
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vishnu D. Rajput | -- | 3612 | 2023-01-17 10:14:34 | | | |
| 2 | Catherine Yang | Meta information modification | 3612 | 2023-01-17 10:28:05 | | | | |
| 3 | Catherine Yang | Meta information modification | 3612 | 2023-01-18 01:42:40 | | |
Video Upload Options
The treatment of wastewater is an expensive and energy-extensive practice that not only ensures the power generation requirements to sustain the current energy demands of an increasing human population but also aids in the subsequent removal of enormous quantities of wastewater that need to be treated within the environment. Thus, renewable energy source-based wastewater treatment is one of the recently developing techniques to overcome power generation and environmental contamination issues. In wastewater treatment, microbial fuel cell (MFC) technology has demonstrated a promising potential to evolve as a sustainable approach, with the simultaneous recovery of energy and nutrients to produce bioelectricity that harnesses the ability of electrogenic microbes to oxidize organic contaminants present in wastewater.
1. General Features, Types, and Designs of MFCs
1.1. Types of MFCs
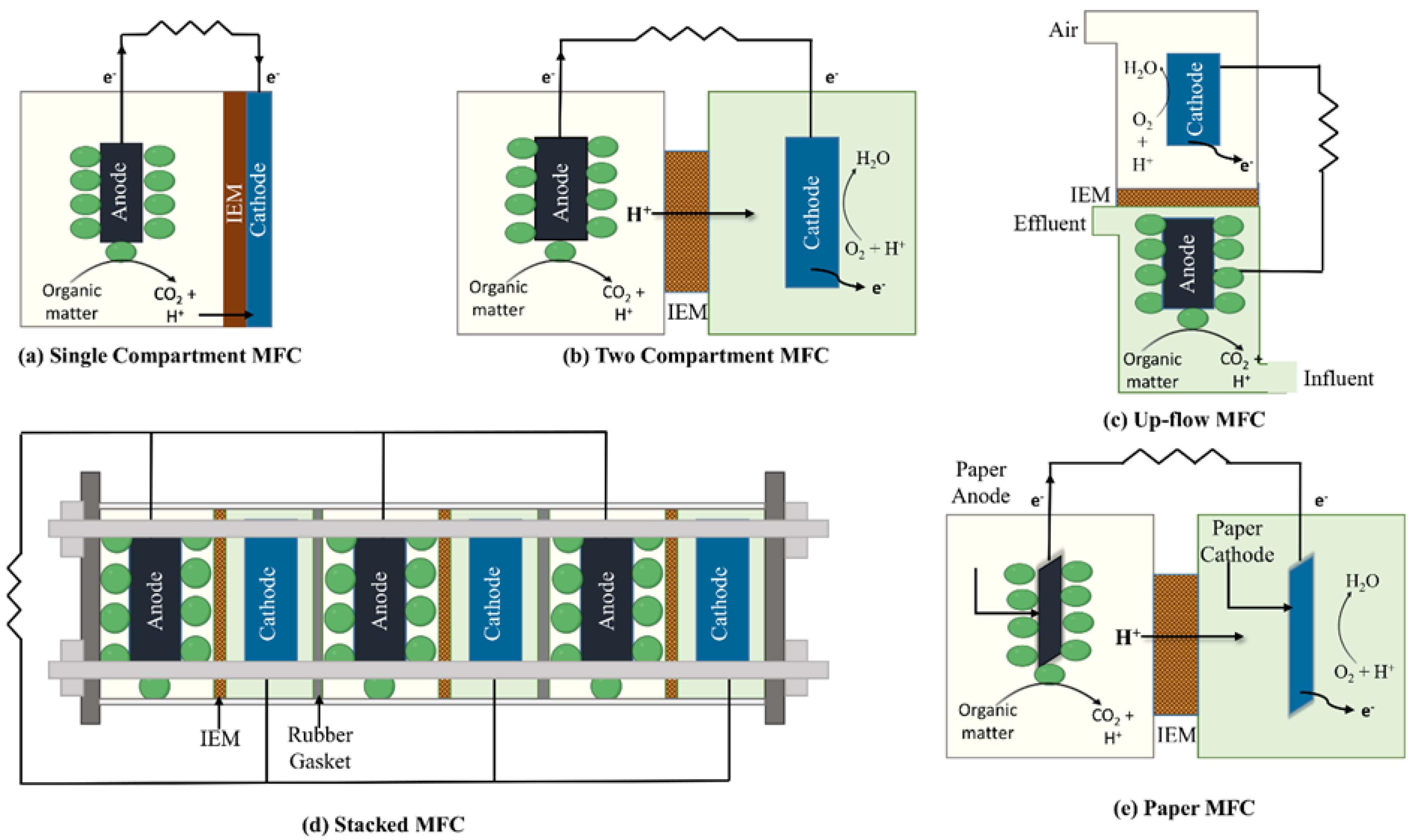
1.1.1. Single-Compartment MFCs
1.1.2. Two-Compartment MFCs
1.1.3. Up-Flow MFCs
1.1.4. Stacked MFCs
1.1.5. Paper MFCs
1.2. Substrate and Microorganisms That Are Used in MFCs
2. Application/Performance of MFCs in Wastewater Treatment
| S. No. | Inoculum and Substrate | Type of MFC | Electrode Material | Power Density/Current Density/Voltage | Treatment Efficiency | Reference |
|---|---|---|---|---|---|---|
| 1 | Swine wastewater manure |
Two-chambered | Carbon cloth | 13 mW/m2 | TCOD: 83%, CE: 0.3% |
[31] |
| 2 | Agriculture wastewater (Human feces wastewater) |
Two-chambered | -Anode: carbon paper -Cathode: carbon paper with 40% platinum |
70.8 mW/m2 | TCOD: 71.0%, SCOD: 88.0%, NH4+: 44.0% |
[32] |
| 3 | Domestic and olive mill wastewater | Single-chambered air cathode | -Anode: graphite fiber brush. -Cathodes 7 cm2 (total exposed surface area) |
124.6 mW m−2 | TCOD: 65.0% BOD: 50.0%, CE: 29% |
[33] |
| 4 | Dairy wastewater (COD of 1000 mg/L) inoculated by activated sludge from the dairy WWTP | Annular single chambered | -Graphite-coated stainless-steel mesh anode -Cathode: carbon cloth type B |
20.2 W/m3 | COD: 91%, CE: 26.87% |
[28] |
| 5 | Synthetic wastewater | Up-flow constructed wetland (UCW-MFC) | -anode: graphite -cathode: magnesium |
15.1 mW/m2 | COD: 92.1%, NH4+: 93.2%, NO3−: 81.1%, CE: 1.64% |
[34] |
| 6 | Industrial wastewater | Dual chambered anaerobic MFCs | Anode and cathode | 260 mW/m2 | TCOD: 87%, SCOD: 79%, TSS: 72% |
[35] |
| 7 | Acetate | Single-chambered MFC | Substrate as a source of carbon to stimulate electroactive bacteria |
506 mW/m2 | CE (72.3%), butyrate (43.0%), propionate (36.0%), and glucose (15%) |
[36] |
| 8 | Arabitol | Single-chambered MFC | Co substrate in a single chamber | 0.68 mA/cm2 | COD: >91% | [37] |
| 9 | Cysteine | MFC with carbon paper electrodes (11.25 cm2) dual chamber | Co-substrate | 36 mW/m2 | - | [36] |
| 10 | Common effluent treatment plant (CETP) wastewater | H-type, dual chamber, mediator-less MFC | graphite plates | 0.6 V | COD: 50% | [38] |
| 11 | Sodium benzoate (0.721 g/L) | H-type, dual chamber, mediator-less MFC | graphite plates | 0.8 V | COD: 89% | [38] |
2.1. Factors Affecting Performances of MFCs during Wastewater Treatment
2.1.1. Electrode Properties
| S. No. | Material Used | Anode/ Cathode |
Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| 1 | Graphite rods | Anode | High conductivity, chemical stability, low cost, and easy to handle | Surface area is difficult to increase | [40] |
| 2 | Graphite brushes |
Anode | Easy to construct and more specific area | Clogging issues | [41] |
| 3 | Carbon cloth | Anode | Large porosity relatively | Not cost efficient | [42] |
| 4 | Carbon paper | Anode | Easy to construct wire connection | Brittle | [43] |
| 5 | Carbon felt | Anode | Enormous surface area | Elevated resistance | [44] |
| 6 | Reticulated vitreous carbon |
Anode | High electrical conductivity | Delicate and large resistance | [8] |
| 7 | Stainless steel | Anode | High conductivity, cost efficient, and easily accessible | Low surface area, compatibility issues, can get corroded |
[45] |
| 8 | Pt-based catalyst | Cathode | High surface area and low potential for the oxygen reduction reaction | pH sensitivity, sulfide poisoning, and non-sustainability |
[46] |
| 9 | Non-Pt-based catalyst | Cathode | pH control, no sulfide poisoning, and non-sustainability | Compromised electron transfer | [47] |
| 10 | Carbon Nano tubes | Cathode | High surface area and power density | Voltage losses | [48] |
| 11 | Palladium | Cathode | Excellent catalytic properties and low cost | Very low oxygen reduction reaction overpotential for catalytic hydrogen production | [49] |
| 12 | Aerobic biocathode | Cathode | Production of methane, ethanol, and formic acid via microbes and application as a biosensor for BOD detection | Loss of electrons through oxygen | [50] |
| 13 | Anaerobic biocathode | Cathode | Prevention of loss of electrons via anodic end | Biofilms catalyze the reduction of chemically active species |
[14] |
| 14 | Cathode with metal-free catalyst | Cathode | Cheap materials, catalytic activity, stability | Superior electrocatalytic activity, with lower overpotential and prolonged stability for ORR |
[47] |
2.1.2. pH
2.1.3. Temperature
2.1.4. Aeration
3. Different Products’ Recovery from Wastewater Using MFCs
References
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003.
- Kumar, R.; Singh, L.; Zularisam, A.; Hai, F.I. Microbial fuel cell is emerging as a versatile technology: A review on its possible applications, challenges and strategies to improve the performances. Int. J. Energy Res. 2018, 42, 369–394.
- ElMekawy, A.; Hegab, H.M.; Vanbroekhoven, K.; Pant, D. Techno-productive potential of photosynthetic microbial fuel cells through different configurations. Renew. Sustain. Energy Rev. 2014, 39, 617–627.
- Kumar, R.; Singh, L.; Zularisam, A.W. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sustain. Energy Rev. 2016, 56, 1322–1336.
- Mercuri, E.G.F.; Kumata, A.Y.J.; Amaral, E.B.; Vitule, J.R.S. Energy by Microbial Fuel Cells: Scientometric global synthesis and challenges. Renew. Sustain. Energy Rev. 2016, 65, 832–840.
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403.
- GajendraPrasad, J.; Panda, S. Microbial Fuel Cells: Types of MFC and Different Source of Substrate. IntJ Latest Technol Eng Mgt App Sc 2018, 7, 158–165.
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzym. Microb. Technol. 2002, 30, 145–152.
- Min, B.; Kim, J.; Oh, S.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968.
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555.
- Pham, C.A.; Jung, S.J.; Phung, N.T.; Lee, J.; Chang, I.S.; Kim, B.H.; Yi, H.; Chun, J. A novel electrochemically active and Fe (III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 2003, 223, 129–134.
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814.
- Tremouli, A.; Antonopoulou, G.; Bebelis, S.; Lyberatos, G. Operation and characterization of a microbial fuel cell fed with pretreated cheese whey at different organic loads. Bioresour. Technol. 2013, 131, 380–389.
- Park, D.H.; Zeikus, J.G. Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol. Bioeng. 2003, 81, 348–355.
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690.
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232.
- Winfield, J.; Gajda, I.; Greenman, J.; Ieropoulos, I. A review into the use of ceramics in microbial fuel cells. Bioresour. Technol. 2016, 215, 296–303.
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous Electricity Generation at High Voltages and Currents Using Stacked Microbial Fuel Cells. Environ. Sci. Technol. 2006, 40, 3388–3394.
- Aelterman, P.; Rabaey, K.; Clauwaert, P.; Verstraete, W. Microbial fuel cells for wastewater treatment. Water Sci. Technol. 2006, 54, 9–15.
- Lee, S.H.; Ban, J.Y.; Oh, C.-H.; Park, H.-K.; Choi, S. A solvent-free microbial-activated air cathode battery paper platform made with pencil-traced graphite electrodes. Sci. Rep. 2016, 6, 28588.
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Microbial fuel cells based on carbon veil electrodes: Stack configuration and scalability. Int. J. Energy Res. 2008, 32, 1228–1240.
- Das, S.; Kungwani, N. Recent developments in microbial fuel cells: A review. J. Sci. Ind. Res. 2010, 69, 727–731.
- Sa’adu, L.; Garba, N.A.; Balarabe, M.D. A Review on Electrode Materials in Microbial Fuel Cell Fabrication. Int. J. Sci. Glob. Sustain. 2019, 5, 5.
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543.
- Luo, J.; Chi, M.; Wang, H.; He, H.; Zhou, M. Electrochemical surface modification of carbon mesh anode to improve the performance of air-cathode microbial fuel cells. Bioprocess Biosyst. Eng. 2013, 36, 1889–1896.
- Choudhury, P.; Uday, U.S.P.; Mahata, N.; Nath Tiwari, O.; Narayan Ray, R.; Kanti Bandyopadhyay, T.; Bhunia, B. Performance improvement of microbial fuel cells for waste water treatment along with value addition: A review on past achievements and recent perspectives. Renew. Sustain. Energy Rev. 2017, 79, 372–389.
- Nosek, D.; Jachimowicz, P.; Cydzik-Kwiatkowska, A. Anode Modification as an Alternative Approach to Improve Electricity Generation in Microbial Fuel Cells. Energies 2020, 13, 6596.
- Mahdi Mardanpour, M.; Nasr Esfahany, M.; Behzad, T.; Sedaqatvand, R. Single chamber microbial fuel cell with spiral anode for dairy wastewater treatment. Biosens. Bioelectron. 2012, 38, 264–269.
- Zhou, M.; Wang, H.; Hassett, D.J.; Gu, T. Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J. Chem. Technol. Biotechnol. 2013, 88, 508–518.
- Jatoi, A.S.; Akhter, F.; Mazari, S.A.; Sabzoi, N.; Aziz, S.; Soomro, S.A.; Mubarak, N.M.; Baloch, H.; Memon, A.Q.; Ahmed, S. Advanced microbial fuel cell for waste water treatment—A review. Environ. Sci. Pollut. Res. 2021, 28, 5005–5019.
- Ma, D.; Jiang, Z.-H.; Lay, C.-H.; Zhou, D. Electricity generation from swine wastewater in microbial fuel cell: Hydraulic reaction time effect. Int. J. Hydrog. Energy 2016, 41, 21820–21826.
- Fangzhou, D.; Zhenglong, L.; Shaoqiang, Y.; Beizhen, X.; Hong, L. Electricity generation directly using human feces wastewater for life support system. Acta Astronaut. 2011, 68, 1537–1547.
- Sciarria, T.P.; Tenca, A.; D’Epifanio, A.; Mecheri, B.; Merlino, G.; Barbato, M.; Borin, S.; Licoccia, S.; Garavaglia, V.; Adani, F. Using olive mill wastewater to improve performance in producing electricity from domestic wastewater by using single-chamber microbial fuel cell. Bioresour. Technol. 2013, 147, 246–253.
- Yakar, A.; Türe, C.; Türker, O.C.; Vymazal, J.; Saz, Ç. Impacts of various filtration media on wastewater treatment and bioelectric production in up-flow constructed wetland combined with microbial fuel cell (UCW-MFC). Ecol. Eng. 2018, 117, 120–132.
- Karuppiah, T.; Uthirakrishnan, U.; Sivakumar, S.V.; Authilingam, S.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. Processing of electroplating industry wastewater through dual chambered microbial fuel cells (MFC) for simultaneous treatment of wastewater and green fuel production. Int. J. Hydrog. Energy 2022, 47, 37569–37576.
- Kong, X.; Sun, Y.; Yuan, Z.; Li, D.; Li, L.; Li, Y. Effect of cathode electron-receiver on the performance of microbial fuel cells. Int. J. Hydrog. Energy 2010, 35, 7224–7227.
- Fadzli, F.S.; Bhawani, S.A.; Adam Mohammad, R.E. Microbial Fuel Cell: Recent Developments in Organic Substrate Use and Bacterial Electrode Interaction. J. Chem. 2021, 2021, 4570388.
- Mukherjee, A.; Patel, V.; Shah, M.T.; Munshi, N.S. Enzymatic and microbial biofuel cells: Current developments and future directions. In Handbook of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 551–576.
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716.
- Catal, T.; Xu, S.; Li, K.; Bermek, H.; Liu, H. Electricity generation from polyalcohols in single-chamber microbial fuel cells. Biosens. Bioelectron. 2008, 24, 849–854.
- Liu, H.; Cheng, S.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493.
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475.
- Ishii, S.I.; Watanabe, K.; Yabuki, S.; Logan, B.E.; Sekiguchi, Y. Comparison of electrode reduction activities of Geobacter sulfurreducens and an enriched consortium in an air-cathode microbial fuel cell. Appl. Environ. Microbiol. 2008, 74, 7348–7355.
- Kim, J.R.; Jung, S.H.; Regan, J.M.; Logan, B.E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 2007, 98, 2568–2577.
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267.
- Dumas, C.; Mollica, A.D. Fe ron, R. Basse guy, L. Etcheverry and A. Bergel. Electrochim. Acta 2007, 53, 468–473.
- Watanabe, K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536.
- Mustakeem, M. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 1–11.
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008.
- Liu, X.-W.; Wang, Y.-P.; Huang, Y.-X.; Sun, X.-F.; Sheng, G.-P.; Zeng, R.J.; Li, F.; Dong, F.; Wang, S.-G.; Tong, Z.-H.; et al. Integration of a microbial fuel cell with activated sludge process for energy-saving wastewater treatment: Taking a sequencing batch reactor as an example. Biotechnol. Bioeng. 2011, 108, 1260–1267.
- Jatoi, A.S.; Tunio, M.; Riaz, S.; Abro, R.; Wajahat, M.H.; Qureshi, K.; Shah, A.; Nizamuddin, S.; Mubarak, N. Utilization of distillery effluent as substrate for power generation with optimized parametric conditions using microbial fuel cell. Eurasian J. Anal. Chem. 2018, 13, em49.
- Puig, S.; Serra, M.; Coma, M.; Cabré, M.; Balaguer, M.D.; Colprim, J. Effect of pH on nutrient dynamics and electricity production using microbial fuel cells. Bioresour. Technol. 2010, 101, 9594–9599.
- Quan, X.-C.; Quan, Y.-P.; Tao, K. Effect of anode aeration on the performance and microbial community of an air–cathode microbial fuel cell. Chem. Eng. J. 2012, 210, 150–156.
- Khan, A.; Chen, Z.; Zhao, S.; Ni, H.; Pei, Y.; Xu, R.; Ling, Z.; Salama, E.-S.; Liu, P.; Li, X. Micro-aeration in anode chamber promotes p-nitrophenol degradation and electricity generation in microbial fuel cell. Bioresour. Technol. 2019, 285, 121291.
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Oon, Y.-S.; Lehl, H.K.; Thung, W.-E.; Nordin, N. Role of macrophyte and effect of supplementary aeration in up-flow constructed wetland-microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2017, 224, 265–275.
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327.
- Malik, S.; Kishore, S.; Prasad, S.; Shah, M.P. A comprehensive review on emerging trends in industrial wastewater research. J. Basic Microbiol. 2022, 62, 296–309.
- Kishore, S.; Malik, S.; Shah, M.P.; Bora, J.; Chaudhary, V.; Kumar, L.; Sayyed, R.Z.; Ranjan, A. A comprehensive review on removal of pollutants from wastewater through microbial nanobiotechnology-based solutions. Biotechnol. Genet. Eng. Rev. 2022, 1–26.
- Malik, S.; Kishore, S.; Bora, J.; Chaudhary, V.; Kumari, A.; Kumari, P.; Kumar, L.; Bhardwaj, A. A Comprehensive Review on Microalgae-Based Biorefinery as Two-Way Source of Wastewater Treatment and Bioresource Recovery. CLEAN—Soil Air Water N/A 2022, 2200044.
- Malik, S.; Kishore, S.; Shah, M.P.; Kumar, S.A. A comprehensive review on nanobiotechnology for bioremediation of heavy metals from wastewater. J. Basic Microbiol. 2022, 62, 361–375.
- Zhang, Y.; He, Q.; Xia, L.; Li, Y.; Song, S. Algae cathode microbial fuel cells for cadmium removal with simultaneous electricity production using nickel foam/graphene electrode. Biochem. Eng. J. 2018, 138, 179–187.
- Hasan, M.N.; Salman, M.S.; Islam, A.; Znad, H.; Hasan, M.M. Sustainable composite sensor material for optical cadmium(II) monitoring and capturing from wastewater. Microchem. J. 2021, 161, 105800.
- Shahat, A.; Kubra, K.T.; Salman, M.S.; Hasan, M.N.; Hasan, M.M. Novel solid-state sensor material for efficient cadmium(II) detection and capturing from wastewater. Microchem. J. 2021, 164, 105967.
- Awual, M.R. A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater. Chem. Eng. J. 2015, 266, 368–375.
- Hasan, M.N.; Shenashen, M.A.; Hasan, M.M.; Znad, H.; Awual, M.R. Assessing of cesium removal from wastewater using functionalized wood cellulosic adsorbent. Chemosphere 2021, 270, 128668.
- Salman, M.S.; Znad, H.; Hasan, M.N.; Hasan, M.M. Optimization of innovative composite sensor for Pb(II) detection and capturing from water samples. Microchem. J. 2021, 160, 105765.
- Wu, Y.; Zhao, X.; Jin, M.; Li, Y.; Li, S.; Kong, F.; Nan, J.; Wang, A. Copper removal and microbial community analysis in single-chamber microbial fuel cell. Bioresour. Technol. 2018, 253, 372–377.
- Rajendran, R.; Dhakshina Moorthy, G.P.; Krishnan, H.; Anappara, S. A Study on Polythiophene Modified Carbon Cloth as Anode in Microbial Fuel Cell for Lead Removal. Arab. J. Sci. Eng. 2021, 46, 6695–6701.
- Tao, Q.; Zhang, X.; Prabaharan, K.; Dai, Y. Separation of cesium from wastewater with copper hexacyanoferrate film in an electrochemical system driven by microbial fuel cells. Bioresour. Technol. 2019, 278, 456–459.
- Choi, C.; Hu, N. The modeling of gold recovery from tetrachloroaurate wastewater using a microbial fuel cell. Bioresour. Technol. 2013, 133, 589–598.
- Hirooka, K.; Ichihashi, O. Phosphorus recovery from artificial wastewater by microbial fuel cell and its effect on power generation. Bioresour. Technol. 2013, 137, 368–375.
- Paucar, N.E.; Sato, C. Microbial fuel cell for energy production, nutrient removal and recovery from wastewater: A review. Processes 2021, 9, 1318.





