Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sathish Kumar Natarajan | -- | 2447 | 2023-01-16 12:58:09 | | | |
| 2 | Conner Chen | + 18 word(s) | 2465 | 2023-01-17 07:22:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Thompson, M.; Ulu, A.; Mukherjee, M.; Yuil-Valdes, A.G.; Thoene, M.; Ormer, M.V.; Slotkowski, R.; Mauch, T.; Anderson-Berry, A.; Hanson, C.K.; et al. Role of FA Metabolites and Inflammation during Pregnancy. Encyclopedia. Available online: https://encyclopedia.pub/entry/40227 (accessed on 08 February 2026).
Thompson M, Ulu A, Mukherjee M, Yuil-Valdes AG, Thoene M, Ormer MV, et al. Role of FA Metabolites and Inflammation during Pregnancy. Encyclopedia. Available at: https://encyclopedia.pub/entry/40227. Accessed February 08, 2026.
Thompson, Maranda, Arzu Ulu, Maheswari Mukherjee, Ana G. Yuil-Valdes, Melissa Thoene, Matthew Van Ormer, Rebecca Slotkowski, Teri Mauch, Ann Anderson-Berry, Corrine K. Hanson, et al. "Role of FA Metabolites and Inflammation during Pregnancy" Encyclopedia, https://encyclopedia.pub/entry/40227 (accessed February 08, 2026).
Thompson, M., Ulu, A., Mukherjee, M., Yuil-Valdes, A.G., Thoene, M., Ormer, M.V., Slotkowski, R., Mauch, T., Anderson-Berry, A., Hanson, C.K., Nordgren, T.M., & Natarajan, S.K. (2023, January 16). Role of FA Metabolites and Inflammation during Pregnancy. In Encyclopedia. https://encyclopedia.pub/entry/40227
Thompson, Maranda, et al. "Role of FA Metabolites and Inflammation during Pregnancy." Encyclopedia. Web. 16 January, 2023.
Copy Citation
Normal pregnancy relies on inflammation for implantation, placentation, and parturition, but uncontrolled inflammation can lead to poor maternal and infant outcomes. Maternal diet is one modifiable factor that can impact inflammation. Omega-3 and -6 fatty acids obtained through the diet are metabolized into bioactive compounds that effect inflammation. The downstream products of omega-3 and -6 fatty acids may influence physiology during pregnancy.
omega-3 fatty acid
omega-6 fatty acid
oxylipins
1. The Relationship between Inflammation and Nutrition during Normal Pregnancy
Normal pregnancies rely on inflammation from implantation of the blastocyst to parturition. Mor et al. categorized pregnancy into three immunological events, denoted as Stages 1–3 in Figure 1 and Figure 2 [1]. Early pregnancy is the first stage which includes the first and second trimester when implantation and placentation occur. During this stage, the body relies heavily on inflammation to allow placental cells to invade and establish the maternal–fetal blood flow. The second stage is mid-pregnancy, where rapid fetal growth and development occur within a characteristically anti-inflammatory environment. Lastly, late pregnancy is an inflammatory process as mothers begin to prepare for delivery [2][3][4].
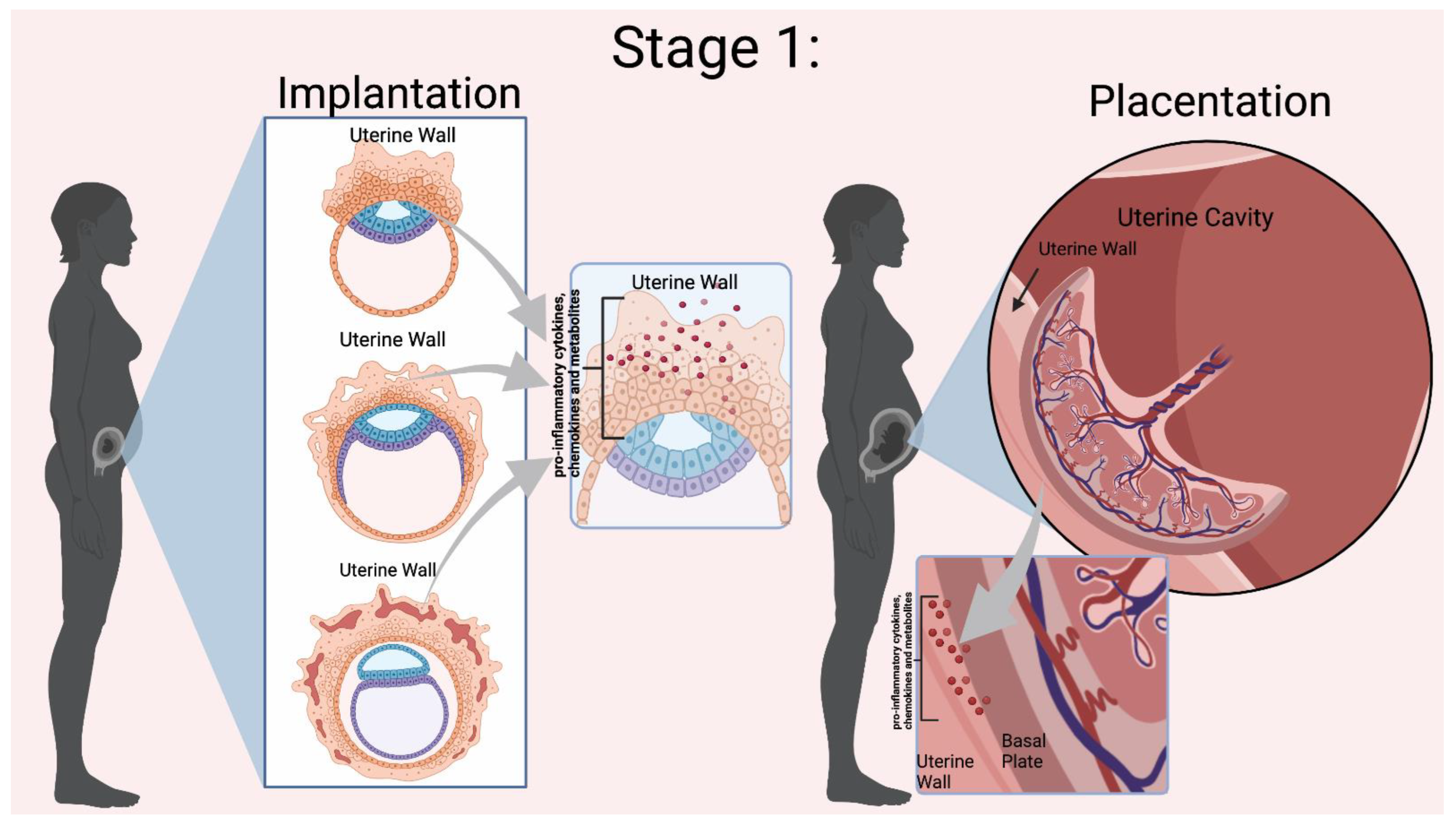
Figure 1. Mor et al. Stage 1 of Immunological Events Occurring During Pregnancy. Implantation relies on the presence of pro-inflammatory cytokines, chemokines, and other mediators to invade the uterine wall [5]. During placentation there is a dynamic relationship between the uterine wall and the placenta for trophoblast differentiation and proper placental development. Inflammation is indicated by the red dots in the Figure. Figure created in BioRender.
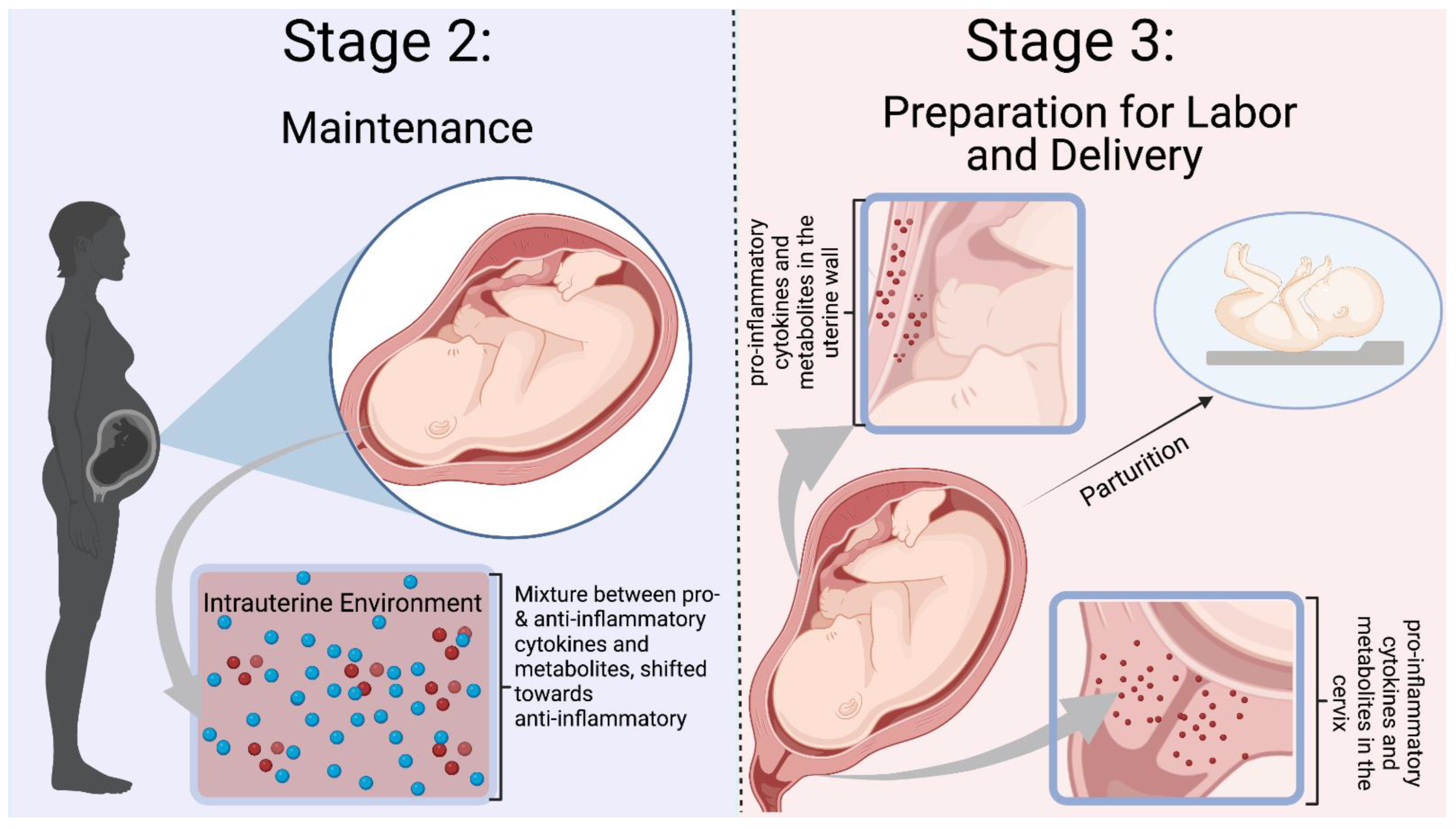
Figure 2. Mor et al. Stages 2–3 of Immunological Events Occurring During Pregnancy. Stage 2 is defined by a balance between pro- and anti-inflammatory mediators, with a shift towards an anti-inflammatory environment. Inflammation is needed to prevent infection and rejection of the fetus. Stage 3 is a pro-inflammatory state with preparation for labor and delivery. Cytokines and mediators remodel the cervix and infiltrate the uterus to participate in uterine contractions [5]. The blue dots represent ant-inflammatory molecules, and the red dots represent pro-inflammatory molecules. Figure was created in Biorender.
During pregnancy, both n-3 and omega-6 fatty acids (n-6 FAs) are necessary for proper fetal development, and their role in the different stages of inflammation was described by Mor et al. For example, the n-3 fatty acids (FA) docosahexaenoic (DHA) is essential for the proper development of the fetal nervous system and retina. Arachidonic acid (AA), an n-6 FA, also accumulates in the fetal brain [1]. However, an imbalance in n-3 and n-6 FA intake can influence the homeostatic equilibrium within the maternal and infant body. There are no consistent guidelines for the recommended daily intake of omega-3 fatty acids (n-3 FAs) during pregnancy, but generally, around 200 g of eicosapentaenoic acid (EPA) + DHA per day is suggested [6]. In the United States, the Western diet is high in n-6 FAs and low in n-3 FAs intake [7][8]. Research has demonstrated that mothers do not reach the suggested daily intake in pregnancy [9][10], which potentially puts them at risk for poor maternal–infant outcomes due to dysregulated inflammation. Therefore, a balance between the intakes of n-3 and n-6 FAs is necessary for normal pregnancy.
Recent evidence suggests that the role of n-6 and n-3 FAs is attributable to their metabolism into bioactive metabolites involved in the inflammatory cascade [11]. The parent n-6 FAs and n-3 FAs competitively compete for the lipoxygenase (LOX), cyclo-oxygenase (COX), or cytochrome P450 enzymes (CYP450), creating eicosanoids. Omega-6 FAs generally produce pro-inflammatory eicosanoids, while n-3 FA metabolites are anti-inflammatory. The amount of n-6 to n-3 FAs influences the types of FAs that are metabolized and ultimately determines the produced eicosanoids [12]. These eicosanoids either enhance the pro-inflammatory environment or move the process toward repair and resolution. Because eicosanoids also have a role in regulating inflammation during pregnancy, more research is needed to understand how these metabolites function “normally.”
AA is a major n-6 FA that produces intermediates with a spectrum of characteristics based on the enzymatic pathway. The COX pathway produces 2-series prostanoids (i.e., prostaglandins and thromboxane). Prostaglandins have been shown to influence T-cell differentiation, platelet aggregation, and inflammatory cytokines [13]. Thromboxane A2 causes platelet aggregation and contributes to vascular constriction [14]. Entry into the LOX pathway creates bioactive leukotrienes and the monohydroxyeicosatetraenoic acids 5–12 (HETEs) from hydroperoxyeicosatetraenoic acid (HpETE). HETEs stimulate chemokinesis and chemotactic responses in eosinophils and neutrophils [15]. Leukotrienes are signaling molecules contributing to vascular inflammation and inflammatory diseases [16]. Lastly, the metabolism of AA through the CYP450 pathway creates hydroxy-eicosatetraenoic acids 16–20 (HETE), epoxides, and epoxyeicosatrienoic acid (EET). A well-studied HETE, 20-HETE, has demonstrated pro-inflammatory effects and influences on the vascular function [17]. EETs are further broken down by epoxide hydrolase into diols or dihyroxyeicosatrienoic acids (DHET) [18]. EETs are being studied for their vasodilation, regulation of cell differentiation, proliferation, migration, and role in cellular inflammation and apoptosis [15].
The other two major n-6 FAs are dihomo γ-linolenic acid (DGLA) and LA. DGLA produces 1-series prostanoids in the COX pathway. Hydroxyeicosatrienoic acids (HETrE) are formed by LOX activity, while CYP-450 (CYP) produces dihydroxyeicosadienoic acid (DiHEDE). HETrE intermediates have been shown to regulate platelets and epidermal cells and the inflammatory response of neutrophils [19]. Linoleic acid (LA) is the substrate for hydroxyoctadecadienoic acids (HODEs) through LOX that regulates platelet activation. Dihyroxdyoctadecenoic acids (DiHOMEs) in the CYP pathway have been found to cause mitochondrial dysfunction and promote allergic inflammation [20][21]. Figure 3 illustrates a condensed schema for the breakdown of n-6 FAs into their intermediates and bioactive compounds through the LOX, COX, and CYP enzymatic pathways.
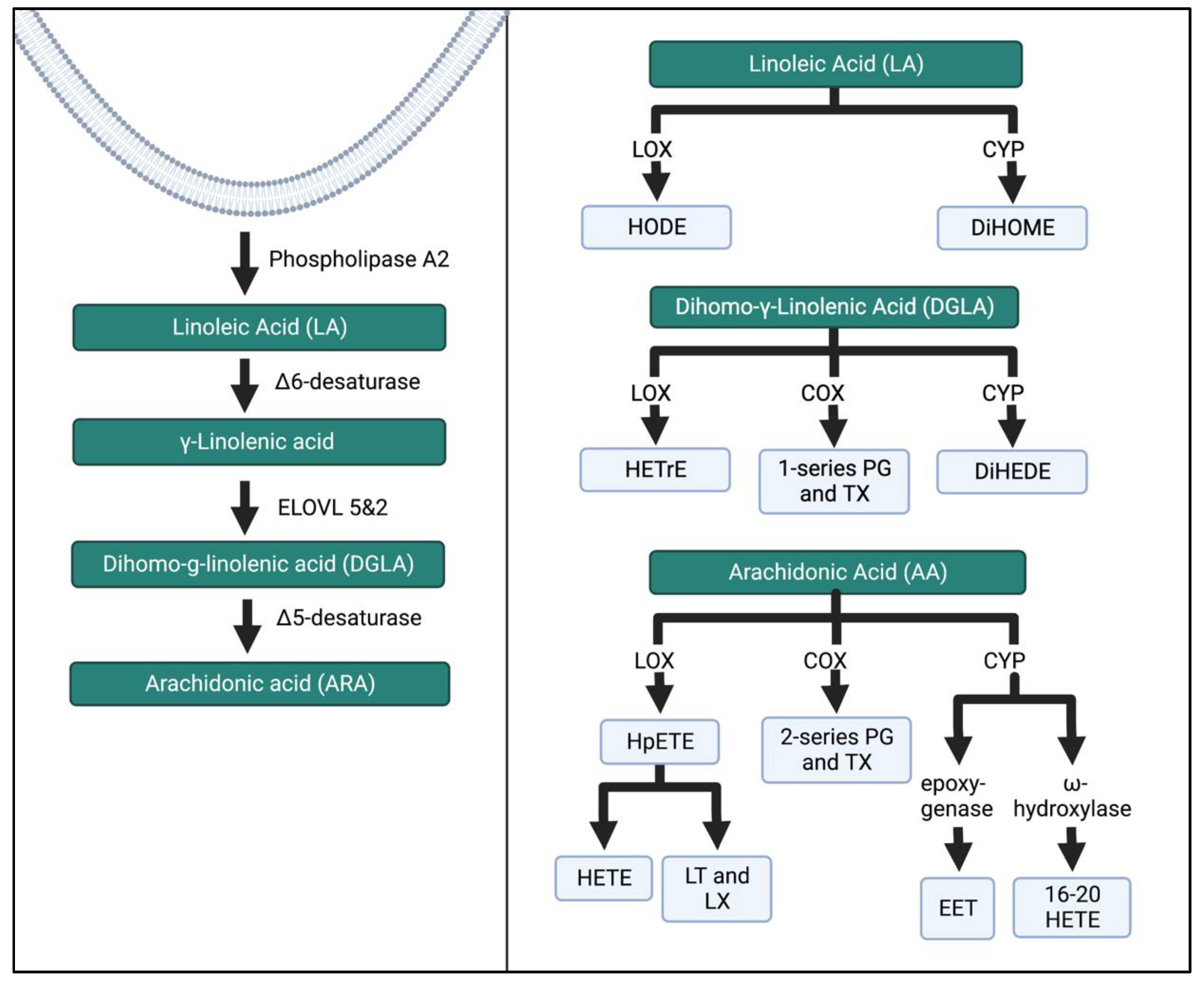
Figure 3. N-6 FA metabolism through the LOX, COX, and CYP enzymatic pathways. These pathways have been simplified from previous research [15][22]. ELOVL, elongation of very long chain fatty acid elongase protein; HODE, hydroxy-octadecadienoic acids; DiHOME, dihydroxy-octadecenoic acids; HETrE, hydroxy-eicosatrienoic acids; DiHEDE, dihydroxy-eicosadienoic acid; PG, prostaglandin; TX, thromboxane; HpETE, hydroperoxy-eicosatetraenoic acid; HETE, creates hydroxyeicosatetraenoic acids; LT, leukotriene; LX, lipoxin; EET, epoxyeicosatrienoic acid. The Figure was created in Biorender.
ALA, EPA, and DHA are major substrates for intermediates and bioactive compounds (Figure 4 and Figure 5). Alpha-linolenic acid (ALA) produces hydroxy-octadecatrienoic acid (HOTrE) via LOX enzymes that produce anti-inflammatory effects through the inactivation of inflammasome complexes, activate autophagy, and induce apoptosis [23]. CYP activity creates dihydroxy-octadecadienoic acid (DiHODE) whose role and tissue distribution are not completely understood [13].
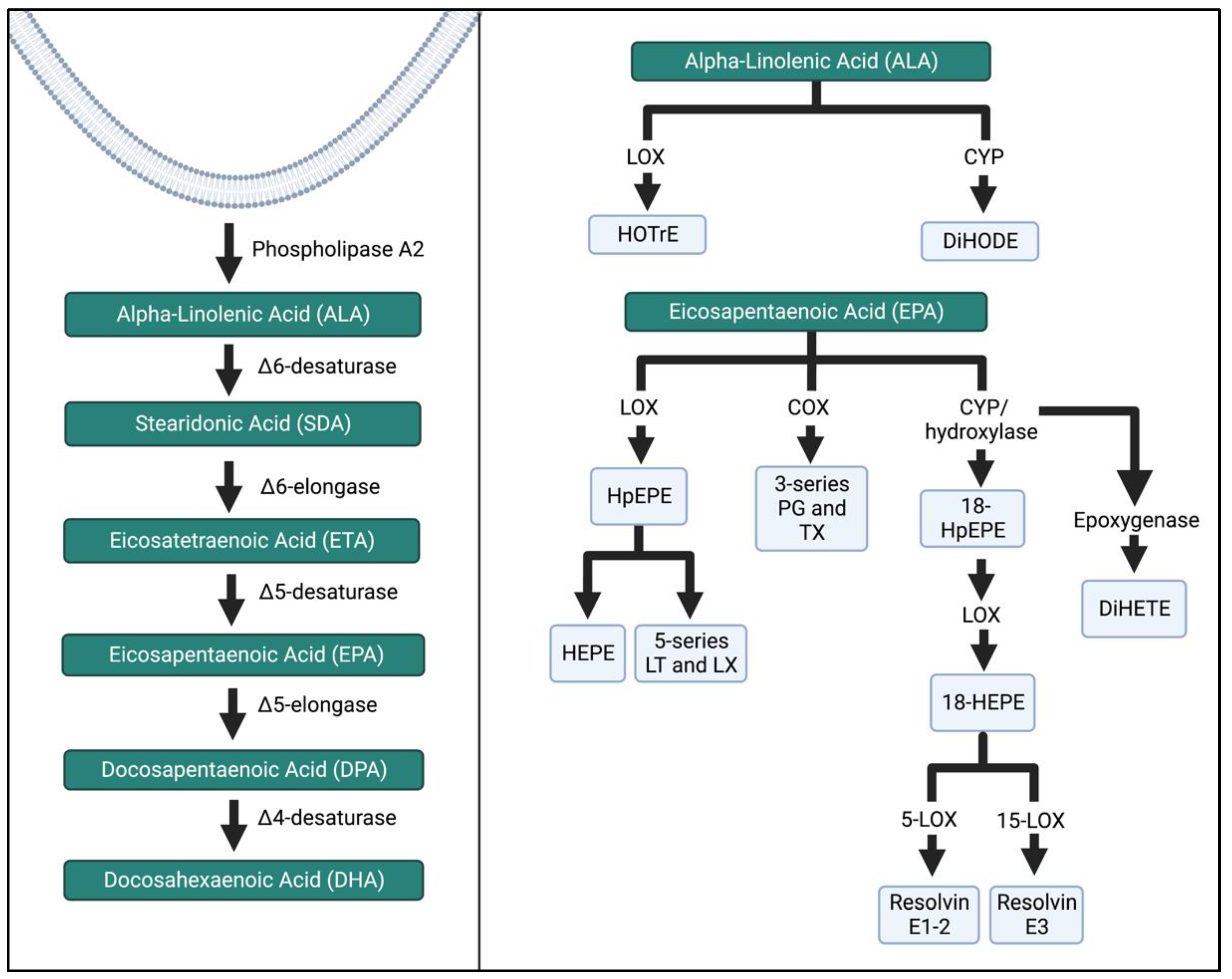
Figure 4. ALA and EPA metabolism by the LOX, COX, and CYP enzymatic pathways. The schema was simplified, and more detailed pathways can be found in the references listed in the Figure above. HOTrE, hydroxy-octadecatrienoic acid; DiHODE, dihydroxy-octadecadienoic acid, HpEPE, hydroperoxyl-eicosapentaenoic acid; HEPE, hydroxyeicosapentaenoic acid; LT, leukotriene; LX, lipoxin; TX, thromboxane; DiHETE, dihydroxy-eicosatetraenoic acid. The Figure was created in Biorender.
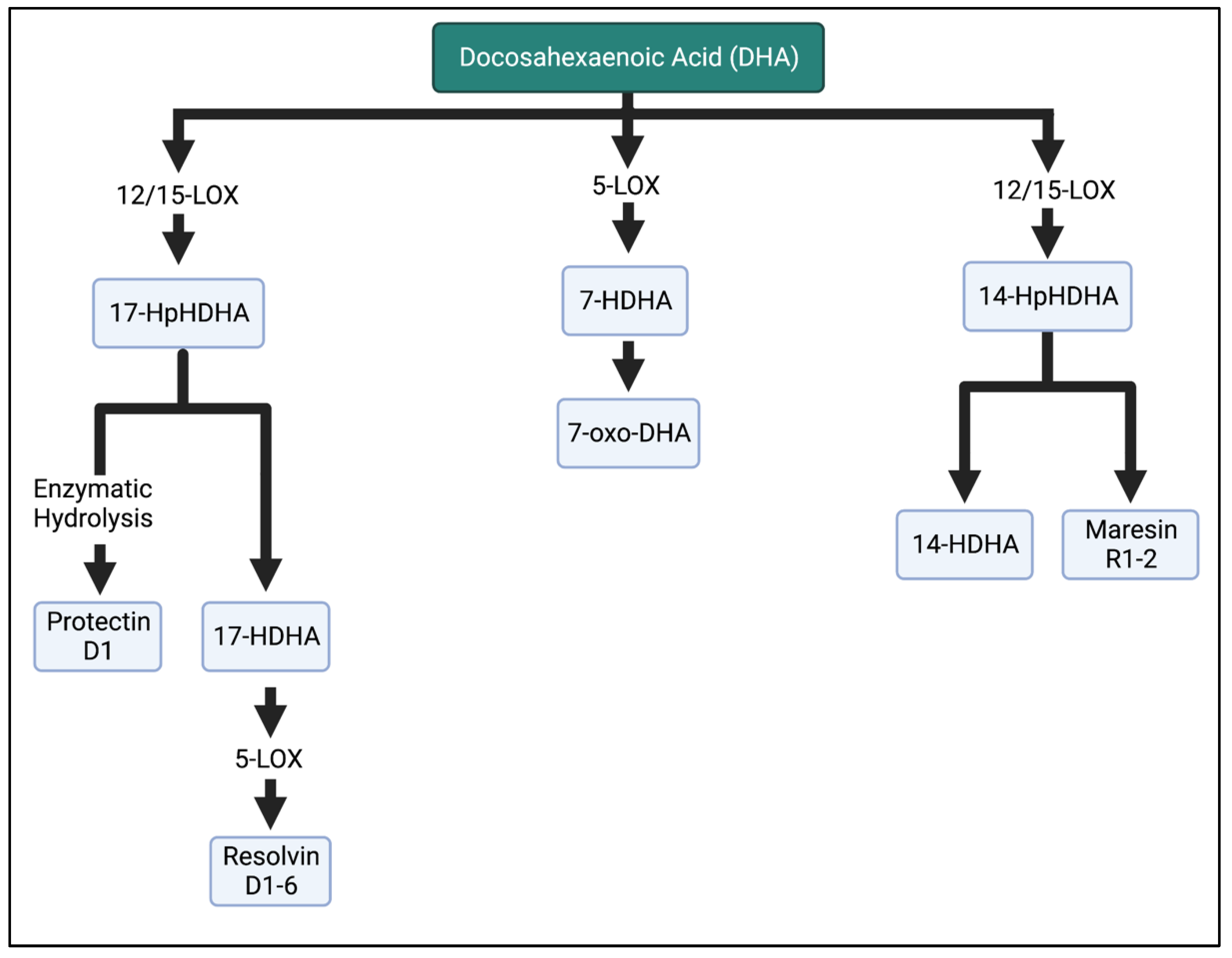
Figure 5. Metabolism of DHA in the LOX enzymatic pathway. DHA is metabolized into resolvins, maresins, and protectins, which are involved in the anti-inflammatory properties of n-3 FAs. HpHDHA, hydroperoxydocosahexaenoic acid; HDHA, hydroxydocosahexaenoic acid; DHA, docosahexaenoic acid. The Figure was created in BioRender.
EPA intermediates and resolvins mediate the effects of the immune system. LOX enzymatic breakdown of EPA yields the intermediates hydroperoxyl-eicosapentaenoic acid (HpEPE) and hydroxyeicosapentaenoic acid (HEPE) and the bioactive compounds leukotrienes (LT) A-E5 and lipoxins (LX) A5 and B5. The metabolite 18-HEPE is produced via CYP, and then further LOX activity reduces the adhesion of monocytes to endothelial cells, while also attenuating fibroblast activation. E-series resolvins are produced via further conversion of 18-HEPE. Resolvins E1–3 inhibit and reduce polymorphonuclear leukocyte infiltration [24][25][26].
2. Implantation, Inflammation, and Fatty Acids’ Role
Using Mor et al. stages of pregnancy, implantation is part of the first stage which requires inflammation to assist in blastocyst adherence to the uterine wall and initiate processes that establish a maternal–fetal connection [3][4]. The fertilized egg, now a blastocyst, must attach to the uterine wall and achieves this through the recruitment of immune cells to the implantation site. Immune cells assist the blastocyst in getting through the epithelial lining of the uterus to the endometrium to implant [3]. In addition, immune cells also help replace the endothelium and vascular smooth muscle cells of the maternal blood vessels to establish the blood flow from the placenta to the fetus [27]. The decidua houses critical immune cells such as natural killer (NK) cells, macrophages, and dendritic cells (DCs). Polarized macrophages (Th-1) and cytokines (e.g., IL-6, IL-8, and TNF-alpha) are important inflammatory players [4]. The communication between the uterus and the blastocyst establishes pregnancy.
Evidence suggests that the different FA enzymatic pathways are key modulators of inflammation during implantation [28]. The 12/15-LOX enzymatic derivatives influence the uterine function during implantation. Li et al. reported in a mouse model an increase in 12-HETE, 15-HETE, and 13-HODE on day 4 of implantation, but the levels of 9-HODE remained low [29]. The potential mechanism is a sequence of events with the upregulation of LOX pathway enzymes by progesterone during implantation, ultimately leading to an increase in the production of eicosanoids locally, such as 12-HETE and 15-HETE. Then, the metabolites activate uterine peroxisome proliferator-activated receptors (PPARs), triggering further expression and the differentiation of cells that regulate the implantation process [28][29]. Lipoxins, synthesized from 13-HODE using the LOX pathway, are also important during implantation, as they allow vessel remodeling and placental abruption due to their ability to separate inflammation and angiogenesis [30][31]. In the 12/15 LOX knockout mice, implantation did not occur due to the necessity for spontaneous inflammation in vessel remodeling and trophoblasts anchoring [29].
The COX pathway has also been shown to influence implantation. COX-1-deficient mice are fertile but have a higher incidence of fetal malformations compared to the control. Sun et al. suggested this is due to the reduced levels of thromboxane A2 (TXA2) synthesized from the AA COX pathway because of its effects on platelet aggregation and vasoconstriction [32]. COX-2 deficiency has been shown to have contradictory results. One study found a failure of implantation and decidualization [32], whereas another study did not observe implantation failures but observed lower rates of ovulation and fertilization due to COX-2 deficiency [33]. A nonhuman primate model studied by Sun et al. suggests that endometrial COX-1 is responsible for prostaglandin (PG) synthesis, and COX-2 may impact the preparation of a receptive endometrium for implantation [32].
Omega-3 and -6 SPM metabolites undergo final metabolism through the cytochrome p450 (CYP450) pathway. Polymorphisms may participate in pregnancy and are implicated in many diseases, including cancer and cardiovascular diseases [34]. Zhou et al. described an increase in CYP2C11, CYP2C23, and CYP2J2 expression in mice on days 6, 12, and 19 of pregnancy [35]. The isoforms CYP2C8 and CYP2C9 contribute to the vasodilating epoxyeicosatrienoic acid (EETs), while CYP2C8, CYP2C9, and CYP2J2 create anti-inflammatory metabolites [34]. CYP2J2 also mediates the formation of anti-inflammatory EETs. CYP11A1 overexpression in animals or placental trophoblast has been shown to decrease trophoblast proliferation [36][37]. These data suggest a unique balance of pro-inflammatory metabolites from the different pathways is necessary for the implantation of the blastocysts.
3. Trophoblast Cells, Placental Unit, and Metabolites during Pregnancy
The proper development of the placenta is essential to establish communication and nutrient transfer between the mother and the fetus. Placental development is considered part of Mor et al.’s stage 1 of pregnancy, which relies on physiological inflammation to induce immune cell differentiation into phenotypes beneficial for trophoblasts [4]. Support for this idea comes from Fest et al., who discovered that the medium conditioned by trophoblast-induced immune cells to release pro-inflammatory cytokines essential for trophoblast development and function [38]. Trophoblast development influences placental development by modulating the maternal immune response through steps referred to as attraction, education and response [3]. First, the trophoblasts help to attract immune cells to the implantation site through the secretion of cytokines such as IL-4, IL-6, and IL-10 [39]. Next, the trophoblasts educate cells through the release of regulatory cytokines such as IL-8 and TNF-α to modulate the differentiation of immune cells [40]. Lastly, educated immune cells can respond to signals from the local environment. Fetal-derived extravillous trophoblasts invade the maternal endometrium and remain in contact with maternal immune effector cells in the decidua, allowing for communication between the mother and the fetus [40]. Normal fetal development relies on the proper development of the placenta because of its crucial role in regulating communication between the mother and the fetus.
The placenta is a modulator and can influence both the maternal and the fetal immune systems. The response of the mother and fetus is mediated by the type of response initiated by the placenta. For example, strong inflammatory responses initiated by placental infections lead to increased levels of pro-inflammatory cytokines (TNF-α, INF-γ, IL-12, and IL-6), causing placenta damage, spontaneous abortion, and preterm labor [41]. Trophoblast cell invasion and fusion are hindered by TNF-α and demonstrate increased apoptosis, increased prostaglandin levels, and increased cortisol production [42]. However, a mild inflammatory response may not terminate the pregnancy and can lead to poor outcomes such as pre-eclampsia [41]. Strong inflammation can cause preterm labor. Abnormal inflammation can influence trophoblast cells’ ability to invade the maternal arteries, leading to poor placental perfusion and the precipitation of poor maternal–fetal outcomes [43].
4. Balance between Anti- and Pro-Inflammatory Mediators in the Middle of Pregnancy
A typical pregnancy relies on a balance between pro- and anti-inflammatory states, and Mor et al. defined the second trimester as a predominantly anti-inflammatory environment. With the progression of pregnancy, placental activity and the production of reactive oxygen species increase due to increased fetal demand and typical development. Sufficient anti-inflammatory mediators are necessary to maintain uterine quiescence and prevent uncontrolled inflammation [44]. However, inflammation is needed to fight infection and allow the mother to maintain the fetus. An intrauterine environment shifted towards pro-inflammation at this stage can lead to intrauterine growth restriction and altered fetal growth [45]. Nutrition during pregnancy programs metabolism and physiological responses in the fetus [44]. As reviewed before, this is the period of rapid growth where n-3 and n-6 FAs are a crucial component of fetal brain development. There are no studies describing n-3 and n-6 FA metabolites’ roles in fetal development in this stage. More studies are needed to understand the baseline levels and their role in maintenance.
5. Inflammation in the Late Stages of Pregnancy and Parturition
The late stages of pregnancy are characterized as the third and final stage of Mor et al.’s categorization, as the mother prepares for the delivery of the fetus [1]. Inflammation in the mother and placenta prepares the body by initiating uterine contraction, ripening, dilating the cervix, and causing the membrane rupture. Prostaglandins, COX pathway metabolites, are vital mediators causing these changes. PGE2 and PGF2a induce contractions, cervical maturation, and fetal membrane rupture. In cord blood, 12-HETE and 15-HETrE were related to the regular course of pregnancy [46]. Further, in the setting of prematurity, the levels of umbilical cord 11-HETE and 15-HETrE were higher compared to those at term delivery. In post-term neonates, 5-HETE levels were lower, while 11-HETE levels were lower than those in preterm and term deliveries [46]. These findings suggest that lower levels of 5-HETE delay labor, whereas higher concentrations of 11-HETE and 15-HETrE accelerate it [28]. As shown by these studies, the contribution of metabolites to inflammation is necessary. However, dysregulation begins to expose the infant to a harmful environment that may increase the risk of morbidity and mortality.
References
- Mor, G.; Cardenas, I. The Immune System in Pregnancy: A Unique Complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433.
- Uauy, R.; Mena, P.; Rojas, C. Essential fatty acids in early life: Structural and functional role. Proc. Nutr. Soc. 2000, 59, 3–15.
- Aukema, H.; Winter, T.; Ravandi, A.; Dalvi, S.; Miller, D.W.; Hatch, G.M. Generation of Bioactive Oxylipins from Exogenously Added Arachidonic, Eicosapentaenoic and Docosahexaenoic Acid in Primary Human Brain Microvessel Endothelial Cells. Lipids 2016, 51, 591–599.
- Gibson, R.A.; Muhlhausler, B.; Makrides, M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern. Child Nutr. 2011, 7, 17–26.
- Lee, E.; Kim, H.; Kim, H.; Ha, E.-H.; Chang, N. Association of maternal omega-6 fatty acid intake with infant birth outcomes: Korean Mothers and Children’s Environmental Health (MOCEH). Nutr. J. 2018, 17, 47.
- Mor, G. Inflammation and Pregnancy. Ann. N. Y. Acad. Sci. 2008, 1127, 121–128.
- Thompson, M.; Hein, N.; Hanson, C.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Bisselou, K.S.; Appiah, A.K.; Kris-Etherton, P.; Skulas-Ray, A.C.; et al. Omega-3 Fatty Acid Intake by Age, Gender, and Pregnancy Status in the United States: National Health and Nutrition Examination Survey 2003–2014. Nutrients 2019, 11, 177.
- Nordgren, T.M.; Lyden, E.; Anderson-Berry, A.; Hanson, C. Omega-3 Fatty Acid Intake of Pregnant Women and Women of Childbearing Age in the United States: Potential for Deficiency? Nutrients 2017, 9, 197.
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87.
- Kalagiri, R.R.; Carder, T.; Choudhury, S.; Vora, N.; Ballard, A.R.; Govande, V.; Drever, N.; Beeram, M.R.; Uddin, M.N. Inflammation in Complicated Pregnancy and Its Outcome. Am. J. Perinatol. 2016, 33, 1337–1356.
- Bannenberg, G.; Serhan, C.N. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim. Biophys. Acta 2010, 1801, 1260–1273.
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216.
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; Souza, F.D.C.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165.
- Smyth, E.M. Thromboxane and the thromboxane receptor in cardiovascular disease. Clin. Lipidol. 2010, 5, 209–219.
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 1–30.
- Jo-Watanabe, A.; Okuno, T.; Yokomizo, T. The Role of Leukotrienes as Potential Therapeutic Targets in Allergic Disorders. Int. J. Mol. Sci. 2019, 20, 3580.
- Tsai, M.-J.; Chang, W.-A.; Tsai, P.-H.; Wu, C.-Y.; Ho, Y.-W.; Yen, M.-C.; Lin, Y.-S.; Kuo, P.-L.; Hsu, Y.-L. Montelukast Induces Apoptosis-Inducing Factor-Mediated Cell Death of Lung Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1353.
- Zeldin, D.C. Epoxygenase Pathways of Arachidonic Acid Metabolism. J. Biol. Chem. 2001, 276, 36059–36062.
- Xi, S.; Pham, H.; Ziboh, V.A. 15-Hydroxyeicosatrienoic acid (15-HETrE) suppresses epidermal hyperproliferation via the modulation of nuclear transcription factor (AP-1) and apoptosis. Arch. Dermatol. Res. 2000, 292, 397–403.
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutr. Biochem. 2020, 86, 108484.
- Levan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; McKean, M.; Ownby, D.R.; Zoratti, E.M.; et al. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 2019, 4, 1851–1861.
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540.
- Kumar, N.; Gupta, G.; Anilkumar, K.; Fatima, N.; Karnati, R.; Reddy, G.V.; Giri, P.V.; Reddanna, P. 15-Lipoxygenase metabolites of α-linolenic acid, , mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci. Rep. 2016, 6, 31649.
- Arita, M.; Ohira, T.; Sun, Y.-P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 Selectively Interacts with Leukotriene B4 Receptor BLT1 and ChemR23 to Regulate Inflammation. J. Immunol. 2007, 178, 3912–3917.
- Oh, S.F.; Dona, M.; Fredman, G.; Krishnamoorthy, S.; Irimia, D.; Serhan, C.N. Resolvin E2 Formation and Impact in Inflammation Resolution. J. Immunol. 2012, 188, 4527–4534.
- Isobe, Y.; Arita, M.; Iwamoto, R.; Urabe, D.; Todoroki, H.; Masuda, K.; Inoue, M.; Arai, H. Stereochemical assignment and anti-inflammatory properties of the omega-3 lipid mediator resolvin E3. J. Biochem. 2013, 153, 355–360.
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792.
- Kikut, J.; Komorniak, N.; Ziętek, M.; Palma, J.; Szczuko, M. Inflammation with the participation of arachidonic (AA) and linoleic acid (LA) derivatives (HETEs and HODEs) is necessary in the course of a normal reproductive cycle and pregnancy. J. Reprod. Immunol. 2020, 141, 103177.
- Li, Q.; Cheon, Y.-P.; Kannan, A.; Shanker, S.; Bagchi, I.C.; Bagchi, M.K. A Novel Pathway Involving Progesterone Receptor, 12/15-Lipoxygenase-derived Eicosanoids, and Peroxisome Proliferator-activated Receptor γ Regulates Implantation in Mice. J. Biol. Chem. 2004, 279, 11570–11581.
- Rinaldi, S.F.; Catalano, R.D.; Wade, J.; Rossi, A.G.; Norman, J.E. 15-epi-lipoxin A4 reduces the mortality of prematurely born pups in a mouse model of infection-induced preterm birth. Mol. Hum. Reprod. 2015, 21, 359–368.
- Chiang, N.; Serhan, C.N.; Dahlén, S.-E.; Drazen, J.M.; Hay, D.W.P.; Rovati, G.E.; Shimizu, T.; Yokomizo, T.; Brink, C. The Lipoxin Receptor ALX: Potent Ligand-Specific and Stereoselective Actions in Vivo. Pharmacol. Rev. 2006, 58, 463–487.
- Sun, T.; Li, S.-J.; Diao, H.-L.; Teng, C.-B.; Wang, H.-B.; Yang, Z.-M. Cyclooxygenases and prostaglandin E synthases in the endometrium of the rhesus monkey during the menstrual cycle. Reproduction 2004, 127, 465–473.
- Cheng, J.-G.; Stewart, C.L. Loss of Cyclooxygenase-2 Retards Decidual Growth but Does Not Inhibit Embryo Implantation or Development to Term. Biol. Reprod. 2003, 68, 401–404.
- Daly, A.K. Polymorphic Variants of Cytochrome P450: Relevance to Cancer and Other Diseases. Adv. Pharmacol. 2015, 74, 85–111.
- Zhou, Y.; Chang, H.-H.; Du, J.; Wang, C.-Y.; Dong, Z.; Wang, M.-H. Renal epoxyeicosatrienoic acid synthesis during pregnancy. Am. J. Physiol. Physiol. 2005, 288, F221–F226.
- Chatuphonprasert, W.; Jarukamjorn, K.; Ellinger, I. Physiology and Pathophysiology of Steroid Biosynthesis, Transport and Metabolism in the Human Placenta. Front. Pharmacol. 2018, 9, 1027.
- He, G.; Xu, W.; Chen, Y.; Liu, X.; Xi, M. Abnormal Apoptosis of Trophoblastic Cells Is Related to the Up-Regulation of CYP11A Gene in Placenta of Preeclampsia Patients. PLoS ONE 2013, 8, e59609.
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412.
- Abrahams, V.M.; Kim, Y.M.; Straszewski, S.L.; Romero, R.; Mor, G. Macrophages and Apoptotic Cell Clearance During Pregnancy. Am. J. Reprod. Immunol. 2004, 51, 275–282.
- Fest, S.; Aldo, P.B.; Abrahams, V.M.; Visintin, I.; Alvero, A.; Chen, R.; Chavez, S.L.; Romero, R.; Mor, G. Trophoblast-Macrophage Interactions: A Regulatory Network for the Protection of Pregnancy. Am. J. Reprod. Immunol. 2007, 57, 55–66.
- McCracken, A.S.; Drury, C.L.; Lee, H.-S.; Morris, J.M. Pregnancy is associated with suppression of the nuclear factor kappa B/I kappa B activation pathway in peripheral blood mononuclear cells. J. Reprod. Immunol. 2003, 58, 27–47.
- Haider, S.; Knöfler, M. Human Tumour Necrosis Factor: Physiological and Pathological Roles in Placenta and Endometrium. Placenta 2009, 30, 111–123.
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179.
- Akerele, O.A.; Cheema, S.K. A balance of omega-3 and omega-6 polyunsaturated fatty acids is important in pregnancy. J. Nutr. Intermed. Metab. 2016, 5, 23–33.
- Boyle, A.K.; Rinaldi, S.F.; Norman, J.E.; Stock, S.J. Preterm birth: Inflammation, fetal injury and treatment strategies. J. Reprod. Immunol. 2017, 119, 62–66.
- Gouveia-Figueira, S.; Martens, D.S.; Nawrot, T.S.; Nording, M.L. Cord blood eicosanoid signatures and newborn gestational age. Prostaglandins Other Lipid Mediat. 2017, 133, 123–127.
More
Information
Subjects:
Pediatrics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
17 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




