Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marlinda Ab Rahman | -- | 1419 | 2023-01-16 07:29:12 | | | |
| 2 | Dean Liu | Meta information modification | 1419 | 2023-01-17 01:45:47 | | | | |
| 3 | Dean Liu | Meta information modification | 1419 | 2023-01-17 01:50:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marlinda, A.R.; Thien, G.S.H.; Shahid, M.; Ling, T.Y.; Hashem, A.; Chan, K.; Johan, M.R. Graphene and Carbon-Based Additives. Encyclopedia. Available online: https://encyclopedia.pub/entry/40196 (accessed on 08 March 2026).
Marlinda AR, Thien GSH, Shahid M, Ling TY, Hashem A, Chan K, et al. Graphene and Carbon-Based Additives. Encyclopedia. Available at: https://encyclopedia.pub/entry/40196. Accessed March 08, 2026.
Marlinda, Ab Rahman, Gregory Soon How Thien, Mehmood Shahid, Ting Yang Ling, Abu Hashem, Kah-Yoong Chan, Mohd Rafie Johan. "Graphene and Carbon-Based Additives" Encyclopedia, https://encyclopedia.pub/entry/40196 (accessed March 08, 2026).
Marlinda, A.R., Thien, G.S.H., Shahid, M., Ling, T.Y., Hashem, A., Chan, K., & Johan, M.R. (2023, January 16). Graphene and Carbon-Based Additives. In Encyclopedia. https://encyclopedia.pub/entry/40196
Marlinda, Ab Rahman, et al. "Graphene and Carbon-Based Additives." Encyclopedia. Web. 16 January, 2023.
Copy Citation
Graphene exhibits remarkable and unparalleled qualities, making it highly desirable for tribological applications due to its exceptionally high mechanical strength, outstanding conductivity, low shear strength, and high surface area. The tribological properties of graphene are controlled by various techniques used for its synthesis and the presence of functional groups, such as residual oxygen functionalities, thickness and lateral dimensions of each sheet, number of atomic lamellae in a sheet, and structural flaws.
graphene
additive lubricant
anti-wear
1. Synthesis of Graphene
Graphene was first isolated and characterised in 2004 by Geim et al. [1] at the University of Manchester. The authors used scotch tape to mechanically exfoliate graphene films from a small mesa of highly oriented pyrolytic graphite. This approach allowed for the preparation of FLG films up to 10 μm in size, with even thicker films that were up to 100 μm across and visible to the naked eye (d ≈ 3 nm). Since then, much research has been devoted to understanding the properties of graphene and developing methods to synthesise it [2][3][4]. The summary of the synthesis technique for graphene is displayed in Figure 1, in which the most common method was chemical vapour deposition (CVD) [5][6][7][8]. CVD is a process where a material is deposited on a substrate by decomposing a gas in a controlled environment. The resulting CVD graphene/graphitic films are often used as a protection layer for micro-electromechanical systems (MEMS)/ nano-electromechanical systems (NEMS) devices [9], gas barriers [10][11], and sensors [12]. In contrast, CVD graphene is usually deposited in situ and may not be suitable for manufacturing graphene for liquid-phase lubricants. The synthesis of liquid-phase graphene additives may be generalised into a few broad steps mechanical exfoliation of graphene oxide or other chemically modified graphene compounds [13][14], followed by chemical reduction or modification [15][16][17] or thermal reduction [18][19][20][21].
One of the most common routes for graphene production involves the mechanical exfoliation of graphite oxide into graphene oxide via a method known as Hummer’s method [16][17][19][20][21]. The Hummers’ method and most of its modified synthesis route involve the oxidation of graphite via a chemical reaction that introduces oxygen molecules to pure graphene layers which make up graphite powder. Usually, sulfuric acid is used as an oxidation agent. Then, potassium permanganate and sodium nitrate are added to act as catalysts for the reaction between the graphene and the concentrated sulfuric acid. The resultant graphene oxide (GO) layers have a weaker inter-layer attraction and thus are easily separated by mechanical agitation in the liquid. Liang et al. [14] introduced an in situ graphene exfoliation method for water-based lubricants. Their method relies on using a non-ionic surfactant (Triton-X) in the exfoliation process. The graphene was mechanically exfoliated via ultrasonic sonification after mixing with the non-ionic surfactant. Liang et al. reported an 80% enhancement in friction properties in water-based lubricants with graphene additives. Alternatively, Patel et al. [15] used off-the-shelf reduced graphene oxide (rGO) nano-platelets as additives to lubricants and reduction of wear and friction by up to 51.86%.
The chemical reduction of GO involves using a chemical agent to remove the oxygen atoms bonded to the GO after some form of mechanical exfoliation is applied to separate the GO layers. Reduced graphene oxide (rGO) is the product of this reduction process, which typically involves using hydrazine hydrate. The rGO has a higher degree of crystallinity than the GO and thus exhibits higher mechanical properties. The rGO has been used as an additive in lubricants that can reduce friction and wear [22]. Due to the toxicity of hydrazine hydrate, various alternatives were proposed [16][17]. Satheesh et al. [17] proposed an alternative reduction route utilising thiourea as a reducing agent. The resulting graphene flakes showed stable thermal performances. Silva et al. [16] summarised using green alternatives to hydrazine hydrate. Amongst the available alternatives, ascorbic acid was known as the most promising reducing agent due to its low toxicity, low cost, and non-carcinogenic properties. Therefore, the resulting graphene demonstrated good electrical and thermal properties.
An alternative method for the reduction of GO involves the use of thermal reduction. Thermal reduction is usually made at an elevated temperature to reduce the GO to graphene. On the contrary, in most cases, the rGO may still have some hydroxyl, carbonyl, and carboxylic acid groups attached to the surface [19][20][21]. Alam et al. reported a modified Hummers method for synthesising rGO with a thermal reduction [21]. The thermal stability of the rGO was the main factor to consider for thermal reduction, as the higher temperature needed to purge the hydroxyl, carbonyl, and carboxylic acid groups may also cause a mass loss in the carbon skeleton due to combustion. The rGO experiences less mass loss at lower temperatures, but the final product may have a higher density of impurities [19][21]. Oliveira et al. [19] suggested that a rapid rate of heating to high temperatures, as opposed to slow annealing, may be the key to creating high-quality rGO. The higher heating rate causes the oxygen-containing group on the graphene surface to detach rapidly, forming high-pressure vapour and pushing the layers apart.
Although the liquid phase exfoliation of graphene may be the best way to produce graphene for lubricant additives, researchers need to be aware of the fundamental limitations and impurities present in the final products [16][17][23]. Ambrosi et al. [23] presented evidence that natural and synthetic graphite contains substantial metallic impurities in the graphite oxide samples after oxidation and in chemically reduced graphene after reduction. Despite some of the impurities being removed during the oxidation process of the graphite, a substantial amount was still present, causing a major impact on the electrochemical properties of the rGO produced [23].
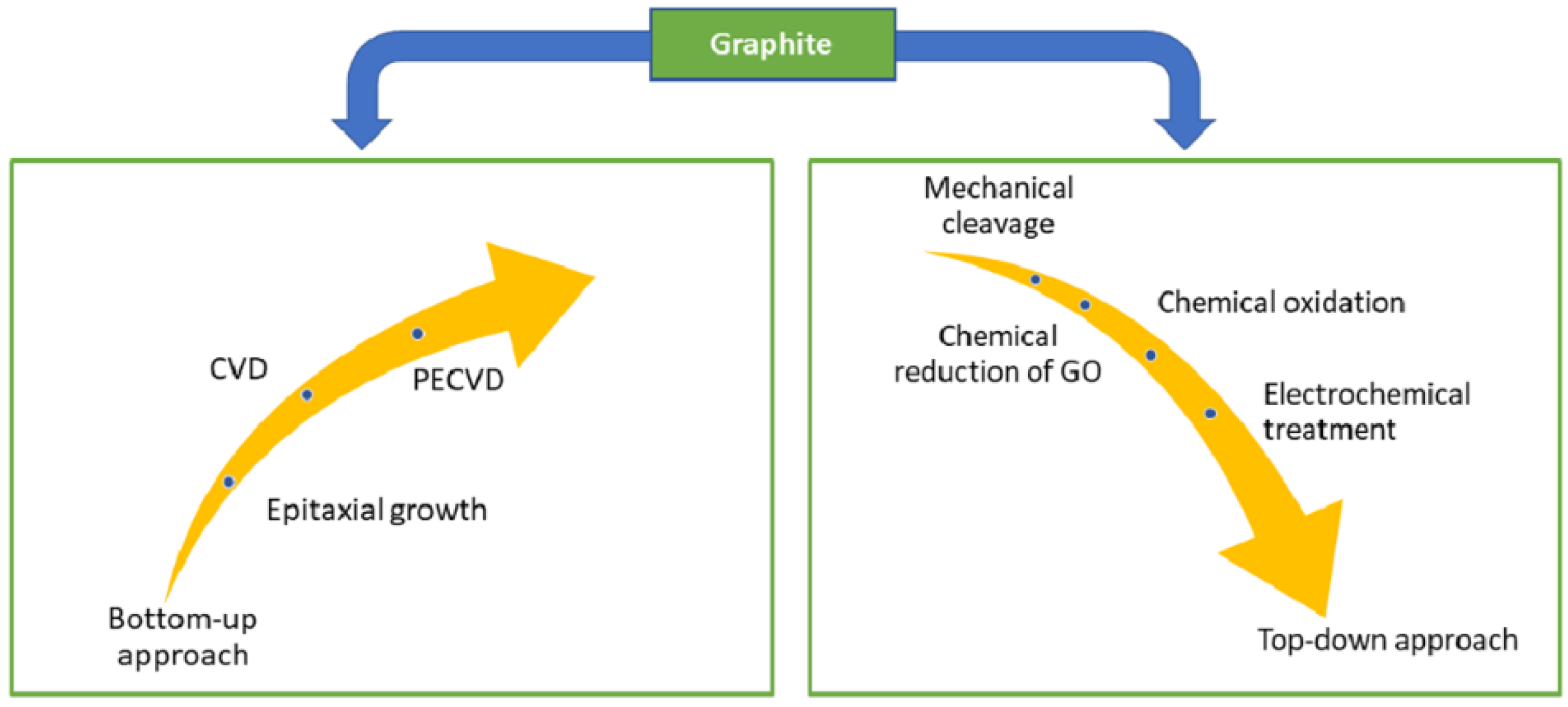
Figure 1. Synthesis approaches of graphene from graphite. Reproduced with permission from [24].
2. Lubrication Mechanisms of Graphene
Graphene-based nanocomposites are used as lubricant additives to reduce friction and wear. The sliding pair wear resistance and friction reduction were greatly enhanced by investigating the tribological behaviour of base oil with graphene lubrication. In invading the rubbing interface, graphene successfully stopped tribo-pairs from making mechanical contact with one another. Thus, with the addition of graphene, base oil viscosity, and film thickness increased. The creation of protective films, interlayer shearing, and surface mending was lubrication mechanisms that helped to lower the friction coefficient and worn scar diameter [25][26][27][28].
Wu et al. [29] investigated graphene’s interlayer shearing and surface mending behaviour. Since the interlayer shearing stress of graphene was smaller than the shear force between sliding pairs, the friction coefficient of a tribo-pair decreased. The graphene interlayer shearing lubrication mechanism that occurred during the running-in stage is shown in Figure 2a, in which the surfaces of the tribo-pairs were covered with many dimples and peaks. Graphene was interlayer sheared during sliding because of the shear stress between tribo-pairs, which reduced the friction coefficient. For the heavy load, the larger graphene nanoplates were broken into smaller ones, which allowed the smaller graphene to reach the rubbing surface much more quickly. Graphene is also used to stop the rubbing surface from oxidising while sliding. After interlayer shearing and tearing during friction, graphene was absorbed into the worn surface to fix the deep dimples and severe scratches. The oil film thickness investigation proved that graphene accessed the contact area and significantly lowered the friction coefficient and wear. Hence, a protective tribofilm can be developed in a constant state of friction.
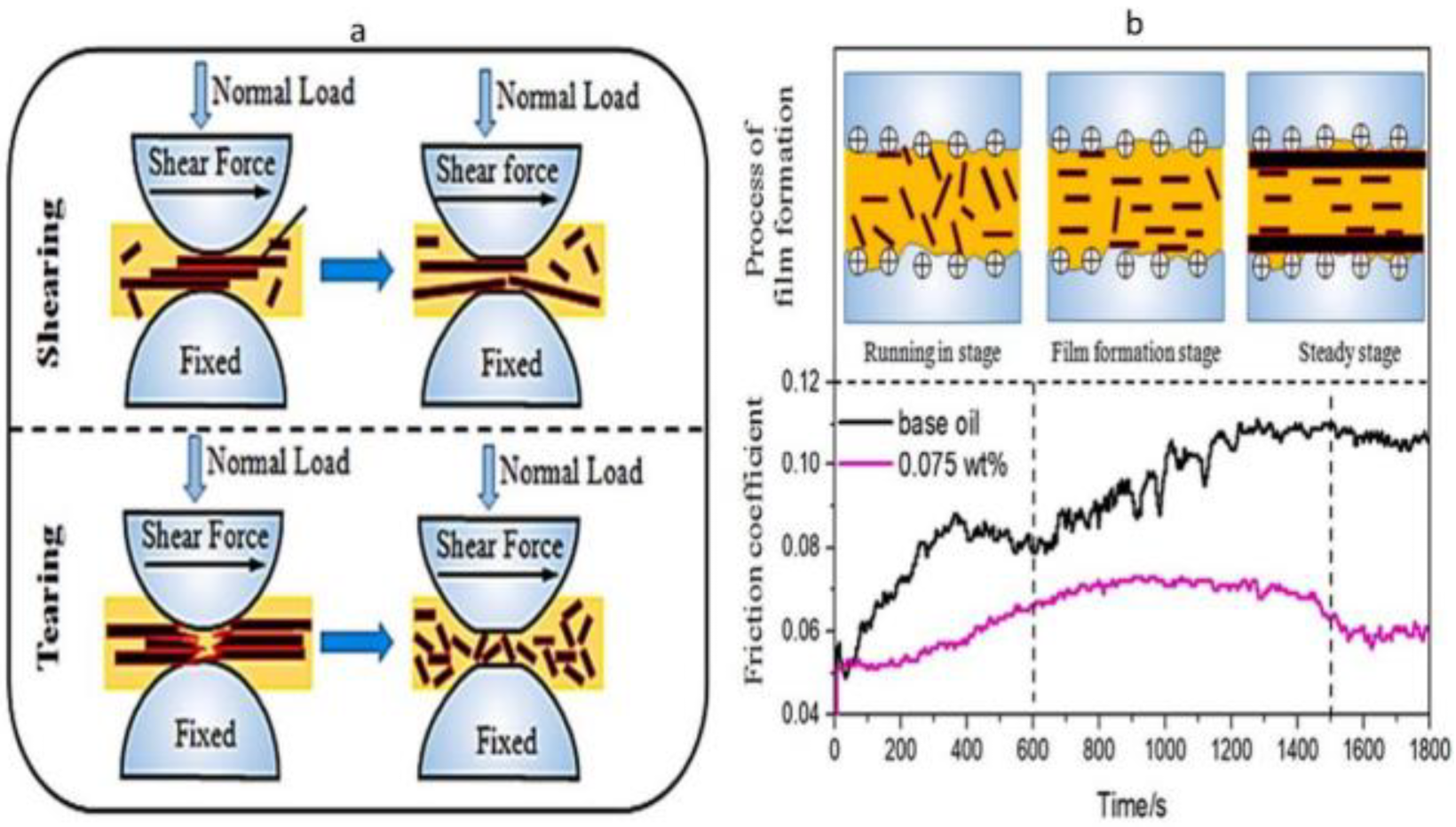
Figure 2. (a) Graphene nanoparticle lubrication mechanism in interlayer shearing. (b) Protective film formation process for graphene lubrication. Reproduced with permission from [29].
Another experiment was conducted to observe the effect of film formation of graphene by Wu et al. [29]. Figure 2b describes the outcome of combining the friction coefficient curves and the protective film formation process. When tribo-pairs were lubricated with aviation lubricant containing 0.075 wt.% of graphene, the friction coefficient significantly decreased compared to the base oil. Three stages, namely, the running-in stage, the protective film formation stage, and the steady stage, were found concerning the increasing sliding time. The interlayer shearing operated when sliding pairs were lubricated by oil containing graphene in the running-in stage, as opposed to lubrication with base oil. Therefore, the graphene nanoplates penetrated the contact area and promiscuously dispersed into the lubricating fluid as the large graphene pieces were broken into little pieces. As the electric charge is combined with shear stress, graphene tends to be parallel to the rubbing surface during the formation of the protective coating. This stage enhanced friction reduction and wear resistance by using graphene to heal severe wear caused by jogging. After adhering graphene to the rubbing surface, the friction coefficient curves remain stable during the steady stage. Finally, protective coatings are created under the impact of average load and shear stress. As a result, sliding pairs’ tribological characteristics were greatly enhanced [29].
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669.
- Bhuyan, M.; Alam, S.; Uddin, M.; Islam, M.; Bipasha, F.A.; Hossain, S.S. Synthesis of Graphene. Int. Nano Lett. 2016, 6, 65–83.
- Lin, L.; Peng, H.; Liu, Z. Synthesis Challenges for Graphene Industry. Nat. Mater. 2019, 18, 520–524.
- Whitener, K.E., Jr.; Sheehan, P.E. Graphene Synthesis. Diam. Relat. Mater. 2014, 46, 25–34.
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene—Synthesis, Characterisation, and Applications: A Review. Molecules 2020, 25, 3856.
- Deng, B.; Liu, Z.; Peng, H. Toward Mass Production of CVD Graphene Films. Adv. Mater. 2019, 31, 1800996.
- Deokar, G.; Avila, J.; Razado-Colambo, I.; Codron, J.-L.; Boyaval, C.; Galopin, E.; Asensio, M.-C.; Vignaud, D. Towards High Quality CVD Graphene Growth and Transfer. Carbon 2015, 89, 82–92.
- Kataria, S.; Wagner, S.; Ruhkopf, J.; Gahoi, A.; Pandey, H.; Bornemann, R.; Vaziri, S.; Smith, A.D.; Ostling, M.; Lemme, M.C. Chemical Vapor Deposited Graphene: From Synthesis to Applications. Phys. Status Solidi 2014, 211, 2439–2449.
- Rana, S.; Reynolds, J.D.; Ling, T.Y.; Shamsudin, M.S.; Pu, S.H.; Chong, H.M.H.; Pamunuwa, D. Nano-Crystalline Graphite for Reliability Improvement in MEM Relay Contacts. Carbon 2018, 133, 193–199.
- Fishlock, S.J.; Pu, S.H.; Bhattacharya, G.; Han, Y.; McLaughlin, J.; McBride, J.W.; Chong, H.M.H.; O’Shea, S.J. Micromachined Nanocrystalline Graphite Membranes for Gas Separation. Carbon 2018, 138, 125–133.
- Seo, T.H.; Lee, S.; Cho, H.; Chandramohan, S.; Suh, E.-K.; Lee, H.S.; Bae, S.K.; Kim, S.M.; Park, M.; Lee, J.K. Tailored CVD Graphene Coating as a Transparent and Flexible Gas Barrier. Sci. Rep. 2016, 6, 24143.
- Ling, T.Y.; Pu, S.H.; Fishlock, S.J.; Han, Y.; Reynolds, J.D.; McBride, J.W.; Chong, H.M.H. Sensing Performance of Nanocrystalline Graphite-Based Humidity Sensors. IEEE Sens. J. 2019, 19, 5421–5428.
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942.
- Liang, S.; Shen, Z.; Yi, M.; Liu, L.; Zhang, X.; Ma, S. In-Situ Exfoliated Graphene for High-Performance Water-Based Lubricants. Carbon 2016, 96, 1181–1190.
- Patel, J.; Kiani, A. Effects of Reduced Graphene Oxide (RGO) at Different Concentrations on Tribological Properties of Liquid Base Lubricants. Lubricants 2019, 7, 11.
- De Silva, K.K.H.; Huang, H.-H.; Joshi, R.K.; Yoshimura, M. Chemical Reduction of Graphene Oxide Using Green Reductants. Carbon 2017, 119, 190–199.
- Satheesh, K.; Jayavel, R. Synthesis and Electrochemical Properties of Reduced Graphene Oxide via Chemical Reduction Using Thiourea as a Reducing Agent. Mater. Lett. 2013, 113, 5–8.
- Nassef, M.G.A.; Soliman, M.; Nassef, B.G.; Daha, M.A.; Nassef, G.A. Impact of Graphene Nano-Additives to Lithium Grease on the Dynamic and Tribological Behavior of Rolling Bearings. Lubricants 2022, 10, 29.
- Oliveira, A.E.F.; Braga, G.B.; Tarley, C.R.T.; Pereira, A.C. Thermally Reduced Graphene Oxide: Synthesis, Studies and Characterization. J. Mater. Sci. 2018, 53, 12005–12015.
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis Route of Reduced Graphene Oxide via Thermal Reduction of Chemically Exfoliated Graphene Oxide. Mater. Chem. Phys. 2018, 204, 1–7.
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (RGO). Graphene 2017, 6, 1–18.
- Rani, A.; Nam, S.; Oh, K.A.; Park, M. Electrical Conductivity of Chemically Reduced Graphene Powders under Compression. Carbon Lett. 2010, 11, 90–95.
- Ambrosi, A.; Chua, C.K.; Khezri, B.; Sofer, Z.; Webster, R.D.; Pumera, M. Chemically Reduced Graphene Contains Inherent Metallic Impurities Present in Parent Natural and Synthetic Graphite. Proc. Natl. Acad. Sci. USA 2012, 109, 12899–12904.
- Rahman, M.A.; Sagadevan, S.; Johan, M.R. Graphene and Its Composites BT—Contemporary Nanomaterials in Material Engineering Applications; Mubarak, N.M., Khalid, M., Walvekar, R., Numan, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 21–35. ISBN 978-3-030-62761-4.
- Wang, L.; Gong, P.; Li, W.; Luo, T.; Cao, B. Mono-Dispersed Ag/Graphene Nanocomposite as Lubricant Additive to Reduce Friction and Wear. Tribol. Int. 2020, 146, 106228.
- Zhai, W.; Shi, X.; Wang, M.; Xu, Z.; Yao, J.; Song, S.; Wang, Y. Grain Refinement: A Mechanism for Graphene Nanoplatelets to Reduce Friction and Wear of Ni3Al Matrix Self-Lubricating Composites. Wear 2014, 310, 33–40.
- Liu, Y.; Shin, D.-G.; Xu, S.; Kim, C.-L.; Kim, D.-E. Understanding of the Lubrication Mechanism of Reduced Graphene Oxide Coating via Dual In-Situ Monitoring of the Chemical and Topographic Structural Evolution. Carbon 2021, 173, 941–952.
- Zhang, J.; Xu, Q.; Gao, L.; Ma, T.; Qiu, M.; Hu, Y.; Wang, H.; Luo, J. A Molecular Dynamics Study of Lubricating Mechanism of Graphene Nanoflakes Embedded in Cu-Based Nanocomposite. Appl. Surf. Sci. 2020, 511, 145620.
- Wu, L.; Gu, L.; Jian, R. Lubrication Mechanism of Graphene Nanoplates as Oil Additives for Ceramics/Steel Sliding Components. Ceram. Int. 2021, 47, 16935–16942.
More
Information
Subjects:
Materials Science, Composites
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
17 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





