Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gergana Zahmanova | -- | 1691 | 2023-01-17 09:24:47 | | | |
| 2 | Sirius Huang | Meta information modification | 1691 | 2023-01-18 03:33:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zahmanova, G.; Aljabali, A.A.; Takova, K.; Toneva, V.; Tambuwala, M.M.; Andonov, A.P.; Lukov, G.L.; Minkov, I. Important Plant Viruses for Plant Biotechnology. Encyclopedia. Available online: https://encyclopedia.pub/entry/40266 (accessed on 08 March 2026).
Zahmanova G, Aljabali AA, Takova K, Toneva V, Tambuwala MM, Andonov AP, et al. Important Plant Viruses for Plant Biotechnology. Encyclopedia. Available at: https://encyclopedia.pub/entry/40266. Accessed March 08, 2026.
Zahmanova, Gergana, Alaa A. Aljabali, Katerina Takova, Valentina Toneva, Murtaza M. Tambuwala, Anton P. Andonov, Georgi L. Lukov, Ivan Minkov. "Important Plant Viruses for Plant Biotechnology" Encyclopedia, https://encyclopedia.pub/entry/40266 (accessed March 08, 2026).
Zahmanova, G., Aljabali, A.A., Takova, K., Toneva, V., Tambuwala, M.M., Andonov, A.P., Lukov, G.L., & Minkov, I. (2023, January 17). Important Plant Viruses for Plant Biotechnology. In Encyclopedia. https://encyclopedia.pub/entry/40266
Zahmanova, Gergana, et al. "Important Plant Viruses for Plant Biotechnology." Encyclopedia. Web. 17 January, 2023.
Copy Citation
Plant viruses have traditionally been studied as pathogens in the context of understanding the molecular and cellular mechanisms of a particular disease affecting crops. In recent years, viruses have emerged as a new alternative for producing biological nanomaterials and chimeric vaccines. Plant viruses were also used to generate highly efficient expression vectors, revolutionizing plant molecular farming (PMF).
plant molecular farming
virus-like particles (VLPs)
protein cages
plant-derived vaccines
biologics
nanomedicine
viral-based nanotechnology
drug delivery
immune therapy
1. Introduction
Since plant viruses were discovered in the late nineteenth century, their impact has changed from harmful pathogens to useful molecular machines with applications in plant molecular farming (PMF) and bio-nanotechnology. Although public perception of a “virus” is often associated with harm, viruses can easily be reorganized into environmentally friendly and safe structures [1][2][3]. The expansion of the overall knowledge and understanding of viral genomes, architecture, and biophysical properties has allowed the use of plant viruses as vectors for recombinant protein expression and the production of virus-based nanoparticles (VNPs) [4]. VLPs are self-assembled, naturally occurring nanomaterials that structurally and morphologically resemble the 3-dimensional structures of virions, but without the viral genome. VLPs modified to carry additional useful payload are defined as VNPs. Some of the many biological applications of VNPs, which are generated from the capsid structural components of various viruses, include vaccine production and innovative delivery mechanisms for therapeutic compounds, diagnostic reagents, enzymes, pesticides, immunogenic peptides, and many others [5].
Historical Review of Plant Molecular Farming
Over the past three decades, several plant systems have been used to produce recombinant proteins. Much interest has been paid to plant-derived antibodies, vaccines, enzymes, microbicides, and viral protein nanocages (Figure 1).
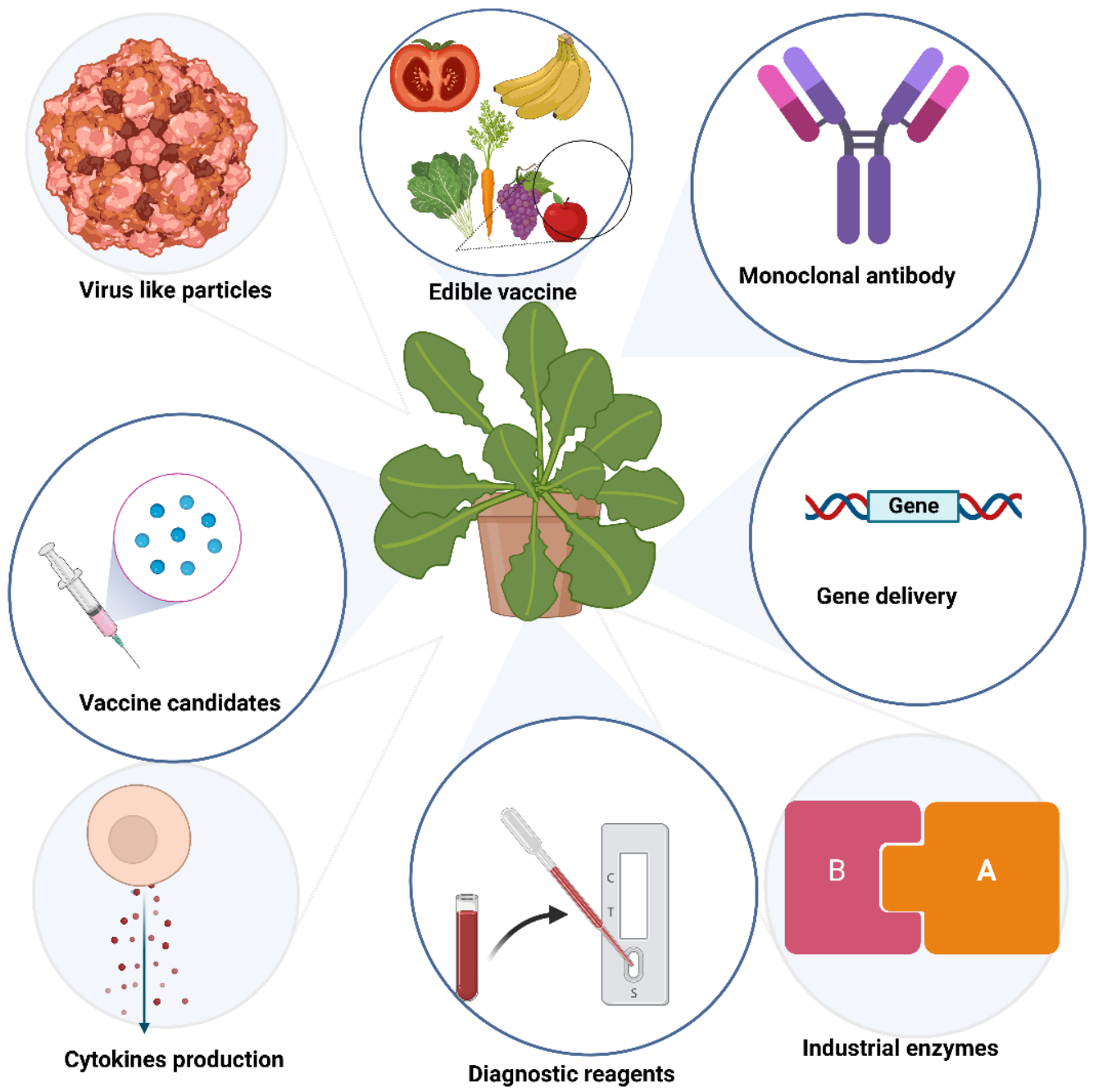
Figure 1. Biologics produced in plants.
Plant molecular farming was born in 1980 when the marker gene for β-glucuronidase (GUS) was successfully transformed in higher plants [6]. Later, β-glucuronidase became one of the successful commercial products of plant molecular farming, together with two other industrial enzymes, avidin and trypsin [7][8][9]. In 1986, human growth hormone (HGH) was produced in transgenic tobacco and sunflowers [10]. In 1988, the first production of human antibodies in plants was reported [11]. The concept of using plants for the production of vaccines, more specifically, edible vaccines, was introduced by Dr. Charles Arntzen in the 1990s [12]. Studies were focused on this topic for many years [13][14][15] until the incident of seed contamination with a transgene expressing a bovine vaccine candidate [16][17]. The new trends in plant molecular farming are to use plants in controlled conditions to produce recombinant products that can either be injected or used orally [18][19][20][21][22].
The initial plant-derived biopharmaceuticals were expressed in stable transgenic plants. This technological approach has three main disadvantages: a long developmental period, low yield, and public concerns about GMO plants. Transient expressions via plant expression virus vectors offer a way to avoid these limitations [23].
Although the production of recombinant proteins in plants by stable gene integration and expression has some limitations, the initial commercialized therapeutic recombinant proteins were produced by this technology. In 2006, stably transformed N. benthamiana cell cultures were used for the production of the first USDA-approved injectable vaccine against the Newcastle disease virus for poultry [24]. In 2012, the FDA approved plant-derived recombinant human β-glucocerebrosidase (marketed as Elelyso®) for the treatment of Gaucher’s disease type 1. The recombinant human β-glucocerebrosidase enzyme was produced in a stably transformed carrot cell suspension culture by Protalix Biotherapeutics Inc. [25] and licensed to Pfizer. In 2022, Health Canada approved the first plant-derived SARS-CoV-2 vaccine, Covifenz [26]. The strategy included transient expression in N. benthamiana of the modified recombinant SARS-CoV-2 S protein with stabilizing point mutations. Medicago Inc. successfully produced and purified modified SARS-CoV-2 S VLPs built from the S protein. Using the same technology, Medicago Inc. successfully produced a vaccine against seasonal influenza [27]. Currently, there are few examples of commercialized plant molecular farming products, and plants cannot replace industrial expression systems. However, PMF has been proven cost-effective and valuable in the production of vaccines, antibodies, enzymes, and biological VNPs under conditions of urgency, such as emerging pandemic viruses or for treating rare diseases.
2. Important Plant Viruses for Plant Biotechnology
Approximately 900 plant viruses, identified mainly in crops, are known [28]. Several of them, such as the tobacco mosaic virus (TMV), potato virus X (PVX), cowpea mosaic virus (CPMV), cowpea chlorotic mottle virus (CCMV), brome mosaic virus (BMV), cucumber mosaic virus (CMV), plum pox virus (PPV), alfalfa mosaic virus (AIMV), papaya mosaic virus (PapMV), tomato bushy stunt virus (TBSV), and many others, are utilized in biotechnology and plant viral-based nanotechnology research and industry (Figure 2) [29][30].
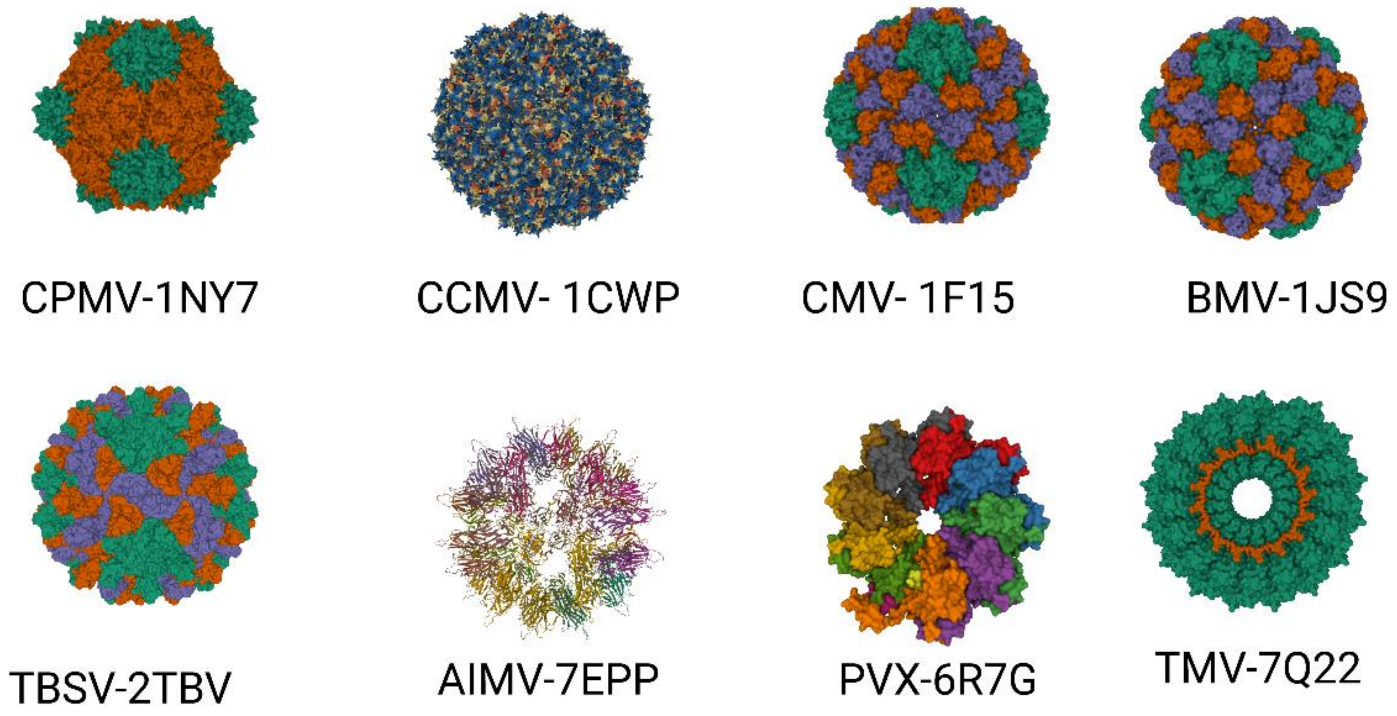
Figure 2. Important plant VLPs with application in biotechnology and plant viral-based nanotechnology. Plant viruses with their corresponding protein databank identification number—CPMV (PDB ID: 1NY7), CCMV (PDB ID: 1CW7), CMV (PDB ID: 1F15), BMV (PDB ID: 1JS9), TBSV (PDB ID: 2TBV), AIMV (PDB ID: 7EPP), PVX (PDB ID: 6R7G), TMV (PDB ID: 7Q22).
Plant viruses have very simple (rod-shaped, spherical, quasi-spherical, or filamentous) structures, consisting of multiple copies of one or a few capsid protein subunits, forming a protein coat around the viral genome. The capsid proteins have the ability to self-assemble, which offers the opportunity to generate a great variety of natural bio-nanomaterials. A better understanding of the structure and biophysical characteristics of plant viruses is vital for developing and producing VNPs, which can be used as nanoparticles for the surface presentation of antigens for recombinant VNP vaccines or as nanocages for “cargo” delivery. VNPs are naturally occurring biological entities, making them both biocompatible and biodegradable. Furthermore, VNPs can be targeted to particular tissue or cells. Finally, the production and purification of VNPs from plants are rapid, cost-effective, and environmentally safe (compared with conventional nanoparticles) and can easily be scaled up depending on demand [31]. There are two main strategies for VNPs production in plants: using a virus infection, allowing the cultivation of plant viruses in their natural host, or the biotechnology approach, using transient expression of the viral genes encoding the capsid proteins in heterologous expression systems [32][33][34].
2.1. Tobacco Mosaic Virus (TMV)
TMV is a member of the genus Tobamovirus in the family of Virgaviridae. TMV has a rod-shaped virion with a diameter of 18 nm, a modal length of 300 nm with a central channel in width, and the viral RNA intercalated between the coat protein turns [35]. TMV coat protein (CP) could self-assemble into the form of a rigid helical RNA-free tube [36]. Tobacco mosaic virus can be easily cultivated in plants, and the yields can be very high, ~4 g/kg wet weight of tobacco. The purified TMV rod-shaped virions can be disassembled in vitro into protein subunits and RNA [37][38]. When nucleic acid is absent, the coat protein can self-assemble into several types of VNPs. The polymerization of the TMV coat protein is temperature and concentration-dependent [39][40]. TMV VNPs are stable and can be used as scaffolds for chemical modifications, coating with biologically active peptides, or loading with drugs [17][41][42][43][44][45][46]. TMV has been widely used as a full-vector and also as a deconstructed virus vector for recombinant protein expression [47].
2.2. Cowpea Mosaic Virus (CPMV)
CPMV belongs to the genus Comovirus in the Comoviridae family. It is a non-enveloped, icosahedral virus with nanoscale dimensions (30 nm). The CPMV protein shell is comprised of 60 copies of the large (L) coat proteins with two domains and 60 copies of the small (S) coat proteins with one domain [48]. The three domains together form the asymmetric unit of the CPMV capsid, with 2 nm channels enabling the exchange of molecules from the exterior to the interior. The internal viral cavity encapsulates two single-stranded RNA molecules [49][50].
This CPMV virus has been widely used in bio-nanotechnology because its structure and particle architecture have been well studied, and the genome can easily be manipulated [51]. Virus particles, which contain viral genomic RNAs, can be produced rapidly in high yield (1–2 g/kg) through the infection of plants [48]. These viral particles have been used for selective attachment of various moieties [51][52][53][54][55] and to display immunogenic epitopes on their surface [56][57]. The drawback is that the CPMV particles containing genomic RNAs raise biosafety and regulatory concerns. They are also ineffective drug containers because they are hard to load with foreign materials such as therapeutic agents or heterologous RNA molecules. Prof. Lomonossoff’s laboratory addressed these problems by developing RNA-free, empty virus-like particles (eVLPs) based on the transient expression of VP60 (precursor of L and S coat proteins) along with the 24K viral proteinase in Nicotiana benthamiana [58][59][60][61]. In addition, the eVLPs are very stable under various conditions, which extends their bio-nanotechnology application range [2][61][62][63]. Lomonossoff’s lab further improved CPMV viral particle packaging, allowing the production of VLPs that can pack up to 6 kb of artificial RNA [64]. Furthermore, CPMV was used for the expression of recombinant proteins by applying full-virus and deconstructed virus strategies [65].
2.3. Cowpea Chlorotic Mottle Virus (CCMV)
CCMV belongs to the Bromoviridae family. The CCMV capsid is composed of 180 identical capsid proteins (CP) that form a ~28 nm diameter icosahedral shell and an 18 nm diameter inner cavity [66]. Native CCMV is stable at pH 5.0. The native CCMV virion can be disassembled, and the RNA genome can be removed by centrifugation under high salt concentrations at neutral pH [67]. In vitro, at pH 5.0, the CCMV capsid proteins can be self-assembled into empty nanoscale structures [68]. CCMV is one of the first viruses used in bio-nanotechnology as a tool for developing drug-delivery vehicles due to its well-studied structure, simple capsid with good biocompatibility, and low toxicity [69]. VNPs based on CCMV have been used to encapsulate guest molecules, such as negatively charged polymers, enzymes, and organic aggregates [70]. A wide variety of ligands, such as small peptides, biotin, fluorescent dyes, organometallic photosensitizers, and intact IgG antibodies, can be attached on the outer surface of the capsid [71][72][73][74].
2.4. Brome Mosaic Virus (BMV)
BMV, a member of the family Bromoviridae, is very similar to CCMV [75]. The native virion has a diameter of 28 nm with T = 3 icosahedral symmetry, and it is built of 180 identical capsid proteins with different conformations (A, B, and C) [76][77]. The A conformation of the coat protein forms pentameric capsomers with small 0.5 nm pores. The B and C conformations of the capsid protein form hexameric capsomer with 0.6 nm pores [76]. The stability of the capsid depends on the interaction between the positively charged inner cavity and the negatively charged encapsulated RNA [78]. BMV, similarly to CCMV, is widely used in bio-nanotechnology due to its well-examined structure and biophysical properties.
References
- Steinmetz, N.F.; Lin, T.; Lomonossoff, G.P.; Johnson, J.E. Structure-Based Engineering of an Icosahedral Virus for Nanomedicine and Nanotechnology. In Viruses and Nanotech; Manchester, M., Steinmetz, N.F., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 23–58. ISBN 978-3-540-69379-6.
- Aljabali, A.A.A.; Shukla, S.; Lomonossoff, G.P.; Steinmetz, N.F.; Evans, D.J. CPMV-DOX Delivers. Mol. Pharm. 2013, 10, 3–10.
- Zeng, Q.; Wen, H.; Wen, Q.; Chen, X.; Wang, Y.; Xuan, W.; Liang, J.; Wan, S. Cucumber Mosaic Virus as Drug Delivery Vehicle for Doxorubicin. Biomaterials 2013, 34, 4632–4642.
- Ibrahim, A.; Odon, V.; Kormelink, R. Plant Viruses in Plant Molecular Pharming: Toward the Use of Enveloped Viruses. Front. Plant Sci. 2019, 10, 803.
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. J. Nanobiotechnol. 2021, 19, 59.
- Fraley, R.T.; Rogers, S.G.; Horsch, R.B.; Sanders, P.R.; Flick, J.S.; Adams, S.P.; Bittner, M.L.; Brand, L.A.; Fink, C.L.; Fry, J.S.; et al. Expression of Bacterial Genes in Plant Cells. Proc. Natl. Acad. Sci. USA 1983, 80, 4803–4807.
- Witcher, D.R.; Hood, E.E.; Peterson, D.; Bailey, M.; Bond, D.; Kusnadi, A.; Evangelista, R.; Nikolov, Z.; Wooge, C.; Mehigh, R.; et al. Commercial Production of β-Glucuronidase (GUS): A Model System for the Production of Proteins in Plants. Mol. Breed. 1998, 4, 301–312.
- Hood, E.E.; Witcher, D.R.; Maddock, S.; Meyer, T.; Baszczynski, C.; Bailey, M.; Flynn, P.; Register, J.; Marshall, L.; Bond, D.; et al. Commercial Production of Avidin from Transgenic Maize: Characterization of Transformant, Production, Processing, Extraction and Purification. Mol. Breed. 1997, 3, 291–306.
- Woodard, S.L.; Mayor, J.M.; Bailey, M.R.; Barker, D.K.; Love, R.T.; Lane, J.R.; Delaney, D.E.; McComas-Wagner, J.M.; Mallubhotla, H.D.; Hood, E.E.; et al. Maize (Zea Mays)-Derived Bovine Trypsin: Characterization of the First Large-Scale, Commercial Protein Product from Transgenic Plants. Biotechnol. Appl. Biochem. 2003, 38, 123–130.
- Barta, A.; Sommergruber, K.; Thompson, D.; Hartmuth, K.; Matzke, M.A.; Matzke, A.J.M. The Expression of a Nopaline Synthase—Human Growth Hormone Chimaeric Gene in Transformed Tobacco and Sunflower Callus Tissue. Plant Mol Biol 1986, 6, 347–357.
- During, K. Wound-Inducible Expression and Secretion of T4 Lysozyme and Monoclonal Antibodies in Nicotiana Tabacum. Ph.D. Thesis, Mathematisch-Naturwissenschaftlichen Fakultat der Universität zu Köln, Köln, Germany, 1988.
- Arntzen, C. Plant-made Pharmaceuticals: From ‘Edible Vaccines’ to Ebola Therapeutics. Plant Biotechnol. J. 2015, 13, 1013–1016.
- Loza-Rubio, E.; Rojas, E.; Gómez, L.; Olivera, M.T.J.; Gómez-Lim, M.A. Development of an Edible Rabies Vaccine in Maize Using the Vnukovo Strain. Dev. Biol. 2008, 131, 477–482.
- Zahmanova, G.; Falzarano, D.; Naimov, S.; Kostova, M.; Boncheva, R.; Dukiandjiev, S.; Minkov, I.; Andonov, A. Oral immunization with truncated hepatitis B virus nucleocapsid expressed in transgenic potatoes. Comptes Rendus L’acade’mie Bulg. Des Sci. 2008, 61, 1293–1300.
- Arntzen, C.; Plotkin, S.; Dodet, B. Plant-Derived Vaccines and Antibodies: Potential and Limitations. Vaccine 2005, 23, 1753–1756.
- Noncompliance History. Available online: https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/compliance-and-inspections/CT_Compliance_history (accessed on 8 December 2022).
- Rybicki, E.P. Plant-Made Vaccines for Humans and Animals. Plant Biotechnol. J. 2010, 8, 620–637.
- Rybicki, E.P. Plant-Produced Vaccines: Promise and Reality. Drug Discov. Today 2009, 14, 16–24.
- Zahmanova, G.; Mazalovska, M.; Toneva, V.; Minkov, I.; Lomonossoff, G. Production of Chimeric Virus-like Particles Bearing M2e Influenza Epitope in Nicotiana Benthamiana Plants. J. Biotechnol. 2015, 208, S109.
- Mardanova, E.S.; Kotlyarov, R.Y.; Stuchinskaya, M.D.; Nikolaeva, L.I.; Zahmanova, G.; Ravin, N.V. High-Yield Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope and Receptor Binding Domain of SARS-CoV-2 in Plants Using Viral Vectors. Int. J. Mol. Sci. 2022, 23, 15684.
- Rebelo, B.A.; Folgado, A.; Ferreira, A.C.; Abranches, R. Production of the SARS-CoV-2 Spike Protein and Its Receptor Binding Domain in Plant Cell Suspension Cultures. Front. Plant Sci. 2022, 13, 995429.
- Jung, J.-W.; Zahmanova, G.; Minkov, I.; Lomonossoff, G.P. Plant-Based Expression and Characterization of SARS-CoV-2 Virus-like Particles Presenting a Native Spike Protein. Plant Biotechnol. J. 2022, 20, 1363–1372.
- Kopertekh, L.; Schiemann, J. Transient Production of Recombinant Pharmaceutical Proteins in Plants: Evolution and Perspectives. Curr. Med. Chem. 2019, 26, 365–380.
- Vermij, P.; Waltz, E. USDA Approves the First Plant-Based Vaccine. Nat. Biotechnol. 2006, 24, 233–234.
- Maxmen, A. Drug-Making Plant Blooms. Nature 2012, 485, 160.
- Medicago Inc Medicago and GSK Announce the Approval by Health Canada of COVIFENZ®, an Adjuvanted Plant-Based COVID-19 Vaccine. Available online: https://medicago.com/en/press-release/covifenz/ (accessed on 26 February 2022).
- Ward, B.J.; Makarkov, A.; Séguin, A.; Pillet, S.; Trépanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, Immunogenicity, and Safety of a Plant-Derived, Quadrivalent, Virus-like Particle Influenza Vaccine in Adults (18–64 Years) and Older Adults (≥65 Years): Two Multicentre, Randomised Phase 3 Trials. Lancet 2020, 396, 1491–1503.
- Roossinck, M.J. Plant Virus Ecology. PLoS Pathog. 2013, 9, e1003304.
- Scholthof, K.-B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 Plant Viruses in Molecular Plant Pathology. Mol. Plant Pathol. 2011, 12, 938–954.
- Balke, I.; Zeltins, A. Use of Plant Viruses and Virus-like Particles for the Creation of Novel Vaccines. Adv. Drug Deliv. Rev. 2019, 145, 119–129.
- Love, A.J.; Makarov, V.; Yaminsky, I.; Kalinina, N.O.; Taliansky, M.E. The Use of Tobacco Mosaic Virus and Cowpea Mosaic Virus for the Production of Novel Metal Nanomaterials. Virology 2014, 449, 133–139.
- Marsian, J.; Lomonossoff, G.P. Molecular Pharming—VLPs Made in Plants. Curr Opin Biotechnol 2016, 37, 201–206.
- Evans, D.J. The Bionanoscience of Plant Viruses: Templates and Synthons for New Materials. J. Mater. Chem. 2008, 18, 3746–3754.
- Sainsbury, F.; Lomonossoff, G.P. Transient Expressions of Synthetic Biology in Plants. Curr. Opin. Plant Biol. 2014, 19, 1–7.
- Clare, D.K.; Orlova, E.V. 4.6Å Cryo-EM Reconstruction of Tobacco Mosaic Virus from Images Recorded at 300keV on a 4k×4k CCD Camera. J. Struct. Biol. 2010, 171, 303–308.
- Schramm, G.; Schumacher, G.; Zillig, W. An Infectious Nucleoprotein from Tobacco Mosaic Virus. Nature 1955, 175, 549–550.
- Harrison, B.D.; Wilson, T.M.A.; Butler, P.J.G. Self–Assembly of Tobacco Mosaic Virus: The Role of an Intermediate Aggregate in Generating Both Specificity and Speed. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1999, 354, 537–550.
- Okada, Y. Molecular Assembly of Tobacco Mosaic Virus in Vitro. Adv. Biophys. 1986, 22, 95–149.
- Shire, S.J.; Steckert, J.J.; Adams, M.L.; Schuster, T.M. Kinetics and Mechanism of Tobacco Mosaic Virus Assembly: Direct Measurement of Relative Rates of Incorporation of 4S and 20S Protein. Proc. Natl. Acad. Sci. USA 1979, 76, 2745–2749.
- Kegel, W.K.; van der Schoot, P. Physical Regulation of the Self-Assembly of Tobacco Mosaic Virus Coat Protein. Biophys. J. 2006, 91, 1501–1512.
- Pitek, A.S.; Hu, H.; Shukla, S.; Steinmetz, N.F. Cancer Theranostic Applications of Albumin-Coated Tobacco Mosaic Virus Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 39468–39477.
- Bruckman, M.A.; Steinmetz, N.F. Chemical Modification of the Inner and Outer Surfaces of Tobacco Mosaic Virus (TMV). Methods Mol. Biol. 2014, 1108, 173–185.
- Bruckman, M.A.; Hern, S.; Jiang, K.; Flask, C.A.; Yu, X.; Steinmetz, N.F. Tobacco Mosaic Virus Rods and Spheres as Supramolecular High-Relaxivity MRI Contrast Agents. J. Mater. Chem. B 2013, 1, 1482–1490.
- Bruckman, M.A.; Czapar, A.E.; VanMeter, A.; Randolph, L.N.; Steinmetz, N.F. Tobacco Mosaic Virus-Based Protein Nanoparticles and Nanorods for Chemotherapy Delivery Targeting Breast Cancer. J. Control. Release 2016, 231, 103–113.
- Kernan, D.L.; Wen, A.M.; Pitek, A.S.; Steinmetz, N.F. Featured Article: Delivery of Chemotherapeutic VcMMAE Using Tobacco Mosaic Virus Nanoparticles. Exp. Biol. Med. 2017, 242, 1405–1411.
- Lee, K.L.; Carpenter, B.L.; Wen, A.M.; Ghiladi, R.A.; Steinmetz, N.F. High Aspect Ratio Nanotubes Formed by Tobacco Mosaic Virus for Delivery of Photodynamic Agents Targeting Melanoma. ACS Biomater. Sci. Eng. 2016, 2, 838–844.
- Takamatsu, N.; Ishikawa, M.; Meshi, T.; Okada, Y. Expression of Bacterial Chloramphenicol Acetyltransferase Gene in Tobacco Plants Mediated by TMV-RNA. EMBO J. 1987, 6, 307–311.
- Lomonossoff, G.P. Cowpea Mosaic Virus. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 569–574. ISBN 978-0-12-374410-4.
- Lomonossoff, G.P.; Johnson, J.E. The Synthesis and Structure of Comovirus Capsids. Prog. Biophys. Mol. Biol. 1991, 55, 107–137.
- Lin, T.; Chen, Z.; Usha, R.; Stauffacher, C.V.; Dai, J.B.; Schmidt, T.; Johnson, J.E. The Refined Crystal Structure of Cowpea Mosaic Virus at 2.8 A Resolution. Virology 1999, 265, 20–34.
- Sainsbury, F.; Cañizares, M.C.; Lomonossoff, G.P. Cowpea Mosaic Virus: The Plant Virus–Based Biotechnology Workhorse. Annu. Rev. Phytopathol. 2010, 48, 437–455.
- Aljabali, A.A.A.; Barclay, J.E.; Steinmetz, N.F.; Lomonossoff, G.P.; Evans, D.J. Controlled Immobilisation of Active Enzymes on the Cowpea Mosaic Virus Capsid. Nanoscale 2012, 4, 5640–5645.
- Aljabali, A.A.A.; Barclay, J.E.; Lomonossoff, G.P.; Evans, D.J. Virus Templated Metallic Nanoparticles. Nanoscale 2010, 2, 2596–2600.
- Evans, D.J. Bionanoscience at the Plant Virus–Inorganic Chemistry Interface. Inorg. Chim. Acta 2010, 363, 1070–1076.
- Steinmetz, N.F.; Evans, D.J. Utilisation of Plant Viruses in Bionanotechnology. Org. Biomol. Chem. 2007, 5, 2891–2902.
- Porta, C.; Spall, V.E.; Loveland, J.; Johnson, J.E.; Barker, P.J.; Lomonossoff, G.P. Development of Cowpea Mosaic Virus as a High-Yielding System for the Presentation of Foreign Peptides. Virology 1994, 202, 949–955.
- Dalsgaard, K.; Uttenthal, Å.; Jones, T.D.; Xu, F.; Merryweather, A.; Hamilton, W.D.O.; Langeveld, J.P.M.; Boshuizen, R.S.; Kamstrup, S.; Lomonossoff, G.P.; et al. Plant–Derived Vaccine Protects Target Animals against a Viral Disease. Nat. Biotechnol. 1997, 15, 248–252.
- Montague, N.P.; Thuenemann, E.C.; Saxena, P.; Saunders, K.; Lenzi, P.; Lomonossoff, G.P. Recent Advances of Cowpea Mosaic Virus-Based Particle Technology. Hum. Vaccines 2011, 7, 383–390.
- Saunders, K.; Sainsbury, F.; Lomonossoff, G.P. Efficient Generation of Cowpea Mosaicvirus Empty Virus-like Particles by the Proteolytic Processing of Precursors in Insect Cells and Plants. Virology 2009, 393, 329–337.
- Hesketh, E.L.; Meshcheriakova, Y.; Thompson, R.F.; Lomonossoff, G.P.; Ranson, N.A. The Structures of a Naturally Empty Cowpea Mosaic Virus Particle and Its Genome-Containing Counterpart by Cryo-Electron Microscopy. Sci. Rep. 2017, 7, 539.
- Huynh, N.T.; Hesketh, E.L.; Saxena, P.; Meshcheriakova, Y.; Ku, Y.-C.; Hoang, L.T.; Johnson, J.E.; Ranson, N.A.; Lomonossoff, G.P.; Reddy, V.S. Crystal Structure and Proteomics Analysis of Empty Virus-like Particles of Cowpea Mosaic Virus. Structure 2016, 24, 567–575.
- Wang, Q.; Kaltgrad, E.; Lin, T.; Johnson, J.E.; Finn, M.G. Natural Supramolecular Building Blocks. Wild-Type Cowpea Mosaic Virus. Chem. Biol. 2002, 9, 805–811.
- Tiu, B.D.B.; Advincula, R.C.; Steinmetz, N.F. Nanomanufacture of Free-Standing, Porous, Janus-Type Films of Polymer-Plant Virus Nanoparticle Arrays. Methods Mol. Biol. 2018, 1776, 143–157.
- Kruse, I.; Peyret, H.; Saxena, P.; Lomonossoff, G.P. Encapsidation of Viral RNA in Picornavirales: Studies on Cowpea Mosaic Virus Demonstrate Dependence on Viral Replication. J. Virol. 2019, 93, e01520-18.
- Peyret, H.; Lomonossoff, G.P. When Plant Virology Met Agrobacterium: The Rise of the Deconstructed Clones. Plant Biotechnol. J. 2015, 13, 1121–1135.
- Caspar, D.L.; Klug, A. Physical Principles in the Construction of Regular Viruses. Cold Spring Harb. Symp. Quant. Biol. 1962, 27, 1–24.
- Douglas, T.; Young, M. Host–Guest Encapsulation of Materials by Assembled Virus Protein Cages. Nature 1998, 393, 152–155.
- Wilts, B.D.; Schaap, I.A.T.; Schmidt, C.F. Swelling and Softening of the Cowpea Chlorotic Mottle Virus in Response to PH Shifts. Biophys. J. 2015, 108, 2541–2549.
- Hema, M.; Vishnu Vardhan, G.P.; Savithri, H.S.; Murthy, M.R.N. Chapter 6—Emerging Trends in the Development of Plant Virus-Based Nanoparticles and Their Biomedical Applications. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 61–82. ISBN 978-0-12-816328-3.
- Minten, I.J.; Ma, Y.; Hempenius, M.A.; Vancso, G.J.; Nolte, R.J.M.; Cornelissen, J.J.L.M. CCMV Capsid Formation Induced by a Functional Negatively Charged Polymer. Org. Biomol. Chem. 2009, 7, 4685–4688.
- Lomonossoff, G.P.; Evans, D.J. Applications of Plant Viruses in Bionanotechnology. In Plant Viral Vectors; Palmer, K., Gleba, Y., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 61–87. ISBN 978-3-642-40829-8.
- Young, M.; Debbie, W.; Uchida, M.; Douglas, T. Plant Viruses as Biotemplates for Materials and Their Use in Nanotechnology. Annu. Rev. Phytopathol. 2008, 46, 361–384.
- Wen, A.M.; Lee, K.L.; Cao, P.; Pangilinan, K.; Carpenter, B.L.; Lam, P.; Veliz, F.A.; Ghiladi, R.A.; Advincula, R.C.; Steinmetz, N.F. Utilizing Viral Nanoparticle/Dendron Hybrid Conjugates in Photodynamic Therapy for Dual Delivery to Macrophages and Cancer Cells. Bioconjug. Chem. 2016, 27, 1227–1235.
- Narayanan, K.B.; Han, S.S. Icosahedral Plant Viral Nanoparticles—Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv. Colloid Interface Sci. 2017, 248, 1–19.
- Van Regenmortel, M.H.V.; Fauquet, C.M.; Bishop, D.H.L.; Carstens, E.B.; Estes, M.K.; Lemon, S.M.; Maniloff, J.; Mayo, M.A.; McGeoch, D.J.; Pringle, C.R.; et al. Classification and Nomenclature of Viruses. In Virus Taxonomy Seventh Report of the International Committee on Taxonomy of Viruses; Academic Press: Cambridge, MA, USA, 2000.
- Lucas, R.W.; Larson, S.B.; McPherson, A. The Crystallographic Structure of Brome Mosaic Virus, Edited by I. A. Wilson. J. Mol. Biol. 2002, 317, 95–108.
- Cuillel, M.; Zulauf, M.; Jacrot, B. Self-Assembly of Brome Mosaic Virus Protein into Capsids: Initial and Final States of Aggregation. J. Mol. Biol. 1983, 164, 589–603.
- Incardona, N.L.; Kaesberg, P. A PH-Induced Structural Change in Bromegrass Mosaic Virus. Biophys. J. 1964, 4, 11–21.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
18 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





