Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José Vega-Baudrit | -- | 2974 | 2023-01-17 15:48:31 | | | |
| 2 | Sirius Huang | Meta information modification | 2974 | 2023-01-18 03:59:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Amores-Monge, V.; Goyanes, S.; Ribba, L.; Lopretti, M.; Sandoval-Barrantes, M.; Camacho, M.; Corrales-Ureña, Y.; Vega-Baudrit, J.R. Main Raw Materials from Pineapple Biomass. Encyclopedia. Available online: https://encyclopedia.pub/entry/40302 (accessed on 07 February 2026).
Amores-Monge V, Goyanes S, Ribba L, Lopretti M, Sandoval-Barrantes M, Camacho M, et al. Main Raw Materials from Pineapple Biomass. Encyclopedia. Available at: https://encyclopedia.pub/entry/40302. Accessed February 07, 2026.
Amores-Monge, Valeria, Silvia Goyanes, Laura Ribba, Mary Lopretti, Manuel Sandoval-Barrantes, Melissa Camacho, Yendry Corrales-Ureña, José Roberto Vega-Baudrit. "Main Raw Materials from Pineapple Biomass" Encyclopedia, https://encyclopedia.pub/entry/40302 (accessed February 07, 2026).
Amores-Monge, V., Goyanes, S., Ribba, L., Lopretti, M., Sandoval-Barrantes, M., Camacho, M., Corrales-Ureña, Y., & Vega-Baudrit, J.R. (2023, January 17). Main Raw Materials from Pineapple Biomass. In Encyclopedia. https://encyclopedia.pub/entry/40302
Amores-Monge, Valeria, et al. "Main Raw Materials from Pineapple Biomass." Encyclopedia. Web. 17 January, 2023.
Copy Citation
Pineapple is a highly demanded fruit in international markets due to its unique appearance and flavor, high fiber content, vitamins, folic acid, and minerals. It makes pineapple production and processing a significant source of income for producing countries. The lignocellulosic biomass obtained from pineapple agro-industrial residues is mainly composed of cellulose, hemicellulose, and lignin; a compilation of information for these three compounds is presented below, with a specific focus on treatments and pretreatments that can be employed to obtain and purify them, as well as the most common applications of each.
pineapple
wastes
biorefinery
1. Pineapple Production
Pineapple (i.e., Ananas comosus, Bromeliaceae family) is a fruit that is native to South America, specifically Brazil, Paraguay, and Argentina. It was discovered on Christopher Columbus’s second visit to this area, and consumption of pineapple spread during the 16th century due to trade along the Spanish and Portuguese sea lanes. Cultivation of this fruit can take between 14 and 20 months and involves three stages: planting and growth of shoots, flowering and harvesting, and the production of new shoots for future plantations [1].
Worldwide, pineapple cultivation has been very successful. There is a high demand for this fruit in the international market, mostly due to its flavor. Its high fiber content and potassium, iodine, carbohydrates, fiber, and vitamins A, B, and C, have prompted recommendations to consume pineapple to treat such diseases as fluid retention due to its diuretic effects, hypertension, cholesterol, anemia, poisoning due to its purifying action, immune system disorders due to its ability to strengthen defenses and assist in the formation of both red and white blood cells, cardiovascular problems, and obesity, among others [2][3].
Due to its tremendous success in the international market, pineapple cultivation has become a global industry. According to FAO, 2019 exports rose to 3.1 million tons, mainly due to the increase in Philippines exports and importations into China. Costa Rica is currently the largest producer and exporter of pineapple, followed by the Philippines and Brazil [4].
It should be noted that there is a variety of pineapples, each of which has different characteristics that make them widely traded. One variety is the Smooth Cayenne, which has a high yield, good potential for conservation, and organoleptic qualities; this pineapple is sensitive to problems such as black spots and other parasites, however, which can affect the overall quality. It is why MD 2, or the “Golden Pineapple,” was developed in the 1980s; this variety has excellent preservation properties, flavor, color, and minimal sensitivity to parasites and discoloration, and it is the most popular variety in commerce and exportation. There are several other widely distributed varieties, including but not limited to Queen Victoria, Sugar Loaf, and Champaka [1].
As was already mentioned, Costa Rica is the largest global producer and exporter of pineapple, followed by Philippines and Brazil. As mentioned before, pineapple exportation began in Costa Rica in the 1980s with the integration of the Pineapple Development Company (i.e., Pindeco), which led to increased production and higher crop yield [5]; this occurred with the use of imported technology and the creation of dependence on chemical inputs, such as herbicides, fungicides, insecticides, and fertilizers [6]. Production began to increase significantly: 24,204 tons of pineapple were produced in 1984, and this increased to 651,000 tons by 1998 [7]; production expanded to plantations in the Huetar Norte, the Atlantic Zone, and throughout the Central and South Pacific area. During this period, pineapple production began to gain additional relevance because of reducing poverty in rural sectors through the generation of direct and indirect jobs; there were 4200 direct jobs in 1995, and these grew to more than 31,000 in 2015 [8].
The Costa Rican pineapple production process (Figure 1) begins with cultivation, which requires the use of agrochemicals, machinery, fuels, and water; this process results in pineapple that can be exported and product that remains for local consumption. In the case of exportation, there is a packaging process before this fruit is exported to different countries and continents, and the United States and the European Union are the main importers of pineapple [9].
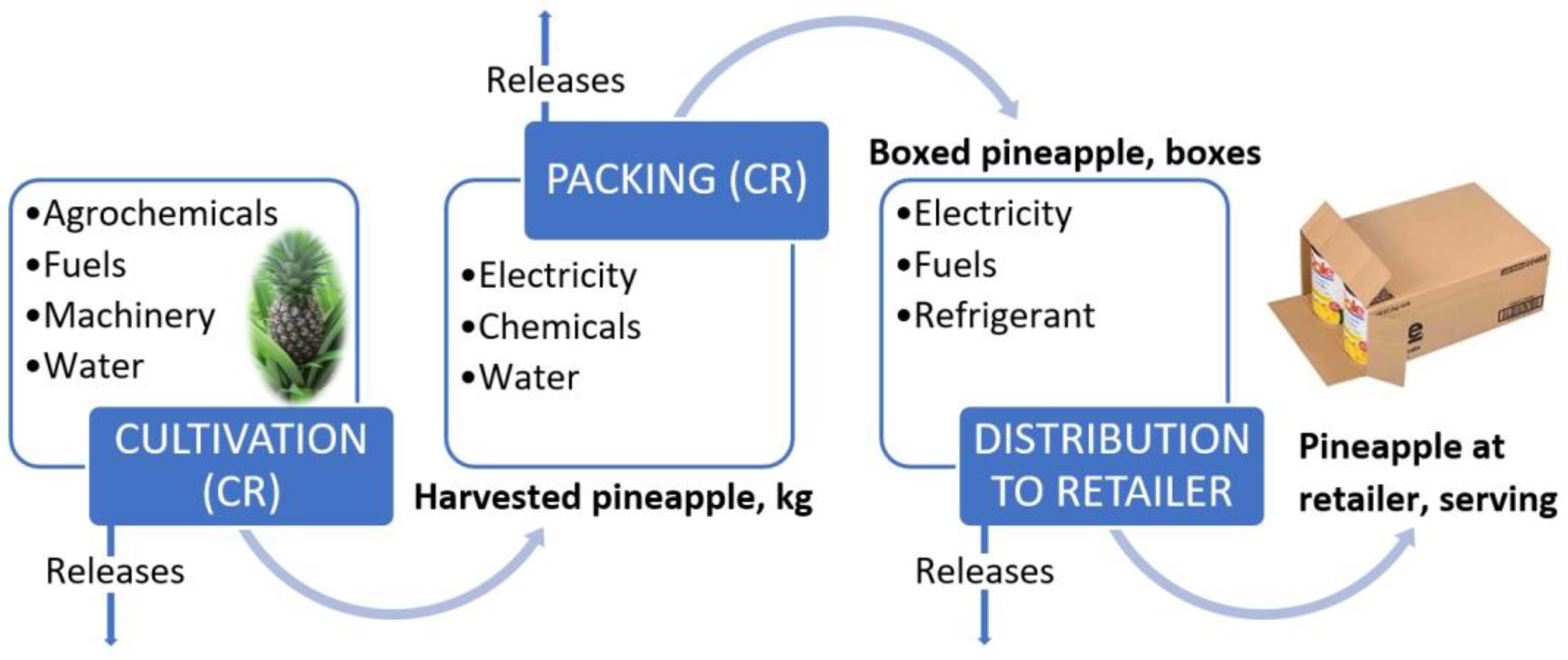
Figure 1. Diagram of fresh pineapple system [9].
2. Main Raw Materials from Pineapple Biomass
2.1. Lignin
Lignin is one of the most abundant biopolymers and is exclusively found in vascular plants, where it fulfills its function by providing structural integrity and mechanical resistance [10][11]. From pineapple peel wastes, lignin is in the range of 15–30% by dry weight [12]. Lignin is formed by a reaction of photosynthesis in cell walls, and it has a resin role because it fills spaces between cellulose and hemicellulose, thereby providing support for lignocellulosic biomass. Lignin is known for its complex structure, and depending on where it was extracted, it has been found multiple unrepeatable structural units [13]. Certain characteristics are typical of most lignins; for example, lignins formed as a result of phenylpropanoid contain most of the methoxyl groups of wood, are resistant to acid hydrolysis and produce vanillin, syringaldehyde, and p hydroxybenzaldehyde when reacting with nitrobenzene in an alkaline solution [10].
As has already been mentioned, lignin comes in different forms and structures, and these can affect the properties and characteristics of its conversion into value-added products. These are usually classified according to the sulfur content thereof, as shown in Figure 2; the lignin with the lowest sulfur content is the most ecologically sound and has more applications due to the smell of other types of lignin [14]. Lignin that contains sulfur is derived from the paper industry and is divided into three categories: Kraft lignin, sulfite lignin, and hydrolyzed lignin. Lignin with no sulfur content is primarily created through biomass conversion technologies and solvent and soda pulping processes; this type of lignin has the highest number of applications in the creation of value-added products [14].
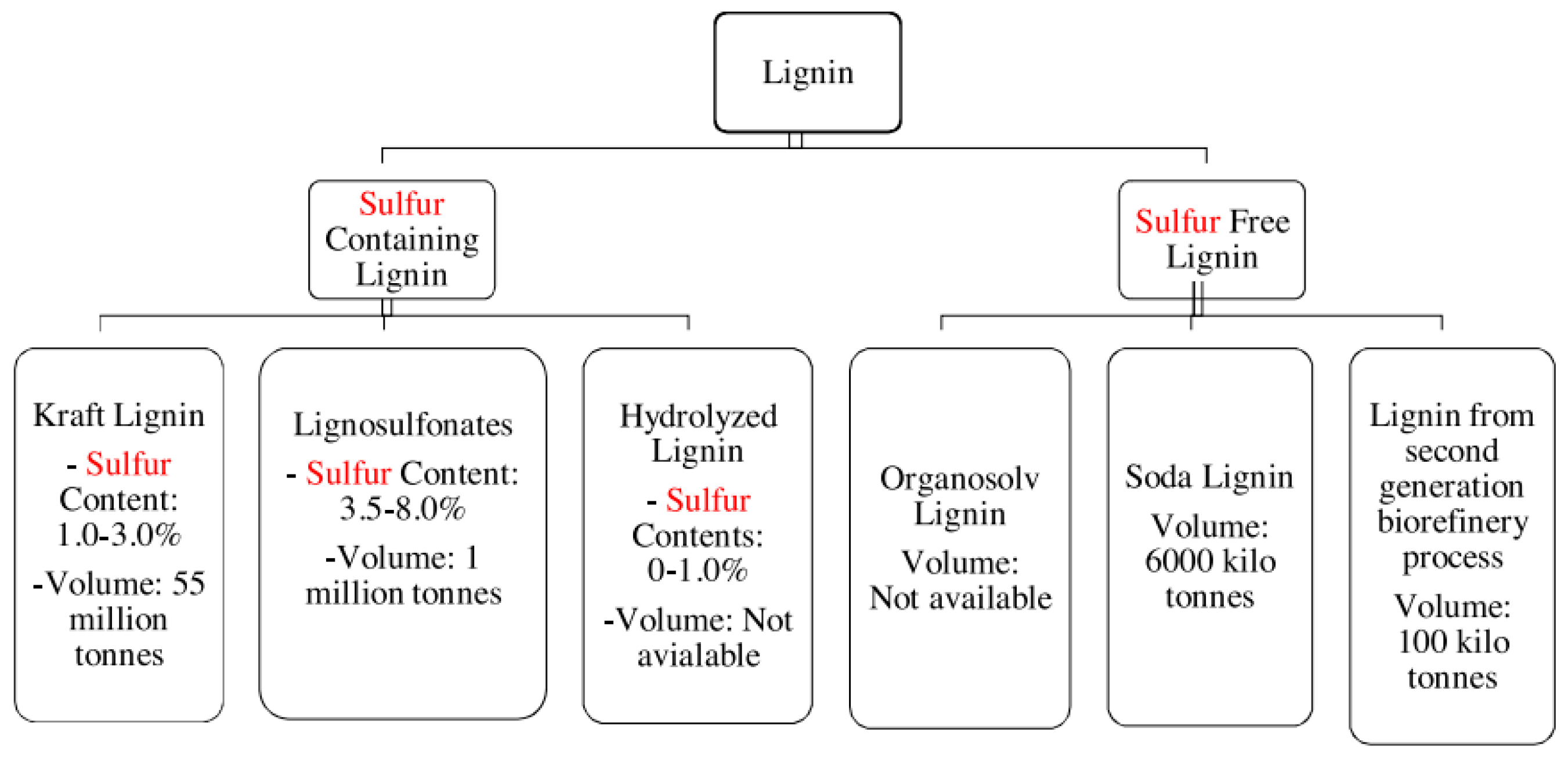
Figure 2. Different sources of lignin and their current volume on scale global [14].
Multiple studies have been conducted to improve lignin extraction from lignocellulosic biomass, devise more effective extraction methods, and enhance the purity of extracted lignin, which employed several different methodologies. One of the greatest challenges in the lignin extraction process is the existence of a lignin–carbohydrate complex, which consists of different reactive components that are covalently bonded to hemicellulose; this is why it is necessary to extract lignin and achieve depolymerization in the most efficient manner possible to break these bonds, even though the reactivity of these components creates new C C bonds after depolymerization that form different intermediates with different properties [12].
There arephysicochemical technology and biological techniques that can be employed during lignin depolymerization: The first is achieved through the use of high temperatures and chemical additives, catalysts, and other compounds, which are commonly used in industrial areas. While the second technique, biological depolymerization, is a slower process, it is more environmentally friendly; this technique uses microorganisms such as bacteria and fungi to degrade lignin due to lignin-modifying enzymes that act by oxidative means, which can be classified according to their specific mechanism-of-action and according to the organism from which they originate [15].
There are also thermochemical techniques that can be employed. The most-used technique is the hydrogenolysis of lignin by means of solvents and catalyst systems. The most-commonly used solvents for this process are water, various alcohols, and compound solvents, which define the efficiency and distribution of products according to the specific characteristics of each compound, such as solubility, polarity, and the capacity of hydrogen supply [16]. There are also a variety of catalyst systems, including but not limited to acidic, basic, metallic, and ionic liquid catalyst systems. In the case of liquid acids, there is significant use of formic acid, sulfuric acid, hydrochloric acid, and phosphoric acid; the most commonly used solid acids are zeolite, metal oxides, metal phosphides, and Lewis acid; and basic catalysts include NaOH, KOH, MgO, and Na2CO3, and metallic catalysts such as Fe, Ni, Cu, and Co are also used [16]. Different systems have been devised that use solvent and catalyst variations, as well as different reaction times and temperatures; some examples of these are shown in Table 1.
Table 1. Conversion of lignin in different solvents [16].
| Feed | Solvent | Catalyst | Reaction Conditions | Major Product | Major Product Yield (%) |
Ref. | |
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Time (h) | ||||||
| Poplar wood sawdust | Alkaline aqueous solution | NiAl alloy | 220 | 3 | Aromatic monomers | 76 | [17] |
| Kraft lignin | Alkaline water | Ni/ZSM-5 zeolite | 200 | 4 | Monomers | 17 | [18] |
| Birch sawdust | water | Pd1Ni4/MIL-100(Fe) | 130–180 | 6 | Phenol + acetophenone | 56.3 | [19] |
| Organosolv lignin | Methanol | Cu20PMO | 300 | 24 | Liquid phase products | 26.3 | [20] |
| Hydrolyzed lignin | Methanol | PD/C, CrCl3 | 300 | 4 | Monomers | 60–86 | [21] |
| Asian lignin | Ethanol | Pt/C | 350 | 2/3 | Lignin-oil | 77.4 | [22] |
| Kraft lignin | Isopropanol | Ni-Cu/H-Beta | 330 | 3 | cycloalkanes | 40.39 | [23] |
| beech dioxasolv lignin |
Methanol | W/C | 200 | 6 | Lignin. Oil | 56.4 | [24] |
| Birch lignin | Aqueous solution | Ru/Nb2O5 | 250 | 20 | Liquid hydrocarbons | 35.5 | [25] |
| Enzymatic lignin | Water | Ru/Nb2O5 | 250 | 20 | Hydrocarbons | 99.6 | [26] |
| Alkali lignin | Ethanol | Ni2P/SiO2 + CuMgAl2O3 | 340 | 4 | Monomers | 53 | [27] |
| Corn stover lignin | N-octane | Ru/Al2O3 + Hf (OTf) | 250 | 4 | Hydrocarbons | >30 | [28] |
The lignin extraction process consists of three steps, which are illustrated in Figure 3. The first step is the extraction of lignin from biomass, which can be achieved by several methods, including hydrolysis, oxidation or reduction fractionation, and a combination of pretreatment methods; followed by this. In the second step, the characterization process is continued by molecular weight determination, nuclear magnetic resonance analysis, and Fourier transform (FTIR). In the third and final step, the conversion process is conducted by different methods to obtain different products [12].
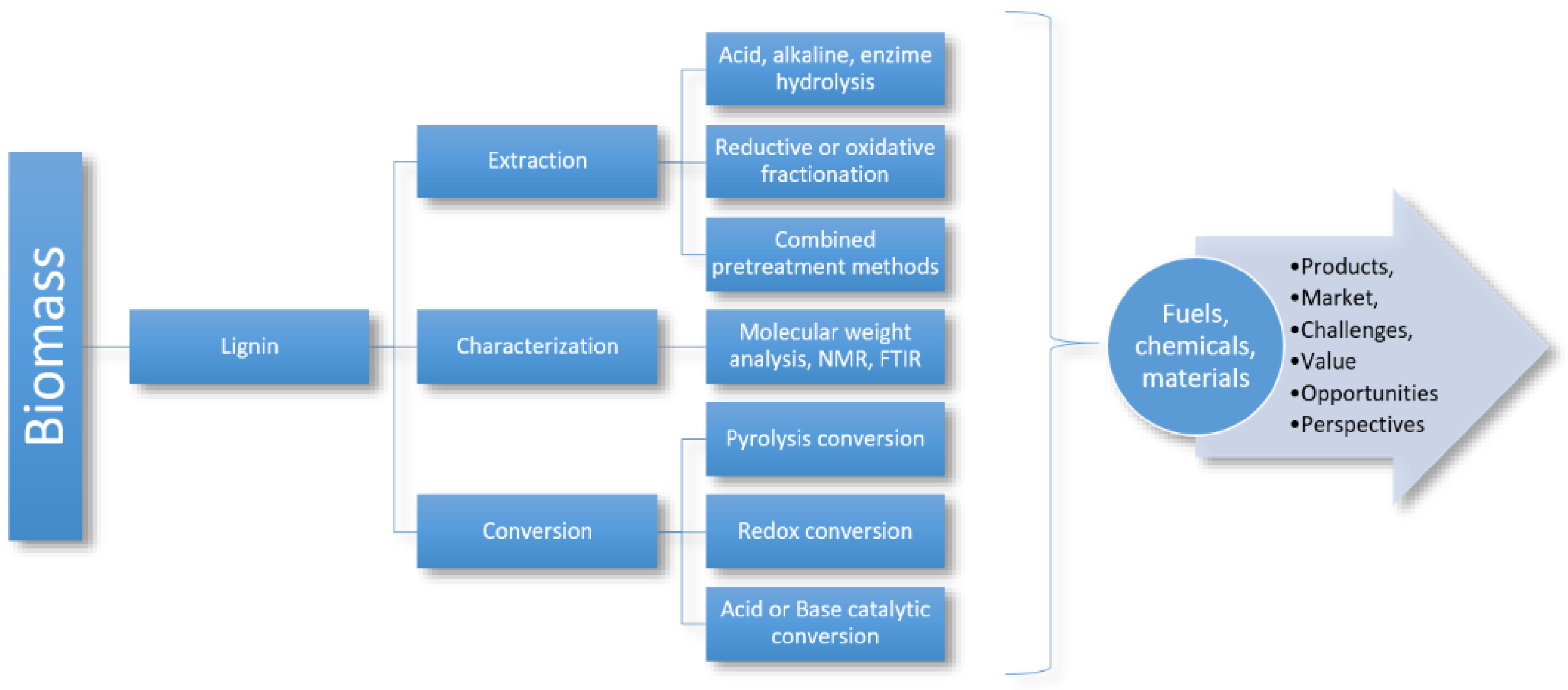
Figure 3. Overview of valorization of lignin to valuable products [12].
Regarding the environmental impact of this process, the use of products made from lignin have been shown to greatly assist efforts to combat climate change and the greenhouse effect, since one of the main by-products reduces the use of fossil fuels. Furthermore, the use of renewable, environmentally friendly biofuels derived from lignin to produce heat, electricity, and automobile fuel can further reduce the carbon footprint of these products and processes [14].
2.2. Cellulose
Like lignin, cellulose is a biopolymer widely found in nature, mostly in plant bodies, some types of algae, the extracellular activities of microorganisms, and the structures of some marine animals. It has a semi-crystalline structure that is based on glycosidic bonds, which vary depending on the source from which the cellulose was obtained, resulting in multiple morphologies and structures that generate different physicochemical properties [29]. Cellulose is a biopolymer composed of groups of two or more repeated glucose units and these can form microfibrils when several cellulose strands are connected to one another. These microfibrils are typically found in the cell walls of trees and plants, and they provide flexibility, strength, and rigidity; the latter is because cellulose is hydrophilic, and absorbing water allows it to create a softer and more flexible wall [30].
The morphology of cellulose is presented in crystalline form, with a strong system of hydrogen bonds in intra and intermolecular forms, which generate a highly ordered, rigid, and strong crystalline structure. Four polymorphic structures of cellulose are currently known—named as I, II, III, and IV—and the mechanisms of each have been studied to understand the different structures of native cellulose, as shown in Figure 4 [31].
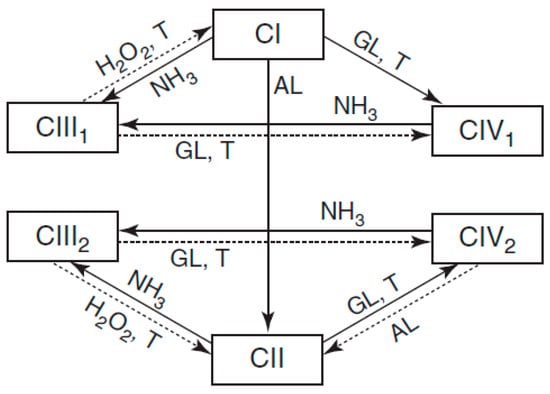
Figure 4. Scheme of transitions between various polymorphic crystalline forms of cellulose CI (native cellulose), CII (cellulose), CIII1 and CIII2 (cellulose III1 and III2), and CIV1 and CIV2 (cellulose IV1 and IV2) [31].
Cellulose is considered a compound with tremendous potential due to certain characteristics it possesses, such as its biodegradable, renewable, hydrophilicity, high absorbent potential, non-toxicity, and mechanical properties and its safety when discarded after use. The wide availability of cellulose in lignocellulosic materials such as wood biomass, coconut, mango, tomato, almonds, and pineapple has made it possible to generate several applications for crude and modified cellulose, with it being most commonly used as an absorbent to eliminate pollutants in wastewater and as a precursor for activated carbon [32]. Cellulose has also been found to have a property that facilitates the separation of solutions of water and oil; this was investigated, and several materials were created to treat oily wastewater in a simpler, more efficient, and environmentally friendly manner than traditional methods that caused other types of pollution and were typically more expensive [33].
Regarding the cellulose present in pineapple waste, more than 55% of the fruit is fibrous waste composed of lignin, hemicellulose, and cellulose, with cellulose representing a large proportion of this waste [34]; as was previously stated, the proportions of each vary according to the source of extraction, as is shown in Table 2 [35].
Table 2. Lignocellulosic material composition [35].
| Lignocellulosic Material | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Pineapple crown (leaves) | 45.53 ± 1.17 | 21.88 ± 0.22 | 13.88 ± 1.70 |
| Pineapple peel | 40.55 ± 1.02 | 28.69 ± 0.35 | 10.01 ± 0.38 |
| Pineapple core | 24.53 ± 1.68 | 28.53 ± 1.37 | 5.78 ± 0.429 |
| Pineapple stubble | 32.2 | 21 | 2.83 |
Due to its great abundance in pineapple residues, several treatments, and pretreatments for cellulose, microcellulose, and nanocellulose have been studied to identify applications in the field of biomaterials. Examples for this are biofuels such as bioethanol that are obtained by extracting cellulose from pineapple bagasse, which can be carried out through initial physical pretreatments of the waste such as drying and washing, followed by acid hydrolysis. After two phases of sulfuric acid for six hours, the fermentation of the obtained cellulose obtained is continued with the use of yeast such as S. cerevisiae, and the bioethanol is finally obtained with a final distillation [36].
The properties of nanocellulose include a high resistance to bending, which is helpful for the reinforcement of polymeric matrices and a great stabilizing potential for suspensions; this is why nanocellulose is used as an excipient in medications for enzymatic immobilization and to provide scaffolding for tissue engineering and biosensors. It is necessary to carry out two different acid hydrolysis processes on cellulose: HCl is added in the first step to obtain microcrystalline cellulose, which is then hydrolyzed with sulfuric acid, thereby reducing the lignin content, and allowing nanocellulose fragments to be obtained [37]. There are several methods to evaluate the treatments, properties, and composition of nanocellulose extracted from pineapple, which make it possible to obtain information of the chemistry, morphology, crystallinity, and thermal properties thereof that are useful to know when considering possible applications in value-added products. Some of the techniques used are high-resolution liquid chromatography, FTIR, scanning electron microscopy (SEM), X-ray diffraction analysis, and thermogravimetric analysis [38].
A study on nanocellulose demonstrated the great thermal stability thereof that makes it an affordable means to create bionanocomposites; it was also shown that cellulose pretreatments could be optimized based on tensile properties, reagent concentrations optimized in hydrolysis increases the amount of cellulose that can be obtained, and an efficient pineapple crushing process can improve mechanical properties of nanocellulose, such as its tensile strength [38]. In another study on the production of nanocellulose from pineapple leaves, acid hydrolysis was used as a pretreatment in conjunction with bleaching to eliminate other hemicellulose and lignin, after which a homogenization process that involved high shear and ultrasounds was carried out to obtain nanocellulose. After the final product was obtained, several studies were conducted, which determined that the degradation temperature of nanocellulose fibers is significantly higher than that of the initial fiber, which indicates that it has great thermal stability and commercial potential [39].
Nanocellulose is currently being investigated for a variety of valuable applications because of its potential for large-scale production, its mechanical properties, and its characteristics such as low thermal expansion, thermal stability, and biodegradability, among others. Some examples of applications include use in adhesives, paper-based materials, cement, food coatings, drilling liquids, transparent and flexible electronics, catalysis support structures, and biomedical materials [40].
2.3. Hemicellulose
Hemicellulose is a mixture of several heteropolysaccharides in branched form, which varies depending on the location origin or extraction. The presence of pentoses, hexoses, and uronic acids stands out because of the amorphous nature of its branched shape, which is why hemicellulose is easier to solubilize and hydrolyze than cellulose and is, therefore, easier to obtain. There are two types of hemicelluloses: xylose, which is mainly found in hardwoods, and glucomannan, mainly found in softwoods. Hemicelluloses are found with lignin and cellulose in the cell walls of plants, where it acts as an interface between these compounds through hydrogen bridges, which allows them to fulfill several functions, such as the storage of substances and regulatory functions, providing structure, and controlling cell expansion [41].
One of the most common applications for all the components of lignocellulosic biomass is the creation of biofuels, such as bioethanol. A study on the role of hemicellulose in this process proved that it can be carried out by enzymatic hydrolysis with a two-phase multiscale process, and it was shown that after an optimal initial mixing that maximizes sugar yields and minimizes costs, said sugars are fermented until bioethanol is obtained with the use of yeasts or bacteria. This process has pretreatment, enzymatic hydrolysis, and fermentation [42]. Associated with this study, a different study indicated that pretreatment could improve the time and efficiency of enzymatic hydrolysis; microwave radiation was employed in conjunction with a hydrothermal-and-alkaline pretreatment, which resulted in a significant improvement in enzyme digestion, and a yield of 89.4% was obtained, compared to untreated residues wherein only 71.6% and 28.4% were obtained [43].
A study on hemicellulose in pineapple peels used an alkali-based method with 5%, 10%, and 15% concentrations and temperatures ranging from 36–65 °C, and a yield of more than 95% of the hemicellulose was obtained under the following conditions: a 15% concentration for 16 h at 45 °C. This study also discussed applying acid hydrolysis directly to the pineapple skin using diluted nitric acid such as xylitol. Finally, the study described the entire process of extracting hemicellulose and converting it into xylooligosaccharides and xylose, which are high-value end products that can be used to create biofuels and other products; this process is presented in Figure 5 [44].
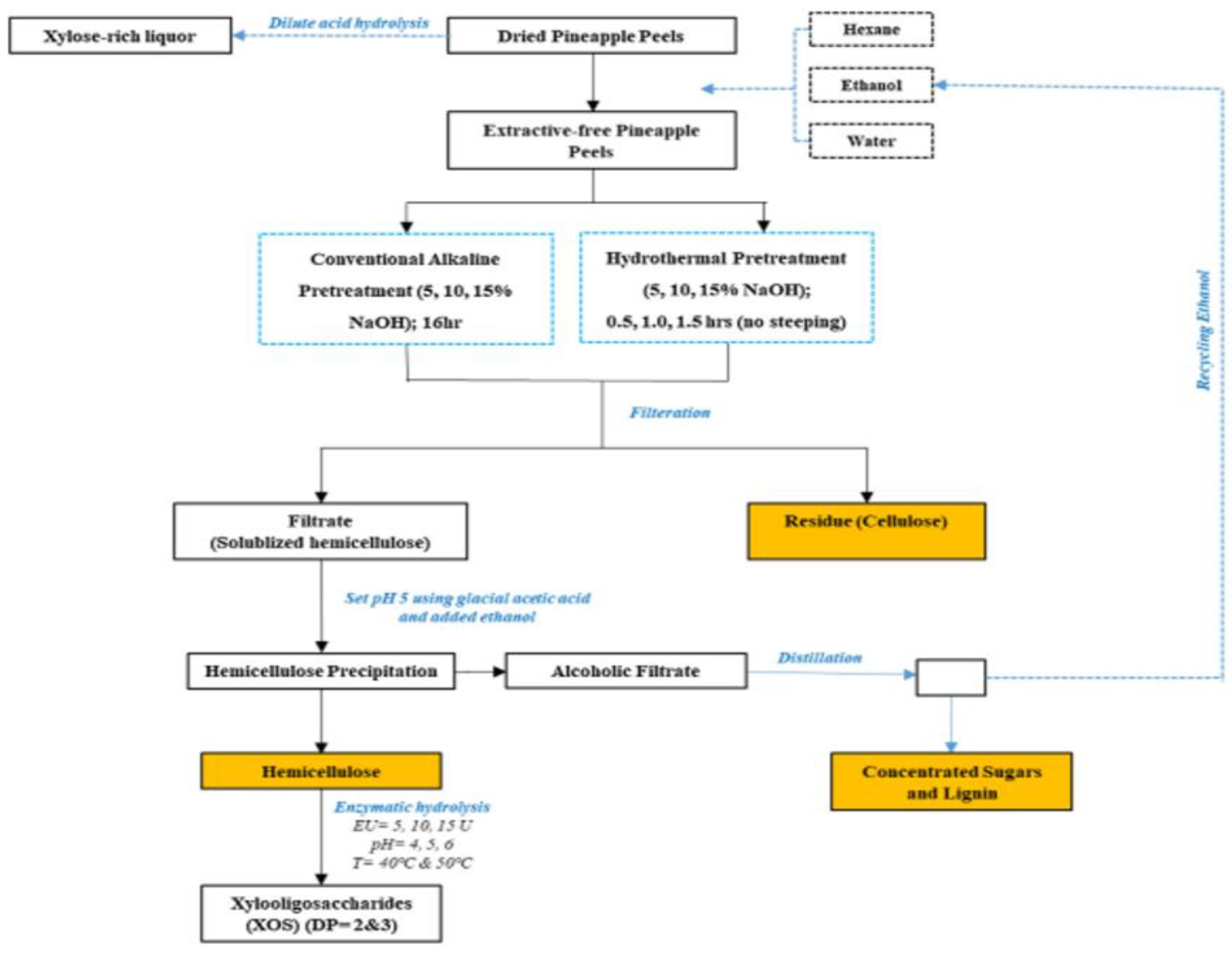
Figure 5. Schematic for extraction of hemicellulose from pineapple peel waste and its valorization into xylooligosaccharides [44].
Associated with this study, a different study affirmed that mass fractionation techniques are necessary to achieve the highest, most efficient recovery of hemicellulose, of which a hydrothermal pretreatment was shown to be the most prominent and valuable due to the use of high temperatures and pressures; this study also discussed several pretreatments of this type, such as supercritical water hydrolysis and subcritical water hydrolysis, as shown in Figure 6; these make it possible to recover a large number of products and result in several applications related to bioresins, bioenergy, hydrogels, bioethanol, food industry, among others [45].
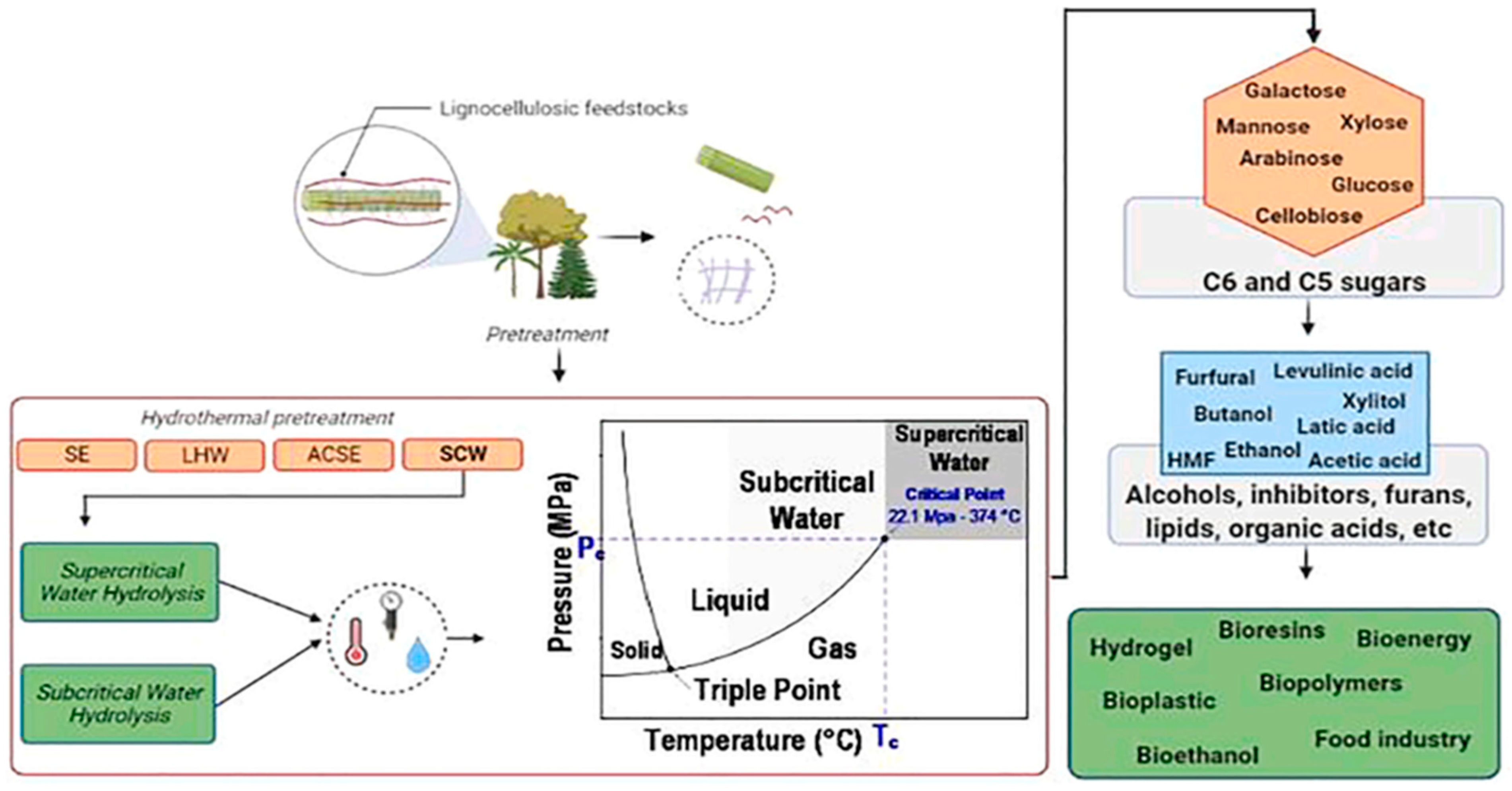
Figure 6. Hydrothermal pretreatments applied to lignocellulosic biomass and the possibility of multi-product recovery [45].
References
- Conferencia de las Naciones Unidas sobre Comercio y Desarrollo-UNCTAD. Available online: https://bit.ly/2W0jbkh (accessed on 30 August 2021).
- Cerrato, I. CEDIA. Available online: https://bit.ly/3lOwtcu (accessed on 30 August 2021).
- Cámara Nacional de Productores y Exportadores de Piña—CANAPEP. Available online: https://bit.ly/3lOQ3VY (accessed on 7 September 2021).
- Food and Agriculture Organization—FAO. Available online: http://www.fao.org/3/cb0834es/CB0834ES.pdf (accessed on 31 August 2021).
- Red Latinoamericana de Investigadores en Cadenas Globales de Mercancías—REDILACG. Available online: http://www.redilacg.org/pina-cr (accessed on 30 August 2021).
- Vinyet, N. PINDECO. Available online: https://bit.ly/3o2DZ6a (accessed on 31 August 2021).
- Morales-Abarca, L.F. Production and yield of the pineapple (Ananas comosus) crop in Costa Rica, 1984–2014 period. Agronegocios 2018, 4, 2.
- Cámara Nacional de Productores y Exportadores de Piña—CANAPEP. Available online: https://bit.ly/3CCeY5R (accessed on 26 August 2021).
- Ingwersen, W.W. Life cycle assessment of fresh pineapple from Costa Rica. J. Clean. Prod. 2012, 35, 152–163.
- Chávez-Sifontes, M.; Domine, M.E. Lignina, estructura y aplicaciones: Métodos de despolimerización para la obtención de derivados aromáticos de interés industrial. Av. Cienc. Ing. 2013, 4, 15–46.
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2019, 56, 75–81.
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461.
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233.
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526.
- Iram, A.; Berenjian, A.; Demirci, A. A Review on the Utilization of Lignin as a Fermentation Substrate to Produce Lignin-Modifying Enzymes and Other Value-Added Products. Molecules 2021, 26, 2960.
- Ye, K.; Liu, Y.; Wu, S.; Zhuang, J. A review for lignin valorization: Challenges and perspectives in catalytic hydrogenolysis. Ind. Crops Prod. 2021, 172, 114008.
- Wang, D.; Wang, Y.; Li, X.; Chen, L.; Li, G.; Li, X. Lignin valorization: A novel in situ catalytic hydrogenolysis method in alkaline aqueous solution. Energy Fuels 2018, 32, 7643–7651.
- Qi, S.-C.; Hayashi, J.-I.; Kudo, S.; Zhang, L. Catalytic hydrogenolysis of kraft lignin to monomers at high yield in alkaline water. Green Chem. 2017, 19, 2636–2645.
- Zhang, J.-W.; Lu, G.-P.; Cai, C. Self-hydrogen transfer hydrogenolysis of β-O-4 linkages in lignin catalyzed by MIL-100 (Fe) supported Pd–Ni BMNPs. Green Chem. 2017, 19, 4538–4543.
- Barta, K.; Matson, T.D.; Fettig, M.L.; Scott, S.L.; Iretskii, A.V.; Ford, P.C. Catalytic disassembly of an organosolv lignin via hydrogen transfer from supercritical methanol. Green Chem. 2010, 12, 1640–1647.
- Shu, R.; Zhang, Q.; Ma, L.; Xu, Y.; Chen, P.; Wang, C.; Wang, T. Insight into the solvent, temperature and time effects on the hydrogenolysis of hydrolyzed lignin. Bioresour. Technol. 2016, 221, 568–575.
- Kim, J.Y.; Park, J.; Kim, U.J.; Choi, J.W. Conversion of lignin to phenol-rich oil fraction under supercritical alcohols in the presence of metal catalysts. Energy Fuels 2015, 29, 5154–5163.
- Kong, L.; Liu, C.; Gao, J.; Wang, Y.; Dai, L. Efficient and controllable alcoholysis of Kraft lignin catalyzed by porous zeolite-supported nickel-copper catalyst. Bioresour. Technol. 2019, 276, 310–317.
- Guo, H.; Qi, Z.; Liu, Y.; Xia, H.; Li, L.; Huang, Q.; Li, C. Tungsten-based catalysts for lignin depolymerization: The role of tungsten species in C–O bond cleavage. Catal. Sci. Technol. 2019, 9, 2144–2151.
- Shao, Y.; Xia, Q.; Dong, L.; Liu, X.; Han, X.; Parker, S.F.; Wang, Y. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 2017, 8, 16104.
- Xin, Y.; Dong, L.; Guo, Y.; Liu, X.; Hu, Y.; Wang, Y. Correlation of the catalytic performance with Nb2O5 surface properties in the hydrodeoxygenation of lignin model compound. J. Catal. 2019, 375, 202–212.
- Korányi, T.I.; Huang, X.; Coumans, A.E.; Hensen, E.J. Synergy in lignin upgrading by a combination of Cu-based mixed oxide and Ni-phosphide catalysts in supercritical ethanol. ACS Sustain. Chem. Eng. 2017, 5, 3535–3543.
- Wang, H.; Wang, H.; Kuhn, E.; Tucker, M.P.; Yang, B. Production of Jet Fuel-Range Hydrocarbons from Hydrodeoxygenation of Lignin over Super Lewis Acid Combined with Metal Catalysts. ChemSusChem 2018, 11, 285–291.
- Gañán, P.; Zuluaga, R.; Castro, C.; Restrepo-Osorio, A.; Cock, J.V.; Osorio, M.; Montoya, Ú.; Vélez, L.; Álvarez, C.; Correa, C.; et al. Celulosa: Un polímero de siempre con mucho futuro. Rev. Colomb. Mater. 2017, 11, 11.
- Keller, S. American Chemical Society—ACS. Available online: https://bit.ly/3vhNf8i (accessed on 15 October 2021).
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for extraction of nanocellulose from various sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 1, pp. 1–51.
- Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076.
- Zhao, X.-Q.; Wahid, F.; Cui, J.-X.; Wang, Y.-Y.; Zhong, C. Cellulose-based special wetting materials for oil/water separation: A review. Int. J. Biol. Macromol. 2021, 185, 890–906.
- Morales-Vázquez, J.G.; López-Zamora, L.; Aguilar-Uscanga, M.G. Ethanol Production from Pineapple Waste. Ph.D. Thesis, Instituto Tecnológico de Orizaba, Orizaba, Mexico, 2020.
- Sánchez-Pardo, M.E.; Ramos-Cassellis, M.E.; Mora-Escobedo, R.; Jiménez-García, E. Chemical characterization of the industrial residues of the pineapple (Ananas comosus). J. Agric. Chem. Environ. 2014, 3, 53–56.
- Segura, A.; Manriquez, A.; Santos, D.; Ambriz, E.; Casas, P.; Muñoz, A.S. Obtención de bioetanol a partir de residuos de cascara de piña (Ananas comosus). Jovenes Cienc. 2020, 8, 1–8.
- Camacho, M.; Ureña, Y.R.C.; Lopretti, M.; Carballo, L.B.; Moreno, G.; Alfaro, B.; Baudrit, J.R.V. Synthesis and characterization of nanocrystalline cellulose derived from pineapple peel residues. J. Renew. Mater. 2017, 5, 271–279.
- Rambabu, N.; PAnthapulakkal, S.; Sain, M.; Dalai, A.K. Production of nanocellulose fibers from pinecone biomass: Evaluation and optimization of chemical and mechanical treatment conditions on mechanical properties of nanocellulose films. Ind. Crops Prod. 2016, 83, 746–754.
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Asrofi, M. Production of nanocellulose from pineapple leaf fibers via high-shear homogenization and ultrasonication. Fibers 2018, 6, 28.
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of cellulose nanomaterials, their capabilities and applications. JOM 2016, 68, 2383–2394.
- Rivas-Siota, S. Biomass Hemicellulose Valorization. Doctoral Dissertation, Universidade de Vigo, Vigo, Spain, 2014.
- Dutta, S.K.; Chakraborty, S. Multiscale dynamics of hemicellulose hydrolysis for biofuel production. Ind. Eng. Chem. Res. 2019, 58, 8963–8978.
- Sun, S.C.; Wang, P.F.; Cao, X.F.; Sun, S.N.; Wen, J.L. An integrated pretreatment for accelerating the enzymatic hydrolysis of poplar and improving the isolation of co-produced hemicelluloses. Ind. Crops Prod. 2021, 173, 114101.
- Banerjee, S.; Patti, A.F.; Ranganathan, V.; Arora, A. Hemicellulose based biorefinery from pineapple peel waste: Xylan extraction and its conversion into xylooligosaccharides. Food Bioprod. Process. 2019, 117, 38–50.
- Scapini, T.; dos Santos, M.S.; Bonatto, C.; Wancura, J.H.; Mulinari, J.; Camargo, A.F.; Klanovicz, N.; Zabot, G.L.; Tres, M.V.; Fongaro, G.; et al. Hydrothermal pretreatment of lignocellulosic biomass for hemicellulose recovery. Bioresour. Technol. 2021, 342, 126033.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
2 times
(View History)
Update Date:
18 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




