Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Reinald Pamplona | -- | 1939 | 2023-01-16 13:58:46 | | | |
| 2 | Peter Tang | -2 word(s) | 1937 | 2023-01-17 01:35:02 | | | | |
| 3 | Peter Tang | Meta information modification | 1937 | 2023-01-17 01:35:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jové, M.; Mota-Martorell, N.; Obis, �.; Sol, J.; Martín-Garí, M.; Ferrer, I.; Portero-Otín, M.; Pamplona, R. Lipid Adaptations in Healthy Adult Human Brain. Encyclopedia. Available online: https://encyclopedia.pub/entry/40230 (accessed on 07 February 2026).
Jové M, Mota-Martorell N, Obis �, Sol J, Martín-Garí M, Ferrer I, et al. Lipid Adaptations in Healthy Adult Human Brain. Encyclopedia. Available at: https://encyclopedia.pub/entry/40230. Accessed February 07, 2026.
Jové, Mariona, Natàlia Mota-Martorell, Èlia Obis, Joaquim Sol, Meritxell Martín-Garí, Isidre Ferrer, Manuel Portero-Otín, Reinald Pamplona. "Lipid Adaptations in Healthy Adult Human Brain" Encyclopedia, https://encyclopedia.pub/entry/40230 (accessed February 07, 2026).
Jové, M., Mota-Martorell, N., Obis, �., Sol, J., Martín-Garí, M., Ferrer, I., Portero-Otín, M., & Pamplona, R. (2023, January 16). Lipid Adaptations in Healthy Adult Human Brain. In Encyclopedia. https://encyclopedia.pub/entry/40230
Jové, Mariona, et al. "Lipid Adaptations in Healthy Adult Human Brain." Encyclopedia. Web. 16 January, 2023.
Copy Citation
Lipids are a diverse and ubiquitous group of compounds with key roles in cell physiology. The multiplicity in lipid functions is achieved by the diversity in the structures of lipid molecules. At least six lipid adaptations against oxidative challenge in the healthy human brain can be discerned.
antioxidants
cholesterol
docosahexaenoic acid
fatty acids
lipidomics
lipid peroxidation
oleic acid
plasmalogens
1. Introduction
Lipids are a diverse and ubiquitous group of compounds with key roles in cell physiology. The multiplicity in lipid functions is achieved by the diversity in the structures of lipid molecules [1]. The exhibited structural diversity of lipids is determined by factors such as variable acyl chain length, number and position of double bonds, head groups, and chemical changes such as oxidations, reductions, ring-forming transformations, and substitutions, as well as modification with carbohydrate residues and other functional chemical groups. There are no reliable estimates of the number of discrete lipid species in nature, but based on alkyl/acyl chain and carbohydrate permutations for glycerolipids, glycerophospholipids, and sphingolipids, the theoretical number of lipid species can be estimated as about 180,000 [2].
Interestingly, in light of available evidence, this extraordinary diversity seems not to be expressed in brain lipid composition, where species seem to be selected during evolution for their specific properties optimizing neural cell structure and functional needs. Currently, the analytical ability of profiling large-scale changes in lipid composition and determining topographical distribution of individual lipid species in neuronal and glial cells has opened a new era for the study of the neurobiology of lipids. Thus, lipidomics has revolutionized the study of lipids in neuroscience, allowing the full characterization of lipid molecular species at all levels of the biological organization (lipidome), and in any condition [3][4].
For lipid classification, the International Lipid Classification and Nomenclature Committee, on the initiative of the LIPID MAPS Consortium, developed and established a comprehensive classification system based on well-defined chemical and biochemical principles, using a framework designed to be compatible with modern informatics technology (for more details about lipid classification and nomenclature, lipid structures, and bioinformatic tools for lipidomic analysis, see [5][6][7]). Based on this classification system, lipids are currently divided into eight categories: fatty acyls (FAs); glycerolipids (GLs); glycerophospholipids (GPs); sphingolipids (SPs); saccharolipids (SLs) and polyketides (PKs); and sterol (ST) and prenol lipids (PRs), which are further divided into classes and subclasses; a unique identification is assigned to each lipid species.
The importance of lipid species in the human brain is clear, both quantitatively and qualitatively. On the one hand, lipids composed about 12% of the fresh weight and half of the dry matter of the human brain [8]. On the other hand, the structural and functional diversity of brain lipids is astonishing. From the seminal studies of the neurochemist J.L.W. Thudichum [9][10] at the turn of the 20th century, a large body of knowledge on the biology of lipids of the brain tissue has been gathered. Thus, most lipid categories, classes, and subclasses are specifically represented in neuronal and glial cells, and the spectrum of lipid molecular species can be considered as the phenotypic expression of the diverse needs and functions ascribed to them. Figure 1 shows the structure of representative lipid species present in the adult human brain, divided into their main categories.
It is assumed that the human brain is especially susceptible to oxidative stress, based on specific traits such as a higher rate of mitochondrial free radical (e.g., reactive oxygen species, ROS) production, a high content in peroxidizable fatty acids (FAs), and a low antioxidative defense (for review, see [11][12]). However, it is also evident that human neurons, although they are post-mitotic cells, survive throughout an entire lifetime. Consequently, to attenuate or avoid the impact of oxidative stress on neuron functionality and survival, they must have evolved adaptive mechanisms to cope with the deleterious effects of oxidative stress. Several of these protective features are derived from lipid adaptations (see Section 5).
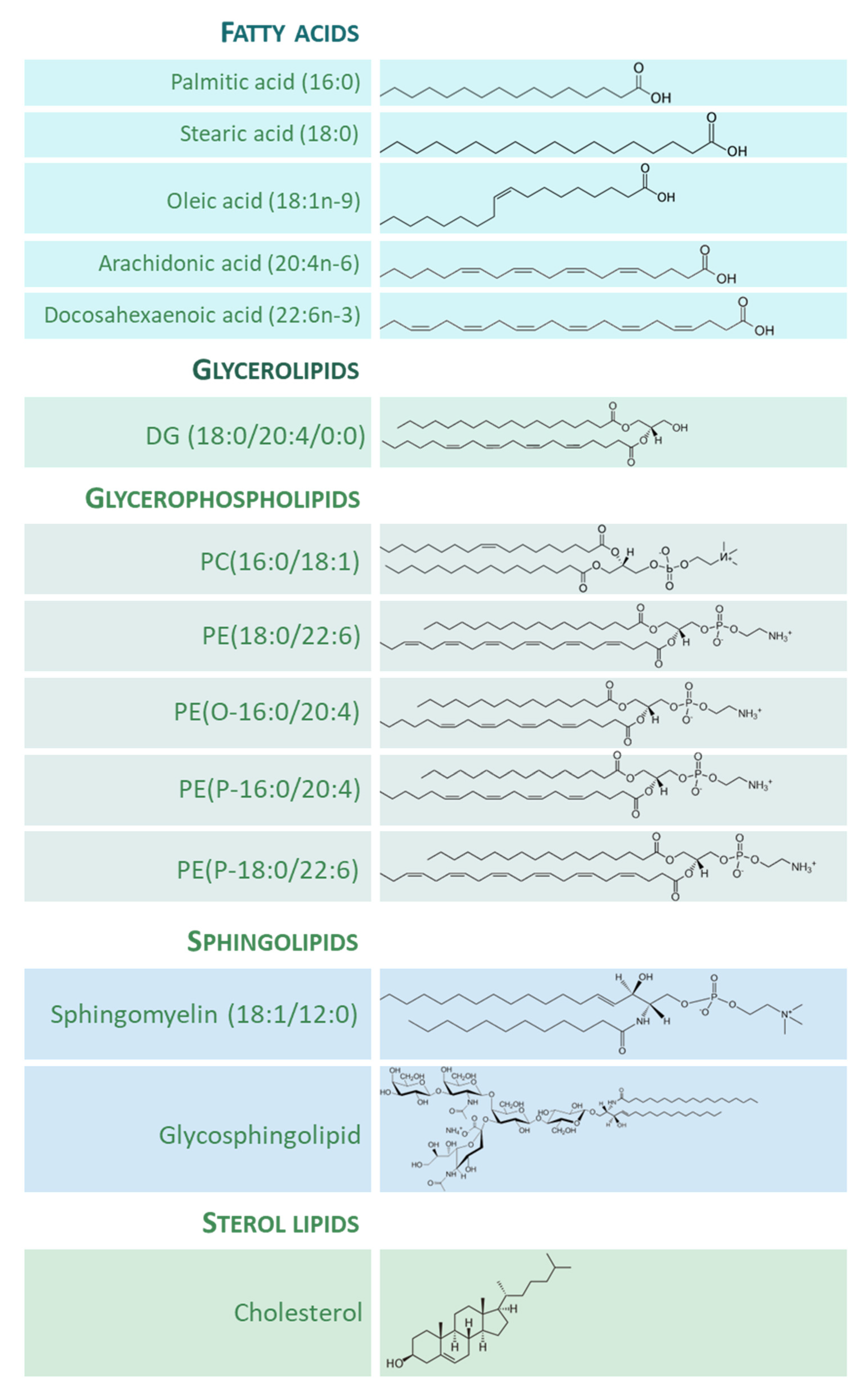
Figure 1. Representative lipid categories and specific molecular species present in the adult human brain. Fatty acids (FAs) shown characterize around 80–90% of total FA profile of the human brain ([13][14]). Glycerolipids (GL) in human brain comprise mono-, di-, and tri-acylglycerols. Glycerophospholipids (GP) are the major components ubiquitously found in neural cells; glycerophosphocholines, glycerophosphoethanolamines, glycerophosphoserines, and glycerophosphoinositols are the main GP present in the human brain [8]; molecular species represented are particularly abundant in human brain, highlighting the presence of ether lipids. Sphingolipids (SP) contain a common sphingoid base moiety; they are acylated to form ceramides, which are modified to generate phosphosphingolipids and glycosphingolipids. Sterol lipids comprise cholesterol and its derivatives.
2. Lipids in the Adult whole Human Brain
The adult whole human brain holds a large concentration of lipids with an amazing diversity of lipid classes and molecular species. The brain lipidome comprises a great diversity of GP classes and subclasses as well as a large portion of SP classes that define a specific human brain “sphingolipidome” [15]. In addition, cholesterol and its metabolites are also abundant in the human brain, containing 25% of the body’s total cholesterol [16].
GPs represent the 5% of the human whole wet brain and are the primary components found in human neural cell membranes [8]. The predominant FAs included in this lipid category are palmitic acid (16:0), palmitoleic acid (16:1n-7), stearic acid (18:0), oleic acid (18:1n-9), linoleic acid (18:2n-6), linolenic acid (18:3n-3), arachidonic acid (AA, 20:4n-6), and docosahexaenoic acid (DHA, 22:6n-3). Diacylglycerophosphates, which are precursors for GPs and neutral lipid (e.g., triradylglycerols) biosynthesis, are in low abundance in the brain (2% of total GPs). Concerning glycerophosphocholines (GPChos), the diacylglycerophosphocholines are the main form, representing 32.8% of the total content, being the primary molecular species PC (16:0-18:1) [17][18][19][20]. Among total GPChos, 2% represent ether lipid species. The glycerophosphoethanolamine (GPEtn) species account for 35.6% of total GPs [20][21]. The 1-(1Z-alkenyl),2-acylglycerophosphoethanolamines (50–60% of the GPEtn class) is the main form of this class, alkylacyl analogue content is low (5% of the GPEtn class), and diacylglycerophosphoethanolamines make up the remaining amount of GPEtns. The sn-1 glycerol position of GPEtn is mainly occupied by 16:0, 18:0, and 18:1n-9 groups; while position-2 consists of PUFA such as 20:4n-6 and 22:6n-3. The glycerophosphoserine species (GPSer) represents 16.6% of total GPs [20]. Among them, more than 90% occur as diacylglycerophosphoserines; the remaining 10% occur as 1-(1Z-alkenyl),2-acylglycerophosphoserine, containing primarily 18:0, 18:1n-9, and 22:6n-3 as FAs. Inositol phosphoglycerides account for about 2.6% of total GPs [19]. Glycerophosphoinositol (GPIn) and glycerophosphoinositol trisphosphate are additional relevant GPs with only trace amounts of glycerophosphoinositol bisphosphates. The brain contains the highest concentrations of GPIn among tissues and the main FA components are 18:0 and 20:4n-6. Finally, 0.2% of the human brain’s GPs are glycerophosphoglycerols (GPGs), and 0.1% are glycerophosphoglycerophosphoglycerols (cardiolipins) [22].
SPs are a category of complex lipids which occur in particularly large concentrations in the human brain [8][23]. This lipid category mainly consists of phosphosphingolipids (including ceramide phosphocholines (sphingomyelins), neutral glycosphingolipids (including cerebrosides of the different series: globo, ganglio, lacto, neolacto, isoglobo, mollu, and arthro), and acidic glycosphingolipids (including gangliosides and sulfoglycosphingolipids, or sulfatides), among others. Sphingomyelins account for about 14.8% of the SP content [17][20] and comprise mainly 18:0, lignoceric (24:0), and nervonic (24:1) acids. Cerebrosides amount to 15.8% of the total lipids [17]. The FA components of the cerebrosides typically contain hydroxyl FAs, and these account for more than 50% of the total FAs. Among the non-hydroxyl FAs, 24:0 and 24:1 are the main components, and cerebronic (24h:0) and hydroxynervonic (24h:1) are the most abundant hydroxyl FAs. Sulfatides are acidic glycosphingolipids and the only sulfoglycosphingolipids present in the brain, accounting for about 6.2% of the brain’s total [17]. The FA composition of sulfatides is similar to that of cerebrosides. Gangliosides, sialic acid-containing glycosphingolipids, occur ubiquitously in cell plasma membranes and are also particularly abundant in the brain [8][24]. The lipid moiety of gangliosides, the ceramide, consists of a long-chain amino alcohol linked to a FA by an amide linkage. The 18 and 20 carbon atom structures containing a trans double bond at position 4–5 are the most abundant long-chain amino alcohol components of brain gangliosides; they are generally referred to as “sphingosine”. The long-chain amino alcohols not containing a double bond at position 4–5 are generally referred to as sphinganine. Stearic acid (18:0) is the main FA of the human brain gangliosides and form over 80% of the total ganglioside FA content [24].
3. Functional Properties of Lipid Species in the Human Brain
Neural cell membranes are asymmetric and dynamic entities that require continuous remodeling in their lipids (chemical structure and molecular shape) in order to respond and adapt to internal and external changes. Brain lipids have different functional properties and actively contribute to guarantee the integrity of neuronal and glial cell membranes and to generate lipid messengers. Furthermore, the FAs present in these lipid species have intrinsic physicochemical properties that determine their chemical reactivity.
4. Evolution of the Human Brain Lipid Composition
The evolution of the human brain has given rise to a structure of enormous complexity. This complexity is also reflected in the cell/tissue lipidome which, although dynamic, is strictly regulated and adapted to all biological organization levels (lipid bilayer domains, subcellular organelles, cell type, tissue, and animal species) [25][26][27][28].
Effectively, diverse studies have revealed the existence of specific traits of the human brain evolution at the lipidome level. Thus, the existence of a specific lipidome of brain tissue and a singular fingerprint of each brain region have been described [29]. This observation can be extended to other animal species (rodents and primates), in which lipidome systematically distinguish the brain from the non-neural tissues. This brain-specific lipidome [29] includes an enrichment in glycosyldiradylglycerols, GPCho, GPEtn, GPG, and neutral glycosphingolipids, and a depletion in fatty amides, triradylglycerols, and sterols. More specifically, the enriched categories are represented in specific lipid subclasses, namely ceramides, dihydroceramides, and, quite particularly, in alkenyl phosphatidylethanolamines (PE(P-) or plasmalogens) [29]. Notably, the extent of differences in the lipidome composition between the brain and non-neural tissues increases in parallel with the increase in the brain function capacity from mice to humans (Figure 2). Within the human brain, inter-regional comparative studies also demonstrated the existence of region-specific differences at the lipidome level [13][14][30]. Significantly, there is an acceleration of lipidome evolution in the neocortical regions that, in addition, specifically affects the lipid subclasses enriched in the brain. This evidence suggests that brain lipidome evolution could contribute to neocortex and brain expansion and the emergence of novel human cognitive functions [29]. Furthermore, it is postulated that lipid species played important roles during evolution that confer self-protection to the brain.
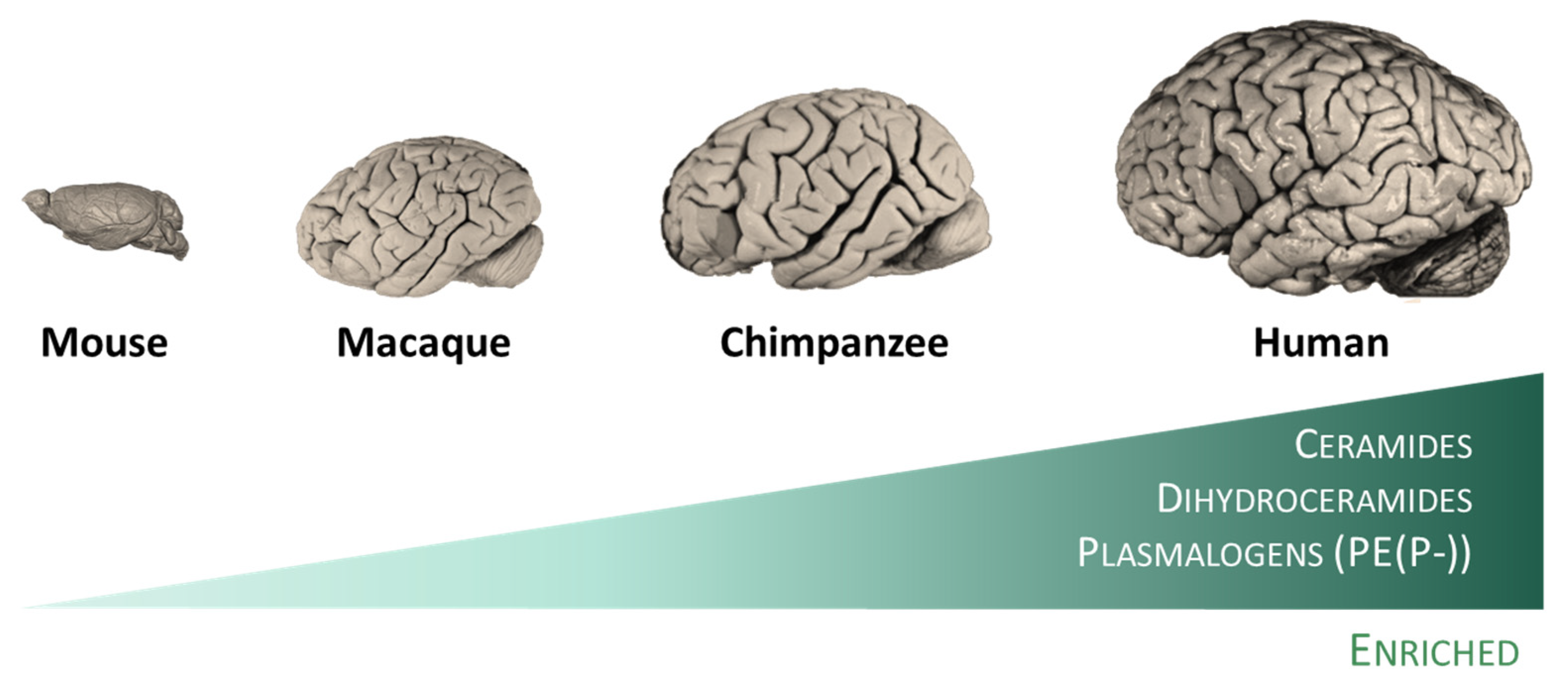
Figure 2. The human brain possesses specific traits at the lipidome level. Among mammalian species, humans included, lipidomes systematically distinguish the brain from the non-neural tissues [29]. This specific lipidome includes an enrichment in ceramides, dihydroceramides, and plasmalogens, and a depletion in fatty amides, triradylglycerols, and sterols. Notably, the extent of differences in the lipidome composition between the brain and non-neural tissues increases in parallel with the increase in the brain function capacity from mice to humans.
5. Lipid Adaptations against Oxidative Challenge in the Healthy Human Brain
It is widely accepted that the brain is highly susceptible to oxidative stress. To support this statement, different factors, such as oxygen consumption and reactive species generation, calcium, glutamate, glucose, mitochondria, generation of free radicals from an endogenous neurotransmitter metabolism, neurotransmitters’ auto-oxidation, modest endogenous antioxidant defense, microglia, redox-active transition metals, use of NOS and NOX for signaling, RNA oxidation, and unsaturated lipid enrichment, have been adduced (for review, see [11][12]). However, the observation that human neurons are functional over an entire lifetime is also irrefutable. Therefore, the existence of mechanisms that preserve function and protect against aging and age-related neurodegeneration can be inferred. In this context, it is proposed that the human brain has evolved to become resistant to stress though lipid-mediated adaptations to preserve neural cells’ integrity and cognitive function across an entire lifespan. These adaptations affect the type and content of lipids that compose neural cell membranes, and derived compounds and signaling pathways with neuroprotective properties.
References
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2011, 1811, 637–647.
- Yetukuri, L.; Ekroos, K.; Vidal-Puig, A.; Orešič, M. Informatics and computational strategies for the study of lipids. Mol. Biosyst. 2008, 4, 121–127.
- Han, X. Neurolipidomics: Challenges and developments. Front. Biosci. 2007, 12, 2601.
- Han, X.; Gross, R.W. The foundations and development of lipidomics. J. Lipid Res. 2022, 63, 100164.
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14.
- Subramaniam, S.; Fahy, E.; Gupta, S.; Sud, M.; Byrnes, R.W.; Cotter, D.; Dinasarapu, A.R.; Maurya, M.R. Bioinformatics and systems biology of the lipidome. Chem. Rev. 2011, 111, 6452–6490.
- Fahy, E.; Subramaniam, S.; Alex Brown, H.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861.
- Sastry, P.S. Lipids of nervous tissue: Composition and metabolism. Prog. Lipid Res. 1985, 24, 69–176.
- Thudichum, J.L. A Treatise on the Chemical Constitution of the Brain; Archon Books: Hamden, CT, USA, 1962.
- Thudichum, J.L.W. A treatise on the chemical constitution of the brain: Based throughout upon original researches. Glasgow Med. J. 1884, 22, 363–364.
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623.
- Cobley, J.N.; Fiorello, M.L.; Miles Bailey, D. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503.
- Naudí, A.; Cabré, R.; Ayala, V.; Jové, M.; Mota-Martorell, N.; Portero-Otín, M.; Pamplona, R. Region-specific vulnerability to lipid peroxidation and evidence of neuronal mechanisms for polyunsaturated fatty acid biosynthesis in the healthy adult human central nervous system. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2017, 1862, 485–495.
- Mota-Martorell, N.; Andrés-Benito, P.; Martín-Gari, M.; Galo-Licona, J.D.; Sol, J.; Fernández-Bernal, A.; Portero-Otín, M.; Ferrer, I.; Jove, M.; Pamplona, R. Selective brain regional changes in lipid profile with human aging. GeroScience 2022, 44, 763–783.
- Merrill, A.H.; Sullards, M.C.; Allegood, J.C.; Kelly, S.; Wang, E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005, 36, 207–224.
- Dietschy, J.M.; Turley, S.D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001, 12, 105–112.
- Rouser, G.; Galli, C.; Kritchevsky, G. Lipid class composition of normal human brain and variations in metachromatic leucodystrophy, tay-sachs, niemann-pick, chronic gaucher’s and alzheimer’s diseases. J. Am. Oil Chem. Soc. 1965, 42, 404–410.
- Rouser, G.; Feldman, G.; Galli, C. Fatty acid compositions of human brain lecithin and sphingomyelin in normal individuals, senile cerebral cortical atrophy, alzheimer’s disease, metachromatic leucodystrophy, tay-sachs and niemann-pick diseases. J. Am. Oil Chem. Soc. 1965, 42, 411–412.
- Rouser, G.; Yamamoto, A. Curvilinear regression course of human brain lipid composition changes with age. Lipids 1968, 3, 284–287.
- O’brien, J.S.; Sampson, E.L. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 537.
- Panganamala, R.V.; Horrocks, L.A.; Geer, J.C.; Cornwell, D.G. Positions of double bonds in the monounsaturated Alk-1-Enyl groups from the plasmalogens of human heart and brain. Chem. Phys. Lipids 1971, 6, 97–102.
- Kahma, K.; Brotherus, J.; Haltia, M.; Renkonen, O. Low and moderate concentrations of lysobisphosphatidic acid in brain and liver of patients affected by some storage diseases. Lipids 1976, 11, 539–544.
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67.
- Sonnino, S.; Chigorno, V. Ganglioside molecular species containing C18- and C20-sphingosine in mammalian nervous tissues and neuronal cell cultures. Biochim. Biophys. Acta-Rev. Biomembr. 2000, 1469, 63–77.
- Aviram, R.; Manella, G.; Kopelman, N.; Neufeld-Cohen, A.; Zwighaft, Z.; Elimelech, M.; Adamovich, Y.; Golik, M.; Wang, C.; Han, X.; et al. Lipidomics analyses reveal temporal and spatial lipid organization and uncover daily oscillations in intracellular organelles. Mol. Cell 2016, 62, 636–648.
- Khrameeva, E.; Kurochkin, I.; Bozek, K.; Giavalisco, P.; Khaitovich, P. Lipidome evolution in mammalian tissues. Mol. Biol. Evol. 2018, 35, 1947–1957.
- Pradas, I.; Huynh, K.; Cabré, R.; Ayala, V.; Meikle, P.J.; Jové, M.; Pamplona, R. Lipidomics reveals a tissue-specific fingerprint. Front. Physiol. 2018, 9, 1165.
- Jové, M.; Mota-Martorell, N.; Pradas, I.; Galo-Licona, J.D.; Martín-Gari, M.; Obis, È.; Sol, J.; Pamplona, R. The Lipidome Fingerprint of Longevity. Molecules 2020, 25, 4343.
- Bozek, K.; Wei, Y.; Yan, Z.; Liu, X.; Xiong, J.; Sugimoto, M.; Tomita, M.; Pääbo, S.; Sherwood, C.C.; Hof, P.R.; et al. Organization and evolution of brain lipidome revealed by large-scale analysis of human, chimpanzee, macaque, and mouse tissues. Neuron 2015, 85, 695–702.
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Lipid compositions of different regions of the human brain during aging. J. Neurochem. 1990, 54, 415–423.
More
Information
Subjects:
Physiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
974
Revisions:
3 times
(View History)
Update Date:
17 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




