürk, G.; Mattiasson, B. Capacitive Biosensors and Molecularly Imprinted Electrodes. Sensors 2017, 17, 390.
- Victorious, A.; Saha, S.; Pandey, R.; Didar, T.F.; Soleymani, L. Affinity-Based Detection of Biomolecules Using Photo-Electrochemical Readout. Front. Chem. 2019, 7, 617.
- Tsouti, V.; Boutopoulos, C.; Zergioti, I.; Chatzandroulis, S. Capacitive Microsystems for Biological Sensing. Biosens. Bioelectron. 2011, 27, 1–11.
- Wang, L.; Veselinovic, M.; Yang, L.; Geiss, B.J.; Dandy, D.S.; Chen, T. A Sensitive DNA Capacitive Biosensor Using Interdigitated Electrodes. Biosens. Bioelectron. 2017, 87, 646–653.
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578.
- Assaifan, A.K.; Alqahtani, F.A.; Alnamlah, S.; Almutairi, R.; Alkhammash, H.I. Detection and Real-Time Monitoring of LDL-Cholesterol by Redox-Free Impedimetric Biosensors. BioChip J. 2022, 16, 197–206.
- Darain, F.; Park, D.-S.; Park, J.-S.; Shim, Y.-B. Development of an Immunosensor for the Detection of Vitellogenin Using Impedance Spectroscopy. Biosens. Bioelectron. 2004, 19, 1245–1252.
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Label-Free Capacitance Based Aptasensor Platform for the Detection of HER2/ErbB2 Cancer Biomarker in Serum. Sens. Actuators B Chem. 2015, 220, 1145–1151.
- Alhoshany, A.; Sivashankar, S.; Mashraei, Y.; Omran, H.; Salama, K.N. A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-Μm CMOS Technology for Cancer Biomarker Detection. Sensors 2017, 17, 1942.
- Daniels, J.S.; Pourmand, N. Label-Free Impedance Biosensors: Opportunities and Challenges. Electroanalysis 2007, 19, 1239–1257.
- Qureshi, A.; Niazi, J.H.; Kallempudi, S.; Gurbuz, Y. Label-Free Capacitive Biosensor for Sensitive Detection of Multiple Biomarkers Using Gold Interdigitated Capacitor Arrays. Biosens. Bioelectron. 2010, 25, 2318–2323.
- Qureshi, A.; Gurbuz, Y.; Kallempudi, S.; Niazi, J.H. Label-Free RNA Aptamer-Based Capacitive Biosensor for the Detection of C-Reactive Protein. Phys. Chem. Chem. Phys. 2010, 12, 9176–9182.
- Berggren, C.; Stålhandske, P.; Brundell, J.; Johansson, G. A Feasibility Study of a Capacitive Biosensor for Direct Detection of DNA Hybridization. Electroanalysis 1999, 11, 156–160.
- Carrara, S.; Bhalla, V.; Stagni, C.; Benini, L.; Ferretti, A.; Valle, F.; Gallotta, A.; Riccò, B.; Samorì, B. Label-Free Cancer Markers Detection by Capacitance Biochip. Sens. Actuators B Chem. 2009, 136, 163–172.
- Berggren, C.; Bjarnason, B.; Johansson, G. An Immunological Interleukine-6 Capacitive Biosensor Using Perturbation with a Potentiostatic Step. Biosens. Bioelectron. 1998, 13, 1061–1068.
- Stagni, C.; Guiducci, C.; Benini, L.; Ricco, B.; Carrara, S.; Samori, B.; Paulus, C.; Schienle, M.; Augustyniak, M.; Thewes, R. CMOS DNA Sensor Array with Integrated A/D Conversion Based on Label-Free Capacitance Measurement. IEEE J. Solid-State Circuits 2006, 41, 2956–2964.
- Stagni, C.; Guiducci, C.; Benini, L.; Ricco, B.; Carrara, S.; Paulus, C.; Schienle, M.; Thewes, R. A Fully Electronic Label-Free DNA Sensor Chip. IEEE Sens. J. 2007, 7, 577–585.
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170.
- Rafael Castiello, F.; Porter, J.; Modarres, P.; Tabrizian, M. Interfacial Capacitance Immunosensing Using Interdigitated Electrodes: The Effect of Insulation/Immobilization Chemistry. Phys. Chem. Chem. Phys. 2019, 21, 15787–15797.
- Berney, H.; Alderman, J.; Lane, W.; Collins, J.K. A Differential Capacitive Biosensor Using Polyethylene Glycol to Overlay the Biolayer. Sens. Actuators B Chem. 1997, 44, 578–584.
- Chen, H.-J.; Chen, R.L.C.; Hsieh, B.-C.; Hsiao, H.-Y.; Kung, Y.; Hou, Y.-T.; Cheng, T.-J. Label-Free and Reagentless Capacitive Aptasensor for Thrombin. Biosens. Bioelectron. 2019, 131, 53–59.
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive Biosensors. Electroanalysis 2001, 13, 173–180.
- Lum, J.; Wang, R.; Lassiter, K.; Srinivasan, B.; Abi-Ghanem, D.; Berghman, L.; Hargis, B.; Tung, S.; Lu, H.; Li, Y. Rapid Detection of Avian Influenza H5N1 Virus Using Impedance Measurement of Immuno-Reaction Coupled with RBC Amplification. Biosens. Bioelectron. 2012, 38, 67–73.
- Wang, R.; Lin, J.; Lassiter, K.; Srinivasan, B.; Lin, L.; Lu, H.; Tung, S.; Hargis, B.; Bottje, W.; Berghman, L.; et al. Evaluation Study of a Portable Impedance Biosensor for Detection of Avian Influenza Virus. J. Virol. Methods 2011, 178, 52–58.
- Rickert, J.; Göpel, W.; Beck, W.; Jung, G.; Heiduschka, P. A ‘Mixed’ Self-Assembled Monolayer for an Impedimetric Immunosensor. Biosens. Bioelectron. 1996, 11, 757–768.
- Jung, H.-W.; Chang, Y.W.; Lee, G.; Cho, S.; Kang, M.-J.; Pyun, J.-C. A Capacitive Biosensor Based on an Interdigitated Electrode with Nanoislands. Anal. Chim. Acta 2014, 844, 27–34.
- Nasrin, F.; Khoris, I.M.; Chowdhury, A.D.; Boonyakida, J.; Park, E.Y. Impedimetric Biosensor of Norovirus with Low Variance Using Simple Bioconjugation on Conductive Polymer-Au Nanocomposite. Sens. Actuators B Chem. 2022, 369, 132390.
- Frias, I.A.M.; Vega Gonzales Gil, L.H.; Cordeiro, M.T.; Oliveira, M.D.L.; Andrade, C.A.S. Self-Enriching Electrospun Biosensors for Impedimetric Sensing of Zika Virus. ACS Appl. Mater. Interfaces 2022, 14, 41–48.
- Park, J.-H.; Lee, G.-Y.; Song, Z.; Bong, J.-H.; Chang, Y.W.; Cho, S.; Kang, M.-J.; Pyun, J.-C. Capacitive Biosensor Based on Vertically Paired Electrodes for the Detection of SARS-CoV-2. Biosens. Bioelectron. 2022, 202, 113975.
- Cheng, C.; Oueslati, R.; Wu, J.; Chen, J.; Eda, S. Capacitive DNA Sensor for Rapid and Sensitive Detection of Whole Genome Human Herpesvirus-1 DsDNA in Serum. Electrophoresis 2017, 38, 1617–1623.
- Zeng, J.; Duarte, P.A.; Ma, Y.; Savchenko, O.; Shoute, L.; Khaniani, Y.; Babiuk, S.; Zhuo, R.; Abdelrasoul, G.N.; Charlton, C.; et al. An Impedimetric Biosensor for COVID-19 Serology Test and Modification of Sensor Performance via Dielectrophoresis Force. Biosens. Bioelectron. 2022, 213, 114476.
- Figueroa-Miranda, G.; Liang, Y.; Suranglikar, M.; Stadler, M.; Samane, N.; Tintelott, M.; Lo, Y.; Tanner, J.A.; Vu, X.T.; Knoch, J.; et al. Delineating Charge and Capacitance Transduction in System-Integrated Graphene-Based BioFETs Used as Aptasensors for Malaria Detection. Biosens. Bioelectron. 2022, 208, 114219.
- Figueroa-Miranda, G.; Chen, S.; Neis, M.; Zhou, L.; Zhang, Y.; Lo, Y.; Tanner, J.A.; Kreidenweiss, A.; Offenhäusser, A.; Mayer, D. Multi-Target Electrochemical Malaria Aptasensor on Flexible Multielectrode Arrays for Detection in Malaria Parasite Blood Samples. Sens. Actuators B Chem. 2021, 349, 130812.
- Lenyk, B.; Figueroa-Miranda, G.; Pavlushko, I.; Lo, Y.; Tanner, J.A.; Offenhäusser, A.; Mayer, D. Dual-Transducer Malaria Aptasensor Combining Electrochemical Impedance and Surface Plasmon Polariton Detection on Gold Nanohole Arrays. ChemElectroChem 2020, 7, 4594–4600.
- Figueroa-Miranda, G.; Feng, L.; Shiu, S.C.-C.; Dirkzwager, R.M.; Cheung, Y.-W.; Tanner, J.A.; Schöning, M.J.; Offenhäusser, A.; Mayer, D. Aptamer-Based Electrochemical Biosensor for Highly Sensitive and Selective Malaria Detection with Adjustable Dynamic Response Range and Reusability. Sens. Actuators B Chem. 2018, 255, 235–243.
- Zhou, Y.-M.; Hu, S.-Q.; Cao, Z.-X.; Shen, G.-L.; Yu, R.-Q. Capacitive Immunosensor for the Determination of Schistosoma Japonicum Antigen. Anal. Lett. 2002, 35, 1919–1930.
- Limbut, W.; Hedström, M.; Thavarungkul, P.; Kanatharana, P.; Mattiasson, B. Capacitive Biosensor for Detection of Endotoxin. Anal. Bioanal. Chem. 2007, 389, 517–525.
- DeSilva, M.S.; Zhang, Y.; Hesketh, P.J.; Maclay, G.J.; Gendel, S.M.; Stetter, J.R. Impedance Based Sensing of the Specific Binding Reaction between Staphylococcus Enterotoxin B and Its Antibody on an Ultra-Thin Platinum Film. Biosens. Bioelectron. 1995, 10, 675–682.
- Loyprasert, S.; Hedström, M.; Thavarungkul, P.; Kanatharana, P.; Mattiasson, B. Sub-Attomolar Detection of Cholera Toxin Using a Label-Free Capacitive Immunosensor. Biosens. Bioelectron. 2010, 25, 1977–1983.
- Srivastava, A.; Creek, D.J. Discovery and Validation of Clinical Biomarkers of Cancer: A Review Combining Metabolomics and Proteomics. Proteomics 2019, 19, e1700448.
- Durkin, T.J.; Barua, B.; Savagatrup, S. Rapid Detection of Sepsis: Recent Advances in Biomarker Sensing Platforms. ACS Omega 2021, 6, 31390–31395.
- Wang, Y.; Ng, K.; Byrd, R.J.; Hu, J.; Ebadollahi, S.; Daar, Z.; deFilippi, C.; Steinhubl, S.R.; Stewart, W.F. Early Detection of Heart Failure with Varying Prediction Windows by Structured and Unstructured Data in Electronic Health Records. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015.
- Arya, S.K.; Zhurauski, P.; Jolly, P.; Batistuti, M.R.; Mulato, M.; Estrela, P. Capacitive Aptasensor Based on Interdigitated Electrode for Breast Cancer Detection in Undiluted Human Serum. Biosens. Bioelectron. 2018, 102, 106–112.
- Tanak, A.S.; Jagannath, B.; Tamrakar, Y.; Muthukumar, S.; Prasad, S. Non-Faradaic Electrochemical Impedimetric Profiling of Procalcitonin and C-Reactive Protein as a Dual Marker Biosensor for Early Sepsis Detection. Anal. Chim. Acta X 2019, 3, 100029.
- Macwan, I.; Aphale, A.; Bhagvath, P.; Prasad, S.; Patra, P. Detection of Cardiovascular CRP Protein Biomarker Using a Novel Nanofibrous Substrate. Biosensors 2020, 10, 72.
- Lin, K.-C.; Kunduru, V.; Bothara, M.; Rege, K.; Prasad, S.; Ramakrishna, B.L. Biogenic Nanoporous Silica-Based Sensor for Enhanced Electrochemical Detection of Cardiovascular Biomarkers Proteins. Biosens. Bioelectron. 2010, 25, 2336–2342.
- Chaocharoen, W.; Suginta, W.; Limbut, W.; Ranok, A.; Numnuam, A.; Khunkaewla, P.; Kanatharana, P.; Thavarungkul, P.; Schulte, A. Electrochemical Detection of the Disease Marker Human Chitinase-3-like Protein 1 by Matching Antibody-Modified Gold Electrodes as Label-Free Immunosensors. Bioelectrochemistry 2015, 101, 106–113.
- Chen, C.; Gopinath, S.C.B.; Anbu, P. Longitudinal Zeolite-Iron Oxide Nanocomposite Deposited Capacitance Biosensor for Interleukin-3 in Sepsis Detection. Nanoscale Res. Lett. 2021, 16, 68.
- Liu, Z.; Huang, S.; Jiang, D.; Liu, B.; Kong, J. A Novel Capacitive Immunosensor Using Electropolymerized Insulating Poly (O-phenylenediamine) Film on a Glass Carbon Electrode for Probing Transferrin. Anal. Lett. 2004, 37, 2283–2301.
- Park, J.-W.; Saravan Kallempudi, S.; Niazi, J.H.; Gurbuz, Y.; Youn, B.-S.; Gu, M.B. Rapid and Sensitive Detection of Nampt (PBEF/Visfatin) in Human Serum Using an SsDNA Aptamer-Based Capacitive Biosensor. Biosens. Bioelectron. 2012, 38, 233–238.
- Sharma, P.K.; Kim, E.-S.; Mishra, S.; Ganbold, E.; Seong, R.-S.; Kim, Y.M.; Jahng, G.-H.; Rhee, H.Y.; Han, H.-S.; Kim, D.H.; et al. Ultrasensitive Probeless Capacitive Biosensor for Amyloid Beta (Aβ1-42) Detection in Human Plasma Using Interdigitated Electrodes. Biosens. Bioelectron. 2022, 212, 114365.
- Radke, S.M.; Alocilja, E.C. A High Density Microelectrode Array Biosensor for Detection of E. coli O157:H7. Biosens. Bioelectron. 2005, 20, 1662–1667.
- Couniot, N.; Flandre, D.; Francis, L.A.; Afzalian, A. Signal-to-Noise Ratio Optimization for Detecting Bacteria with Interdigitated Microelectrodes. Sens. Actuators B Chem. 2013, 189, 43–51.
- Brosel-Oliu, S.; Mergel, O.; Uria, N.; Abramova, N.; van Rijn, P.; Bratov, A. 3D Impedimetric Sensors as a Tool for Monitoring Bacterial Response to Antibiotics. Lab Chip 2019, 19, 1436–1447.
- Zhang, Y.; Liu, Y. A Digital Microfluidic Device Integrated with Electrochemical Impedance Spectroscopy for Cell-Based Immunoassay. Biosensors 2022, 12, 330.
- Posseckardt, J.; Schirmer, C.; Kick, A.; Rebatschek, K.; Lamz, T.; Mertig, M. Monitoring of Saccharomyces Cerevisiae Viability by Non-Faradaic Impedance Spectroscopy Using Interdigitated Screen-Printed Platinum Electrodes. Sens. Actuators B Chem. 2018, 255, 3417–3424.
- Figueroa-Miranda, G.; Wu, C.; Zhang, Y.; Nörbel, L.; Lo, Y.; Tanner, J.A.; Elling, L.; Offenhäusser, A.; Mayer, D. Polyethylene Glycol-Mediated Blocking and Monolayer Morphology of an Electrochemical Aptasensor for Malaria Biomarker Detection in Human Serum. Bioelectrochemistry 2020, 136, 107589.
- Kirchhain, A.; Bonini, A.; Vivaldi, F.; Poma, N.; Di Francesco, F. Latest Developments in Non-Faradic Impedimetric Biosensors: Towards Clinical Applications. TrAC Trends Anal. Chem. 2020, 133, 116073.
- Wu, Z.-S.; Li, J.-S.; Deng, T.; Luo, M.-H.; Shen, G.-L.; Yu, R.-Q. A Sensitive Immunoassay Based on Electropolymerized Films by Capacitance Measurements for Direct Detection of Immunospecies. Anal. Biochem. 2005, 337, 308–315.
- Mirsky, V.M.; Riepl, M.; Wolfbeis, O.S. Capacitive Monitoring of Protein Immobilization and Antigen–Antibody Reactions on Monomolecular Alkylthiol Films on Gold Electrodes. Biosens. Bioelectron. 1997, 12, 977–989.
- Quoc, T.V.; Ngoc, V.N.; Bui, T.T.; Jen, C.-P.; Duc, T.C. High-Frequency Interdigitated Array Electrode-Based Capacitive Biosensor for Protein Detection. BioChip J. 2019, 13, 403–415.
- Luka, G.; Samiei, E.; Dehghani, S.; Johnson, T.; Najjaran, H.; Hoorfar, M. Label-Free Capacitive Biosensor for Detection of Cryptosporidium. Sensors 2019, 19, 258.
- Lee, K.-H.; Lee, J.-O.; Sohn, M.-J.; Lee, B.; Choi, S.-H.; Kim, S.K.; Yoon, J.-B.; Cho, G.-H. One-Chip Electronic Detection of DNA Hybridization Using Precision Impedance-Based CMOS Array Sensor. Biosens. Bioelectron. 2010, 26, 1373–1379.
- Dijksma, M.; Kamp, B.; Hoogvliet, J.C.; van Bennekom, W.P. Development of an Electrochemical Immunosensor for Direct Detection of Interferon-γ at the Attomolar Level. Anal. Chem. 2001, 73, 901–907.
- Niyomdecha, S.; Limbut, W.; Numnuam, A.; Kanatharana, P.; Charlermroj, R.; Karoonuthaisiri, N.; Thavarungkul, P. Phage-Based Capacitive Biosensor for Salmonella Detection. Talanta 2018, 188, 658–664.
- Wongkittisuksa, B.; Limsakul, C.; Kanatharana, P.; Limbut, W.; Asawatreratanakul, P.; Dawan, S.; Loyprasert, S.; Thavarungkul, P. Development and Application of a Real-Time Capacitive Sensor. Biosens. Bioelectron. 2011, 26, 2466–2472.
- Limbut, W.; Kanatharana, P.; Mattiasson, B.; Asawatreratanakul, P.; Thavarungkul, P. A Comparative Study of Capacitive Immunosensors Based on Self-Assembled Monolayers Formed from Thiourea, Thioctic Acid, and 3-Mercaptopropionic Acid. Biosens. Bioelectron. 2006, 22, 233–240.
- Hedström, M.; Galaev, I.Y.; Mattiasson, B. Continuous Measurements of a Binding Reaction Using a Capacitive Biosensor. Biosens. Bioelectron. 2005, 21, 41–48.
- Yagati, A.K.; Behrent, A.; Beck, S.; Rink, S.; Goepferich, A.M.; Min, J.; Lee, M.-H.; Baeumner, A.J. Laser-Induced Graphene Interdigitated Electrodes for Label-Free or Nanolabel-Enhanced Highly Sensitive Capacitive Aptamer-Based Biosensors. Biosens. Bioelectron. 2020, 164, 112272.
- Subramani, I.G.; Ayub, R.M.; Gopinath, S.C.B.; Perumal, V.; Fathil, M.F.M.; Md Arshad, M.K. Lectin Bioreceptor Approach in Capacitive Biosensor for Prostate-Specific Membrane Antigen Detection in Diagnosing Prostate Cancer. J. Taiwan Inst. Chem. Eng. 2021, 120, 9–16.
- De Vasconcelos, E.A.; Peres, N.G.; Pereira, C.O.; da Silva, V.L.; da Silva, E.F.; Dutra, R.F. Potential of a Simplified Measurement Scheme and Device Structure for a Low Cost Label-Free Point-of-Care Capacitive Biosensor. Biosens. Bioelectron. 2009, 25, 870–876.
- Hwang, C.; Park, N.; Kim, E.S.; Kim, M.; Kim, S.D.; Park, S.; Kim, N.Y.; Kim, J.H. Ultra-Fast and Recyclable DNA Biosensor for Point-of-Care Detection of SARS-CoV-2 (COVID-19). Biosens. Bioelectron. 2021, 185, 113177.
- Zhurauski, P.; Arya, S.K.; Jolly, P.; Tiede, C.; Tomlinson, D.C.; Ko Ferrigno, P.; Estrela, P. Sensitive and Selective Affimer-Functionalised Interdigitated Electrode-Based Capacitive Biosensor for Her4 Protein Tumour Biomarker Detection. Biosens. Bioelectron. 2018, 108, 1–8.
- Varlan, A.R.; Suls, J.; Sansen, W.; Veelaert, D.; De Loof, A. Capacitive Sensor for the Allatostatin Direct Immunoassay. Sens. Actuators B Chem. 1997, 44, 334–340.
- Mantzila, A.G.; Prodromidis, M.I. Performance of Impedimetric Biosensors Based on Anodically Formed Ti/TiO2 Electrodes. Electroanalysis 2005, 17, 1878–1885.
- Kallempudi, S.S.; Gurbuz, Y. A Nanostructured-Nickel Based Interdigitated Capacitive Transducer for Biosensor Applications. Sens. Actuators B Chem. 2011, 160, 891–898.
- Liao, W.; Cui, X.T. Reagentless Aptamer Based Impedance Biosensor for Monitoring a Neuro-Inflammatory Cytokine PDGF. Biosens. Bioelectron. 2007, 23, 218–224.
- Choudhury, S.; Nautiyal, R.; Thakkar, D.K.; Betty, C.A. Thickness Dependence of Nanocrystalline Tin Oxide Thin Films in Capacitive Biosensor Characterization. J. Electroanal. Chem. 2020, 877, 114742.
- Wei, F.; Sun, B.; Guo, Y.; Zhao, X.S. Monitoring DNA Hybridization on Alkyl Modified Silicon Surface through Capacitance Measurement. Biosens. Bioelectron. 2003, 18, 1157–1163.
- Betty, C.A. Highly Sensitive Capacitive Immunosensor Based on Porous Silicon-Polyaniline Structure: Bias Dependence on Specificity. Biosens. Bioelectron. 2009, 25, 338–343.
- Gebbert, A.; Alvarez-Icaza, M.; Stoecklein, W.; Schmid, R.D. Real-Time Monitoring of Immunochemical Interactions with a Tantalum Capacitance Flow-through Cell. Anal. Chem. 1992, 64, 997–1003.
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-Assembled Monolayers of Thiols and Dithiols on Gold: New Challenges for a Well-Known System. Chem. Soc. Rev. 2010, 39, 1805–1834.
- Bhalla, V.; Carrara, S.; Stagni, C.; Samorì, B. Chip Cleaning and Regeneration for Electrochemical Sensor Arrays. Thin Solid Film. 2010, 518, 3360–3366.
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488.
- Zander, Z.K.; Becker, M.L. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices. ACS Macro Lett. 2018, 7, 16–25.
- Chen, X.; Noy, A. Antifouling Strategies for Protecting Bioelectronic Devices. APL Mater. 2021, 9, 020701.
- Bevilacqua, P.; Nuzzo, S.; Torino, E.; Condorelli, G.; Salvatore, M.; Grimaldi, A.M. Antifouling Strategies of Nanoparticles for Diagnostic and Therapeutic Application: A Systematic Review of the Literature. Nanomaterials 2021, 11, 780.
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167.
- Bhalla, V.; Carrara, S.; Sharma, P.; Nangia, Y.; Raman Suri, C. Gold Nanoparticles Mediated Label-Free Capacitance Detection of Cardiac Troponin I. Sens. Actuators B Chem. 2012, 161, 761–768.
- Dykstra, P.H.; Roy, V.; Byrd, C.; Bentley, W.E.; Ghodssi, R. Microfluidic Electrochemical Sensor Array for Characterizing Protein Interactions with Various Functionalized Surfaces. Anal. Chem. 2011, 83, 5920–5927.
- Numnuam, A.; Kanatharana, P.; Mattiasson, B.; Asawatreratanakul, P.; Wongkittisuksa, B.; Limsakul, C.; Thavarungkul, P. Capacitive Biosensor for Quantification of Trace Amounts of DNA. Biosens. Bioelectron. 2009, 24, 2559–2565.
- Wang, K.; Jiang, D.; Kong, J.; Zhang, S.; Liu, B.; Lu, T. Sensitively Detecting Recombinant Hirudin Variant-2 with Capacitive Immunoassay Based on Self-Assembled Monolayers. Anal. Lett. 2003, 36, 2571–2583.
- Ianeselli, L.; Grenci, G.; Callegari, C.; Tormen, M.; Casalis, L. Development of Stable and Reproducible Biosensors Based on Electrochemical Impedance Spectroscopy: Three-Electrode versus Two-Electrode Setup. Biosens. Bioelectron. 2014, 55, 1–6.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
18 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




