
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Junpei Zhou | -- | 1225 | 2023-01-17 15:24:36 | | | |
| 2 | Conner Chen | + 2 word(s) | 1227 | 2023-01-18 04:38:23 | | | | |
| 3 | Conner Chen | + 6 word(s) | 1233 | 2023-01-19 02:02:34 | | |
Video Upload Options
Although there are multiple catalytic strategies for the conversion of major ginsenoside, biotransformation using ginsenosidases is more advantageous for the targeted production of minor ginsenosides due to the high specificity, high selectivity, high catalytic efficiency, high product purity, mild reaction conditions and clear catalytic process, which is one of the current research topics in ginsenoside biotransformation. Ginsenosidases are commonly classified as type I, type II, type III, type IV and type V according to the hydrolysis site, residues and types of sugar moieties of ginsenosides. Ginsenosidase type I simultaneously hydrolyze the glycosyl residues linked to C-20 and C-3 positions in the PPD-type ginsenosides, yielding minor ginsenosides with only one glucose residue or other glycosyl residues. Ginsenosidase type II hydrolyzes the glycosyl residues at the C-20 position of protopanaxadiol (PPD)-and protopanaxatriol (PPT)-type saponins. Ginsenosidase type III hydrolyzes the sugar moieties attached to the C-3 position of PPD-type ginsenosides. Ginsenosidase type IV only hydrolyzes the sugar moieties linked to C-6 in PPT-type ginsenosides. Ginsenosidase type V hydrolyzes the glycosyl moieties attached to the C-20 and C-6 positions of PPT-type ginsenosides to yield the corresponding aglycones.
1. Introduction
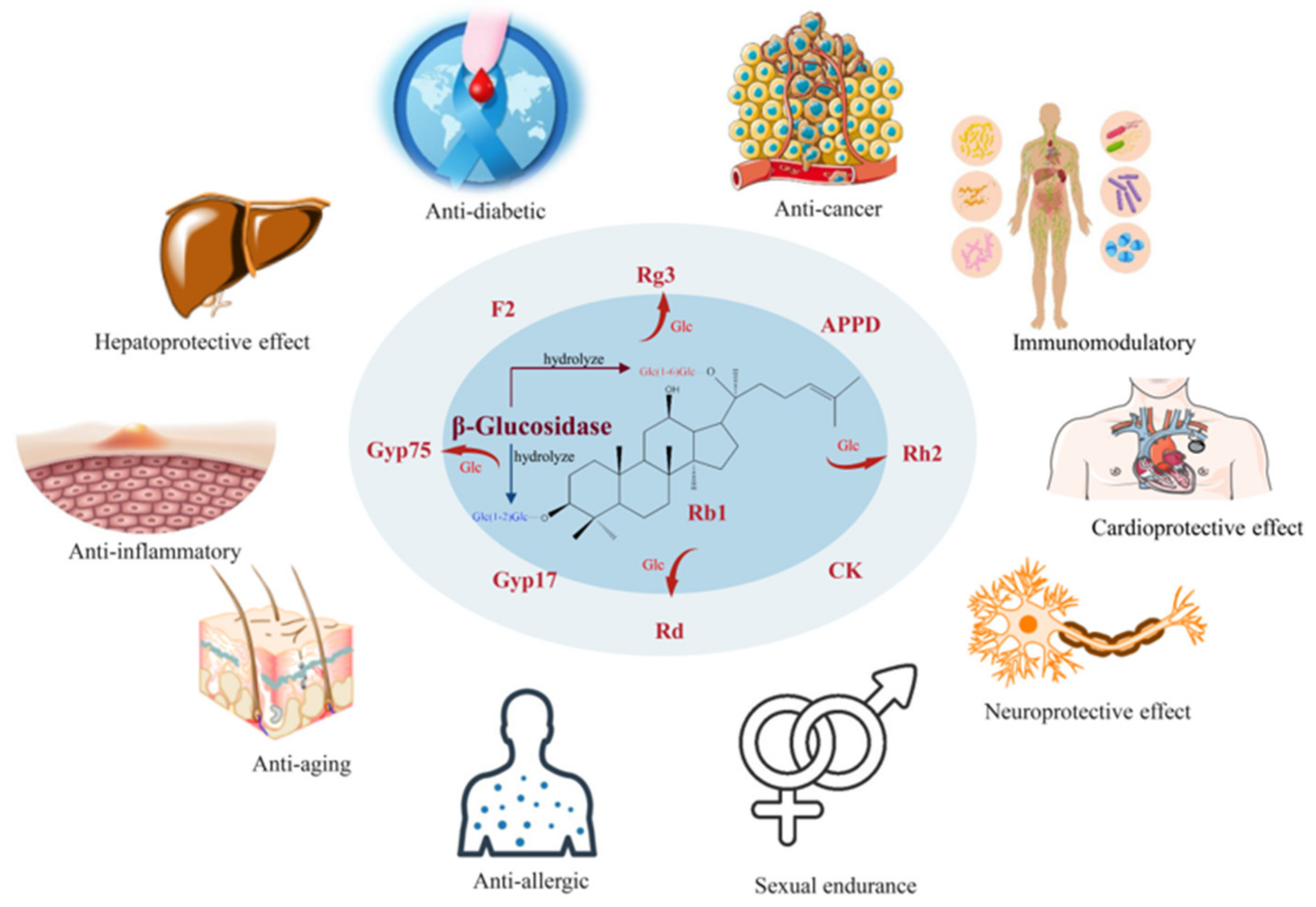
2. Classification of Ginsenosidases
References
- Chu, L.L.; Bae, H. Bacterial endophytes from ginseng and their biotechnological application. J. Ginseng Res. 2022, 46, 1–10.
- Liu, H.B.; Lu, X.; Hu, Y.; Fan, X.H. Chemical constituents of Panax ginseng and Panax notoginseng explain why they differ in therapeutic efficacy. Pharmacol. Res. 2020, 161, 105263.
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and their biosynthesis. Acta Pharm. Sin. B 2021, 11, 1813–1834.
- Zhang, J.J.; Tong, Y.L.; Lu, X.; Dong, F.M.; Ma, X.X.; Yin, S.Y.; He, Y.; Liu, Y.H.; Liu, Q.C.; Fan, D.D. A derivant of ginsenoside CK and its inhibitory effect on hepatocellular carcinoma. Life Sci. 2022, 304, 120698.
- Zhou, P.; Xie, W.; He, S.; Sun, Y.; Meng, X.; Sun, G.; Sun, X. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells 2019, 8, 204.
- Wang, J.; Zeng, L.; Zhang, Y.; Qi, W.X.; Wang, Z.Y.; Tian, L.; Zhao, D.Q.; Wu, Q.B.; Li, X.Y.; Wang, T. Pharmacological properties, molecular mechanisms and therapeutic potential of ginsenoside Rg3 as an antioxidant and anti-inflammatory agent. Front. Pharmacol. 2022, 13, 975784.
- Chen, Y.Y.; Liu, Q.P.; An, P.; Jia, M.; Luan, X.; Tang, J.Y.; Zhang, H. Ginsenoside Rd: A promising natural neuroprotective agent. Phytomedicine 2022, 95, 153883.
- Liu, T.; Zhu, L.; Wang, L. A narrative review of the pharmacology of ginsenoside compound K. Ann. Transl. Med. 2022, 10, 234.
- Chopra, P.; Chhillar, H.; Kim, Y.J.; Jo, I.H.; Kim, S.T.; Gupta, R. Phytochemistry of ginsenosides: Recent advancements and emerging roles. Crit. Rev. Food Sci. Nutr. 2021, 1–28.
- Ma, Z.; Mi, Y.; Han, X.; Li, H.; Tian, M.; Duan, Z.; Fan, D.; Ma, P. Transformation of ginsenoside via deep eutectic solvents based on choline chloride as an enzymatic reaction medium. Bioprocess Biosyst. Eng. 2020, 43, 1195–1208.
- Geraldi, A.; Ni’matuzahroh, F.; Cui, C.H.; Nguyen, T.T.; Kim, S.C. Enzymatic biotransformation of ginsenoside Rb1 by recombinant β-glucosidase of bacterial isolates from Indonesia. Biocatal. Agric. Biotechnol. 2020, 23, 101449.
- Wu, L.; Jin, Y.; Yin, C.; Bai, L. Co-transformation of Panax major ginsenosides Rb1 and Rg1 to minor ginsenosides C-K and F1 by Cladosporium cladosporioides. J. Ind. Microbiol. Biotechnol. 2012, 39, 521–527.
- Won, H.J.; Kim, H.I.; Park, T.; Kim, H.; Jo, K.; Jeon, H.; Ha, S.J.; Hyun, J.M.; Jeong, A.; Kim, J.S.; et al. Non-clinical pharmacokinetic behavior of ginsenosides. J. Ginseng Res. 2019, 43, 354–360.
- Yang, L.; Zou, H.; Gao, Y.; Luo, J.; Xie, X.; Meng, W.; Zhou, H.; Tan, Z. Insights into gastrointestinal microbiota-generated ginsenoside metabolites and their bioactivities. Drug Metab. Rev. 2020, 52, 125–138.
- Li, W.; Jiang, Y.; Liu, Y.; Li, C.; Fan, D. Biocatalytic strategies in producing ginsenoside by glycosidase-A review. Chin. J. Biotechnol. 2019, 35, 1590–1606.
- Wong, A.S.; Che, C.M.; Leung, K.W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272.
- Li, W.N.; Fan, D.D. Biocatalytic strategies for the production of ginsenosides using glycosidase: Current state and perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 3807–3823.
- Renchinkhand, G.; Magsar, U.; Bae, H.C.; Choi, S.H.; Nam, M.S. Identification of β-glucosidase activity of Lentilactobacillus buchneri URN103L and its potential to convert ginsenoside Rb1 from Panax ginseng. Foods 2022, 11, 529.
- Kim, D.H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J. Ginseng Res. 2018, 42, 255–263.
- Yang, X.D.; Yang, Y.Y.; Ouyang, D.S.; Yang, G.P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220.
- Ketudat Cairns, J.R.; Esen, A. β-Glucosidases. Cell Mol. Life Sci 2010, 67, 3389–3405.
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049.
- Zheng, M.M.; Xu, F.X.; Li, Y.J.; Xi, X.Z.; Cui, X.W.; Han, C.C.; Zhang, X.L. Study on transformation of ginsenosides in different methods. Biomed Res. Int. 2017, 2017, 8601027.
- Quan, L.H.; Min, J.W.; Jin, Y.; Wang, C.; Kim, Y.J.; Yang, D.C. Enzymatic biotransformation of ginsenoside Rb1 to compound K by recombinant β-glucosidase from Microbacterium esteraromaticum. J. Agric. Food Chem. 2012, 60, 3776–3781.
- Yu, H.; Liu, Q.; Zhang, C.; Lu, M.; Fu, Y.; Im, W.T.; Lee, S.T.; Jin, F. A new ginsenosidase from Aspergillus strain hydrolyzing 20-O-multi-glycoside of PPD ginsenoside. Process Biochem. 2009, 44, 772–775.
- Jin, X.F.; Yu, H.S.; Wang, D.M.; Liu, T.Q.; Liu, C.Y.; An, D.S.; Im, W.T.; Kim, S.G.; Jin, F.X. Kinetics of a cloned special ginsenosidase hydrolyzing 3-O-glucoside of multi-protopanaxadiol-type ginsenosides, named ginsenosidase type III. J. Microbiol. Biotechnol. 2012, 22, 343–351.
- Wang, D.M.; Yu, H.S.; Song, J.G.; Xu, Y.F.; Liu, C.Y.; Jin, F.X. A novel ginsenosidase from an Aspergillus strain hydrolyzing 6-O-multi-glycosides of protopanaxatriol-type ginsenosides, named ginsenosidase type IV. J. Microbiol. Biotechnol. 2011, 21, 1057–1063.
- Cui, C.H.; Kim, S.C.; Im, W.T. Characterization of the ginsenoside-transforming recombinant β-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl. Microbiol. Biotechnol. 2013, 97, 649–659.





