
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Palak Sondhi | -- | 4314 | 2023-01-17 03:24:09 | | | |
| 2 | Conner Chen | + 4 word(s) | 4318 | 2023-01-18 03:51:41 | | | | |
| 3 | Conner Chen | + 2 word(s) | 4320 | 2023-01-18 09:59:40 | | | | |
| 4 | Conner Chen | + 6 word(s) | 4326 | 2023-01-18 10:10:58 | | | | |
| 5 | Conner Chen | + 6 word(s) | 4332 | 2023-01-18 10:27:29 | | | | |
| 6 | Conner Chen | Meta information modification | 4332 | 2023-01-29 07:13:53 | | |
Video Upload Options
Nanoporous gold (np-Au) has promising applications in therapeutic delivery. The promises arise from its high surface area-to-volume ratio, ease of tuning shape and size, ability to be modified by organic molecules including drugs, and biocompatibility. For the demands of a real patient, light-triggered on-demand pulsatile release from a reservoir containing highly enriched medicines has been demonstrated to be provided by versatile drug delivery devices using nanoporous membranes made of gold nanorods and dendrimers.
1. Introduction
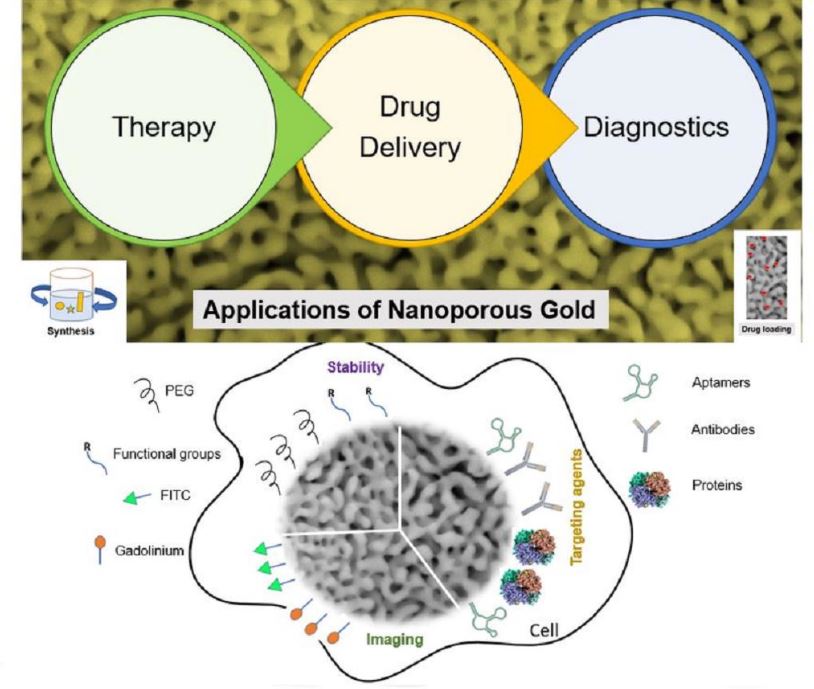
2. Nanoporous Gold-Based Platforms as Future Drug Reservoirs
2.1. Factors Affecting the Therapeutic Efficacy
2.2. Mechanism of Targeting
2.3. Application to Neurological Conditions and Mental Health
3. Emerging Biomedical Applications of Nanoporous Gold-based Structures
Nanoporous gold (np-Au) is a metallic structure with pores and ligaments on the nanoscale. Since these particles can be considered as a combination of nanomaterials, inert metals, and nanoporous framework, np-Au provides a wide range of applicability to the field of biomedicine [49]. Due to quantum mechanical principles, nanoparticles with diameters between 1 and 10 nm (between the size of molecules and that of bulk metal properties) exhibit electronic band structure. Nanoparticles can engage in quantum tunneling in this small size range. The physical characteristics that result rely significantly on the particle size, interparticle spacing, kind of organic shell that surrounds them, and shape of the nanoparticles. They are neither those of bulk metal nor those of molecular compounds. A few “last metallic electrons” are employed in nearby particle tunneling [50]. Tunneling effects start to interfere with the interaction between the surface plasmons when the interparticle distance is less than 1 nm, according to theoretical research by Nordlander and colleagues. The interaction between surface plasmons is disrupted by a quantum tunneling phenomenon when the interparticle distance (d) is smaller than 1 nm, which results in the red shift absorption. Tunneling of electrons to and from immobilized biomolecules may enhance the efficiency of biosensors if the biomolecule interacts with gold nanoparticles or structural features in this size range.
There is additional research being done using gold nanoparticles as biological probes. It can be mixed with a variety of biological macromolecules, including nucleic acids, heavy metal ions, and protein, thanks to its unique optical features, macroscopic quantum tunneling effect, surface effect, and strong biocompatibility [51][52]. Nanoscale pores and framework provides enhanced physical, chemical, and biological activities due to their nano-size, enhanced surface, and quantum tunneling effects. The increased surface area also works for the betterment of the adsorption capacity of np-Au by providing more binding surfaces of biomolecules. The porous structures also play a vital role in the transfer of biomolecules through their increased permeability which can catalytically help to increase the reaction rates too [53]. Better electrical conductivity and the energy absorption capacity of np-Au is another main characteristic that facilitates the transfer of electrons which makes it more important for biomedical applications. In addition, the tunability of np-Au in case of size, shape, and pore, makes np-Au more practical in this applied field [54]. Due to these special characteristics, applications of np-Au have been increasing in recent years in the field of biomedicine, such as biosensing, drug delivery, and catalysis. Biosensing has become an important part of research for the analysis and detection of biomedical elements due to its necessity-driven demand. The challenges in the biomedical field due to the surge of known and unknown biological elements are growing continuously thereby increasing the demand for diverse types of biosensors [55]. Researchers in recent years are focusing on the development of simple, low-cost, real-time, and efficient biosensors. For these purposes, np-Au has proven its standing as a promising tool with its unique and excellent characteristics. Different studies have been done to fabricate np-Au-based biosensors using its various forms such as bare np-Au, surface-functionalized np-Au, shape-controlled np-Au, and other np-Au with hybrid structures [56].
3.1. Plasmonics-Based Applications
Plasmonic metal nanostructures have numerous uses in fields including optics, medicine, and catalysis. Their composition, configuration, environment around nanostructures, shape, and size have a major impact on their plasmonic characteristics, such as surface plasmon resonance (SPR) and localized surface plasmon resonance (LSPR). Due to the distinctive 3-dimensional bicontinuous nanostructure with a significant surface area, strong catalytic activity, and tunable plasmonic resonance, nanoporous gold (NPG) has recently received a lot of attention [57]. It is believed that a key factor in LSPR sensing and surface-enhanced optical phenomena like surface-enhanced Raman scattering surface-enhanced Raman scattering (SERS) and surface-enhanced fluorescence is the enhanced electromagnetic (EM) fields of LSPR excited in the ligaments. By changing the morphology of porous nanostructures such the pore and ligament size by dealloying time and thermal annealing, it is possible to achieve limited tunability in plasmonic resonance [58][59]. By dealloying ultra-dilute Au-Ag alloys with a low gold content of 1–5% at.%, an ultralow density nanoporous gold (ULDNPG) with better plasmonic photocatalytic SERS performances was created. To achieve the dealloying of such diluted solid solutions, a sandwich dealloying strategy was developed. Excellent SERS characteristics of these ULDNPG structures include high sensitivity, good repeatability, and low cost [60]. Small molecule label-free sensing has been accomplished using the morphological characteristics of NPG as a capturing scaffold. Recently, DNA topologically functionalized plasmonic nanostructures were used in SERS sensing systems. Target molecules, such as malachite green, can be attracted to NPG disks (NPGD) surfaces by stacking and electrostatic forces by using guanine quadruplex (G4) moieties. The collected molecules generated a remarkable SERS signal because of the high-density plasmonic hot-spots on NPG disks [61][62]. A microfluidic device with NPGD monolithically embedded inside has been used to produce a microfluidic SERS sensor. The three-dimensionally distributed nanoscale pores and ligaments in the NPGD, which appear as high-density SERS hot-spots, are what contributed to the enhanced surface area. Further ensuring extensive coverage of these hotspots on the microchannel floor are high-density NPGD arrays [63][64].
3.2. Hybrid Structures Involving np-Au
Electrochemical biosensors have been widely used in clinical research for recognizing biological analytes through a catalytic or binding event occurring at the electrode’s interface [65][66]. Tremendous demand for enhancing charge transport in the biosensors to significantly increase its sensitivity and reliability along with faster response times have stimulated intensive research on developing versatile materials with ultrahigh activities towards catalysis. For this reason, composite materials are being designed to combine highly electrocatalytic materials with a conductive material [67]. Recently, a hybrid electrode with ultra-thin, ultra-light, and flexible characteristics was created with graphitic carbon nitride (g-C3N4) nanosheets that have been electrochemically deposited on the surface of nanoporous gold film (NPGF). The hybrid electrode has shown a striking enhancement of supercapacitive performance (specific capacitance of 440 F g−1 at 2A g−1 in 0.5 M Na2SO4 solution). The superior property has been attributed to the strong interfacial effect between the defected gold atoms of np-Au and g-C3N4 [68]. Metal oxides supported three-dimensional (3D) hierarchical porous np-Au/Ni foam electrode has shown exceptionally high catalytic activity resulting mainly from its open and porous structure facilitating the mass transport and charge transfer [69]. Np-Au-modified biosensors with hybrid structures have been found more promising for meeting the demands of a highly sensitive and reliable biosensor with rapid response and better selectivity. Many related studies have mentioned the synergistic effect of hybrid structures behind this enhanced sensitivity and selectivity. X. Y. Lang et al. (2013) have reported a flexible and self-supported microelectrode having np-Au/cobalt oxide hybrid structure for electrochemical detection of glucose [67]. As per the study, the synergistic approach of gold skeleton, np-Au and cobalt oxide nanoparticles leads to the enhanced oxidation of glucose and thereby resulting in ultrahigh sensitivity. The sensitivity of up to 12.5 mA mM−1 cm−2 at a very short response time of less than a second was reported by the researchers with a very low detection limit of 5nM. A study performed by Y. Pei et al. (2018) used a hybrid structure of highly surface-roughened np-Au/Au-Sn alloy to increase the performance of a glucose biosensor [70].
References
- Shulga, O.V.; Zhou, D.; Demchenko, A.V.; Stine, K.J. Detection of free prostate specific antigen (fPSA) on a nanoporous gold platform. Analyst 2008, 133, 319–322.
- Chapman, C.A.; Chen, H.; Stamou, M.; Biener, J.; Biener, M.M.; Lein, P.J.; Seker, E. Nanoporous gold as a neural interface coating: Effects of topography, surface chemistry, and feature size. ACS Appl. Mater. Interfaces 2015, 7, 7093–7100.
- Kurtulus, O.; Daggumati, P.; Seker, E. Molecular release from patterned nanoporous gold thin films. Nanoscale 2014, 6, 7062–7071.
- Lee, M.N.; Santiago-Cordoba, M.A.; Hamilton, C.E.; Subbaiyan, N.K.; Duque, J.G.; Obrey, K.A.D. Developing Monolithic Nanoporous Gold with Hierarchical Bicontinuity Using Colloidal Bijels. J. Phys. Chem. Lett. 2014, 5, 809–812.
- Şeker, E.; Shih, W.-C.; Stine, K.J. Nanoporous metals by alloy corrosion: Bioanalytical and biomedical applications. MRS Bull. 2018, 43, 49–56.
- Tao, A.R.; Habas, S.; Yang, P. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325.
- Marino, A.; Arai, S.; Hou, Y.; Degl’Innocenti, A.; Cappello, V.; Mazzolai, B.; Chang, Y.-T.; Mattoli, V.; Suzuki, M.; Ciofani, G. Gold nanoshell-mediated remote myotube activation. ACS Nano 2017, 11, 2494–2508.
- Chapman, C.A.; Chen, H.; Stamou, M.; Lein, P.J.; Seker, E. Mechanisms of Reduced Astrocyte Surface Coverage in Cortical Neuron-Glia Co-cultures on Nanoporous Gold Surfaces. Cell. Mol. Bioeng. 2016, 9, 433–442.
- Kurtulus, O.; Seker, E. Nanotopography effects on astrocyte attachment to nanoporous gold surfaces. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 6568–6571.
- Chapman, C.A.R.; Wang, L.; Chen, H.; Garrison, J.; Lein, P.J.; Seker, E. Nanoporous Gold Biointerfaces: Modifying Nanostructure to Control Neural Cell Coverage and Enhance Electrophysiological Recording Performance. Adv. Funct. Mater. 2017, 27, 1604631.
- Goshi, N.; Morgan, R.K.; Lein, P.J.; Seker, E. A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J. Neuroinflamm. 2020, 17, 155.
- Tan, Y.H.; Terrill, S.E.; Paranjape, G.S.; Stine, K.J.; Nichols, M.R. The influence of gold surface texture on microglia morphology and activation. Biomater. Sci. 2014, 2, 110–120.
- Stine, K.J. Nanoporous Gold and Other Related Materials. Nanomaterials 2019, 9, 1080.
- Garcia-Gradilla, V.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Yardımcı, C.; Wiitala, D.; Galarnyk, M.; Wang, J. Ultrasound-Propelled Nanoporous Gold Wire for Efficient Drug Loading and Release. Small 2014, 10, 4154–4159.
- Polat, O.; Seker, E. Halide-Gated Molecular Release from Nanoporous Gold Thin Films. J. Phys. Chem. C 2015, 119, 24812–24818.
- Li, Z.; Seker, E. Configurable microfluidic platform for investigating therapeutic delivery from biomedical device coatings. Lab. Chip 2017, 17, 3331–3337.
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Demchenko, A.V.; Stine, K.J. Nanoporous Gold Monolith for High Loading of Unmodified Doxorubicin and Sustained Co-Release of Doxorubicin-Rapamycin. Nanomaterials 2021, 11, 208.
- Neupane, D.; Bhattarai, J.K.; Demchenko, A.V.; Stine, K.J. A pH sensitive thiolated β-cyclodextrin-modified nanoporous gold for controlled release of doxorubicin. J. Drug Deliv. Sci. Technol. 2020, 60, 101985.
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167.
- Pagneux, Q.; Ye, R.; Chengnan, L.; Barras, A.; Hennuyer, N.; Staels, B.; Caina, D.; Osses, J.I.A.; Abderrahmani, A.; Plaisance, V.; et al. Electrothermal patches driving the transdermal delivery of insulin. Nanoscale Horiz. 2020, 5, 663–670.
- Kim, K.; Jo, M.-C.; Jeong, S.; Palanikumar, L.; Rotello, V.M.; Ryu, J.-H.; Park, M.-H. Externally controlled drug release using a gold nanorod contained composite membrane. Nanoscale 2016, 8, 11949–11955.
- Daggumati, P.; Kurtulus, O.; Chapman, C.A.R.; Dimlioglu, D.; Seker, E. Microfabrication of nanoporous gold patterns for cell-material interaction studies. JoVE (J. Vis. Exp.) 2013, e50678.
- Palanisamy, B.; Goshi, N.; Seker, E. Chemically-Gated and Sustained Molecular Transport through Nanoporous Gold Thin Films in Biofouling Conditions. Nanomaterials 2021, 11, 498.
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20.
- Song, J.; Yang, X.; Yang, Z.; Lin, L.; Liu, Y.; Zhou, Z.; Shen, Z.; Yu, G.; Dai, Y.; Jacobson, O.; et al. Rational Design of Branched Nanoporous Gold Nanoshells with Enhanced Physico-Optical Properties for Optical Imaging and Cancer Therapy. ACS Nano 2017, 11, 6102–6113.
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1603–1611.
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120.
- Xuan, J.; Jia, X.-d.; Jiang, L.-P.; Abdel-Halim, E.S.; Zhu, J.-J. Gold nanoparticle-assembled capsules and their application as hydrogen peroxide biosensor based on hemoglobin. Bioelectrochemistry 2012, 84, 32–37.
- Radhakrishnan, D.; Mohanan, S.; Choi, G.; Choy, J.-H.; Tiburcius, S.; Trinh, H.T.; Bolan, S.; Verrills, N.; Tanwar, P.; Karakoti, A.; et al. The emergence of nanoporous materials in lung cancer therapy. Sci. Technol. Adv. Mater. 2022, 23, 225–274.
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Structure and applications of gold in nanoporous form. In Noble and Precious Metals-Properties, Nanoscale Effects and Applications; Seehra, M.S., Bristow, A., Eds.; IntechOpen: London, UK, 2018; pp. 341–365.
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238.
- Huynh, E.; Zheng, G. Cancer nanomedicine: Addressing the dark side of the enhanced permeability and retention effect. Nanomedicine 2015, 10, 1993–1995.
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1252–1276.
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193.
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release Off. J. Control. Release Soc. 2016, 244, 108–121.
- Lee, T.; Bang, D.; Chang, Y.W.; Choi, Y.; Park, K.; Oh, A.; Han, S.; Kim, S.H.; Lee, K.; Suh, J.-S.; et al. Cancer theranosis using mono-disperse, mesoporous gold nanoparticles obtained via a robust, high-yield synthetic methodology. RSC Adv. 2016, 6, 13554–13561.
- Liao, Y.-H.; Chang, Y.-J.; Yoshiike, Y.; Chang, Y.-C.; Chen, Y.-R. Negatively Charged Gold Nanoparticles Inhibit Alzheimer’s Amyloid-β Fibrillization, Induce Fibril Dissociation, and Mitigate Neurotoxicity. Small 2012, 8, 3631–3639.
- Hou, K.; Zhao, J.; Wang, H.; Li, B.; Li, K.; Shi, X.; Wan, K.; Ai, J.; Lv, J.; Wang, D.; et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 4790.
- Moore, K.A.; Pate, K.M.; Soto-Ortega, D.D.; Lohse, S.; van der Munnik, N.; Lim, M.; Jackson, K.S.; Lyles, V.D.; Jones, L.; Glassgow, N.; et al. Influence of gold nanoparticle surface chemistry and diameter upon Alzheimer’s disease amyloid-β protein aggregation. J. Biol. Eng. 2017, 11, 5.
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Qu, X. Gold-nanoparticle-based multifunctional amyloid-β inhibitor against Alzheimer’s disease. Chemistry 2015, 21, 829–835.
- Dos Santos Tramontin, N.; da Silva, S.; Arruda, R.; Ugioni, K.S.; Canteiro, P.B.; de Bem Silveira, G.; Mendes, C.; Silveira, P.C.L.; Muller, A.P. Gold Nanoparticles Treatment Reverses Brain Damage in Alzheimer’s Disease Model. Mol. Neurobiol. 2020, 57, 926–936.
- Neely, A.; Perry, C.; Varisli, B.; Singh, A.K.; Arbneshi, T.; Senapati, D.; Kalluri, J.R.; Ray, P.C. Ultrasensitive and Highly Selective Detection of Alzheimer’s Disease Biomarker Using Two-Photon Rayleigh Scattering Properties of Gold Nanoparticle. ACS Nano 2009, 3, 2834–2840.
- Dykman, L.A.; Khlebtsov, N.G. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55.
- Liu, D.; Chen, W.; Tian, Y.; He, S.; Zheng, W.; Sun, J.; Wang, Z.; Jiang, X. A Highly Sensitive Gold-Nanoparticle-Based Assay for Acetylcholinesterase in Cerebrospinal Fluid of Transgenic Mice with Alzheimer’s Disease. Adv. Healthc Mater. 2012, 1, 90–95.
- Rajendran, R.; Menon, K.N.; Nair, S.C. Nanotechnology Approaches for Enhanced CNS Drug Delivery in the Management of Schizophrenia. Adv. Pharm. Bull. 2022, 12, 490–508.
- Nivedhini Iswarya, C.; Kiruba Daniel, S.C.G.; Sivakumar, M. Studies on l-histidine capped Ag and Au nanoparticles for dopamine detection. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 393–401.
- Yang, S.; Feng, W.; Xue, L.; Yin, M.; Li, B.; Lu, L.; Dai, F.; Jiao, J.; Chen, Q. Multifunctional amino acids empowering bifunctional biosensing platform for depression study. Biosens. Bioelectron. 2022, 201, 113972.
- Patel, R.B.; Rao, H.R.; Thakkar, D.V.; Patel, M.R. Comprehending the potential of metallic, lipid, and polymer-based nanocarriers for treatment and management of depression. Neurochem. Int. 2022, 153, 105259.
- Wittstock, A.; Wichmann, A.; Bäumer, M. Nanoporous Gold as a Platform for a Building Block Catalyst. ACS Catalysis 2012, 2, 2199-2215.
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chemical Reviews 2004, 104, 293-346.
- Sakonsinsiri, C.; Puangmali, T.; Sreejivungsa, K.; Koowattanasuchat, S.; Thanan, R.; Chompoosor, A.; Kulchat, S.; Sithithaworn, P. Aptamer-based colorimetric detection of the DNA damage marker 8-oxo-dG using cysteamine-stabilised gold nanoparticles. RSC Advances 2022, 12, 25478-25486.Liu, S.; Tang, L.; Wang, J.; Fu, H.; Wan, X.; Li, Y. Label-free and sensitive detection of microalgae protein using GNRs-based resonance light scattering system. RSC Advances 2017, 7, 14039-14045.
- Liu, S.; Tang, L.; Wang, J.; Fu, H.; Wan, X.; Li, Y. Label-free and sensitive detection of microalgae protein using GNRs-based resonance light scattering system. RSC Advances 2017, 7, 14039-14045.
- Xiao, S.; Wang, S.; Wang, X.; Xu, P. Nanoporous gold: A review and potentials in biotechnological and biomedical applications. Nano Select 2021, 2, 1437-1458.
- Zhang, J.; Li, C.M. Nanoporous metals: fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chemical Society Reviews 2012, 41, 7016-7031.
- Srinivasan, B.; Tung, S. Development and Applications of Portable Biosensors. SLAS Technology 2015, 20, 365-389.
- Malik, P.; Gupta, R.; Malik, V.; Ameta, R.K. Emerging nanomaterials for improved biosensing. Measurement: Sensors 2021, 16, 100050.
- Zeng, J.; Zhao, F.; Li, M.; Li, C.-H.; Lee, T.R.; Shih, W.-C. Morphological control and plasmonic tuning of nanoporous gold disks by surface modifications. Journal of Materials Chemistry C 2015, 3, 247-252.
- Zeng, J.; Zhao, F.; Qi, J.; Li, Y.; Li, C.-H.; Yao, Y.; Lee, T.R.; Shih, W.-C. Internal and external morphology-dependent plasmonic resonance in monolithic nanoporous gold nanoparticles. RSC Advances 2014, 4, 36682-36688.
- Koya, A.N. Plasmonic Nanoarchitectures for Single-Molecule Explorations: An Overview. Advanced Photonics Research 2022, 3, 2100325.
- Huang, J.; Tang, C.; Chen, G.; He, Z.; Wang, T.; He, X.; Yi, T.; Liu, Y.; Zhang, L.; Du, K. Toward the Limitation of Dealloying: Full Spectrum Responsive Ultralow Density Nanoporous Gold for Plasmonic Photocatalytic SERS. ACS Applied Materials & Interfaces 2021, 13, 7735-7744.
- Qiu, S.; Zhao, F.; Zenasni, O.; Li, J.; Shih, W.-C. Nanoporous Gold Disks Functionalized with Stabilized G-Quadruplex Moieties for Sensing Small Molecules. ACS Applied Materials & Interfaces 2016, 8, 29968-29976.
- Arnob, M.; Shih, W.-C. Plasmonic metasurfaces for sensing, typing, and killing of pathogens. In Proceedings of Photonic Diagnosis and Treatment of Infections and Inflammatory Diseases II; pp. 43-48.
- Li, M.; Zhao, F.; Zeng, J.; Qi, J.; Lu, J.; Shih, W.C. Microfluidic surface-enhanced Raman scattering sensor with monolithically integrated nanoporous gold disk arrays for rapid and label-free biomolecular detection. Journal of biomedical optics 2014, 19, 111611.
- Misbah, I.; Shih, W.-C. Plasmonic Sensors on Invisible Substrates. In Proceedings of Biophotonics Congress: Optics in the Life Sciences Congress 2019 (BODA,BRAIN,NTM,OMA,OMP), Tucson, Arizona, 2019/04/14; p. DW2B.2.
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chemical Society Reviews 2010, 39, 1747-1763.
- Chen, R.J.; Choi, H.C.; Bangsaruntip, S.; Yenilmez, E.; Tang, X.; Wang, Q.; Chang, Y.-L.; Dai, H. An investigation of the mechanisms of electronic sensing of protein adsorption on carbon nanotube devices. Journal of the American Chemical Society 2004, 126, 1563-1568.
- Lang, X.-Y.; Fu, H.-Y.; Hou, C.; Han, G.-F.; Yang, P.; Liu, Y.-B.; Jiang, Q. Nanoporous gold supported cobalt oxide microelectrodes as high-performance electrochemical biosensors. Nature Communications 2013, 4, 1-8.
- Chen, A.Y.; Zhang, T.T.; Qiu, Y.J.; Wang, D.; Wang, P.; Li, H.J.; Li, Y.; Yang, J.H.; Wang, X.Y.; Xie, X.F. Construction of nanoporous gold/g-C3N4 heterostructure for electrochemical supercapacitor. Electrochimica Acta 2019, 294, 260-267.
- Li, Z.; He, Y.; Ke, X.; Gan, L.; Zhao, J.; Cui, G.; Wu, G. Three-dimensional nanoporous gold–cobalt oxide electrode for high-performance electroreduction of hydrogen peroxide in alkaline medium. Journal of Power Sources 2015, 294, 136-140.
- Pei, Y.; Hu, M.; Tu, F.; Tang, X.; Huang, W.; Chen, S.; Li, Z.; Xia, Y. Ultra-rapid fabrication of highly surface-roughened nanoporous gold film from AuSn alloy with improved performance for nonenzymatic glucose sensing. Biosensors and Bioelectronics 2018, 117, 758-765.




