Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dong-Wook Han | -- | 1705 | 2023-01-15 23:48:29 | | | |
| 2 | Sirius Huang | Meta information modification | 1705 | 2023-01-16 02:08:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kang, M.S.; Jang, J.; Jo, H.J.; Kim, W.; Kim, B.; Chun, H.; Lim, D.; Han, D. 3D Bioprinting Skin. Encyclopedia. Available online: https://encyclopedia.pub/entry/40187 (accessed on 06 March 2026).
Kang MS, Jang J, Jo HJ, Kim W, Kim B, Chun H, et al. 3D Bioprinting Skin. Encyclopedia. Available at: https://encyclopedia.pub/entry/40187. Accessed March 06, 2026.
Kang, Moon Sung, Jinju Jang, Hyo Jung Jo, Won-Hyeon Kim, Bongju Kim, Heoung-Jae Chun, Dohyung Lim, Dong-Wook Han. "3D Bioprinting Skin" Encyclopedia, https://encyclopedia.pub/entry/40187 (accessed March 06, 2026).
Kang, M.S., Jang, J., Jo, H.J., Kim, W., Kim, B., Chun, H., Lim, D., & Han, D. (2023, January 15). 3D Bioprinting Skin. In Encyclopedia. https://encyclopedia.pub/entry/40187
Kang, Moon Sung, et al. "3D Bioprinting Skin." Encyclopedia. Web. 15 January, 2023.
Copy Citation
3D bioprinting is considered to have a significant impact in the field of tissue engineering, as tissue-scaled large analogs can be fabricated with submicron fidelity. 3D bioprinted skin equivalents are highlighted as the new gold standard for alternative models to animal testing, as well as full-thickness wound healing.
3D bioprinting
skin tissue engineering
biomaterials
skin regeneration
wound healing
1. Introduction
Restoration of damaged skin has been a long-desired goal for modern medicine. Biocomposite dressings have been adopted to improve wound healing in partial thickness wound, and made them the most used strategies for superficial burns and epidermal necrolysis [1]. However, the repair of large-scale deep injuries such as deep burns cannot be handled with conventional wound dressing [2]. To date, full-thickness skin replacement generally adopts graft implantation (e.g., allograft and autograft), but the shortage of skin sources, donor site morbidity, immune reactions, and infection risk limit their use [3]. Moreover, conventional grafts have lowered biological functions and are aesthetically not coordinated with existing tissues, hence, they are unable to recapitulate the full function of skin [4][5]. Therefore, novel approaches based on tissue engineering have been highlighted. The goal of tissue engineering is to develop autologous tissue grafts through a tailored combination of cells and biomaterials, for future full-thickness skin restoration. Various methods to fabricate skin tissue engineering scaffolds have been developed, including foam casting, electrospinning, phase separation, and decellularization [6][7][8][9]. In particular, 3D bioprinting is considered to have a significant impact in the field of tissue engineering, as tissue-scaled large analogs can be fabricated with submicron fidelity [10][11]. Contemporary 3D bioprinted skins have limited use in wound healing applications because long-term stability has not yet been established through many clinical evaluations. However, they have been vigorously used for drug screening platforms within the last decades. To meet the demand for screening platforms without animal testing, in vitro toxicity screening platforms such as skin-on-a-chip have been developed and commercialized. L’OREAL groups (Clichy, France) have developed several kinds of 3D bioprinted skins, including EpiskinTM (Clichy, France) and SkinethicTM (Clichy, France), to use for the evaluation of toxicity, irritation, or regeneration of developed cosmetics. EpidermTM from MatTek (Ashland, MA), Epi-model 24 from J-Tek (Aichi, Japan), and KeraskinTM from Biosolution (Seoul, Republic of Korea) are 3D bioprinted skin tissues which have been widely used for skin irritation testing and drug efficiency screening.
The eye-opening progress of recent studies is classified as advances in bioink materials, structures, and functions of 3D bioprinting skin (Figure 1). Natural hydrogels provide a sufficient microenvironment to laden skin cells with excellent printability, while synthetic polymers are often added to strengthen the biological and mechanical properties of bioink. Mammalian-derived materials such as decellularized extracellular matrix (dECM) have been highlighted as they have a high affinity to native tissues, and can mimic the natural niche [12][13][14]. Based on these novel materials, recent studies have focused on the development of structurally advanced skin models such as full-thickness models, hair induction, and vascularized models for drug screening platforms and directly implantable grafts. Thereafter, technological and regulatory hurdles are discussed, which limit the clinical application of 3D bioprinted skin.
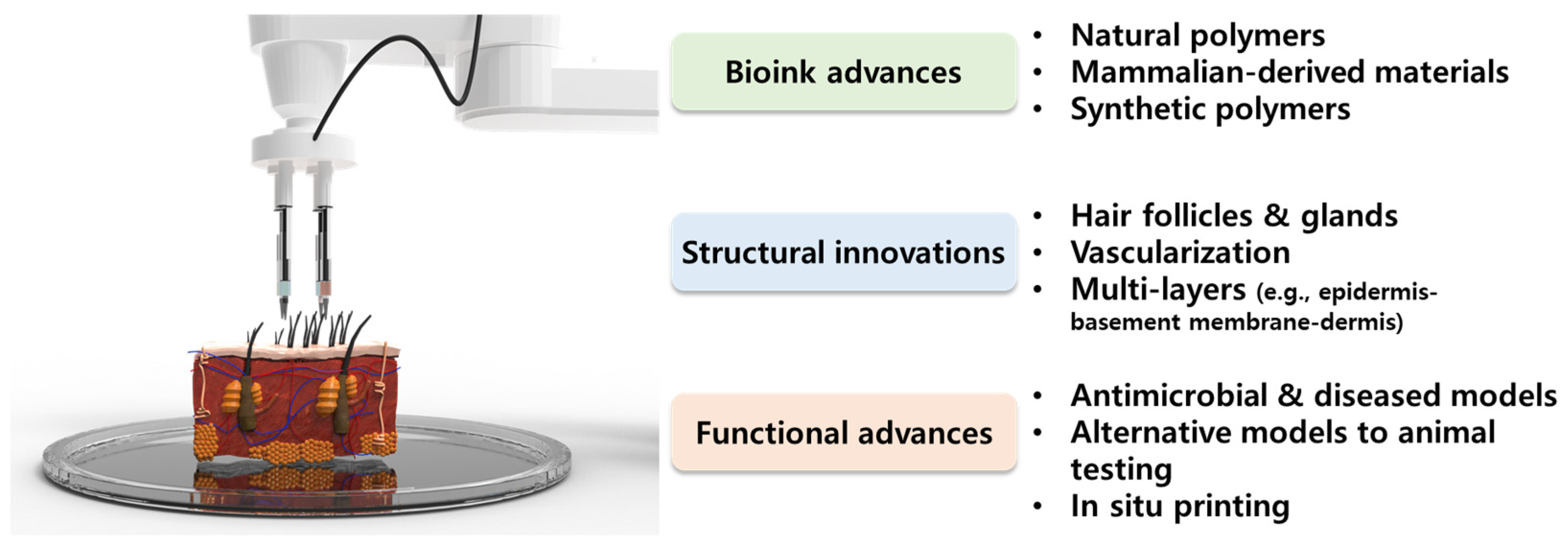
Figure 1. Schematic diagram of recent advances and innovations of bioink materials as well as the structure and function of three-dimensional (3D) bioprinting skin.
2. Skin Anatomy and Functional Replacement of Damaged Skin
The skin is the largest organ of the body, accounting for about 15% of the total body weight in adults [15]. The skin has a hierarchical multi-layered structure that is composed of several components including cells, fibrous matrices, extracellular matrix (ECM), blood vessels, nerves, glands, and hair follicles. Innumerable body functions are regulated by the skin such as physical protection, thermoregulation, sensation, homeostasis, and immunity [16][17]. To perform these tasks, skin mostly consists of three different functional layers, namely the epidermis, dermis, and hypodermis, which are divided into subsections, respectively (Figure 2).
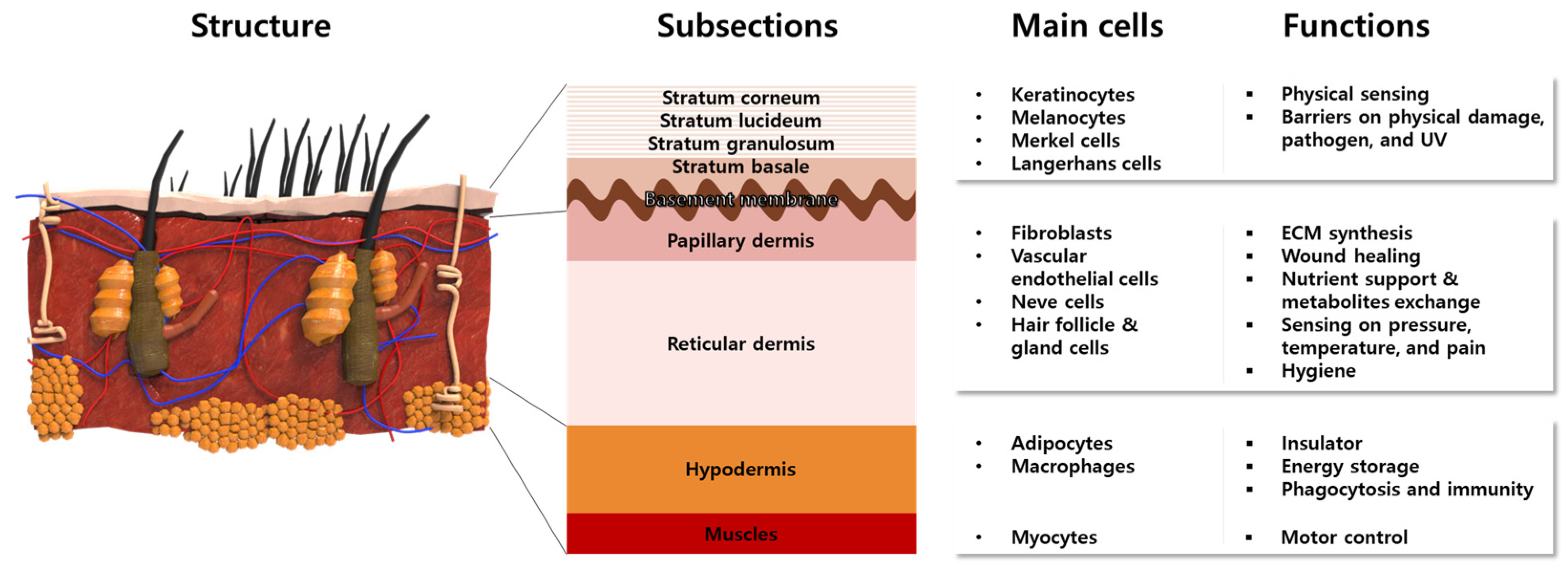
Figure 2. Anatomical structures of multi-layered skin composed of representative cells with intrinsic functions.
The epidermis is a 50 μm–1.5 mm-thick outermost protective layer, consisting of 90% keratinocytes (KCs) and stratified layers containing different types of cells [18]. The KCs locate at the full layer of the epidermis to synthesize the keratin that constitutes the skin barriers. The melanocytes and Merkel cells at the stratum basale produce melanosomes and sense physical stimuli, respectively [19]. The Langerhans cells are known to serve the immunologic roles of antigen-presenting cells [19]. The lowest epidermis, named the basement membrane, mainly consisted of laminin, to connect the dermis and epidermis. On the other hand, the dermis is composed of 2 layers named the papillary dermis and reticular dermis. The biggest population of cells in the dermis consists of fibroblasts (FBs), which synthesize ECM components such as collagen (Col) and elastic fibers, to provide skin elasticity and mechanical support. Moreover, FBs secrete various cytokines to promote the wound healing process [20][21][22]. The blood vessels exist in the dermis and supply oxygen and nutrients. Along with the arterioles and venules, nerves are widely distributed to sense pressure, temperature, and pain. Hair follicles and sweat glands exist in the reticular dermis to conduct body temperature control and physical buffering, as well as the prevention of microbial activity on the skin surfaces. Beneath the dermis and above the muscle, the hypodermis, which is mainly composed of adipocytes, acts as an insulator and energy storage. The macrophage in the hypodermis is involved in the wound healing process by phagocytosis and adaptive immunity.
The skin often suffers from functional or aesthetic disturbances such as toxic epidermal necrolysis, large chronic ulcers, acute wounds, and burns. Physiological regulation of wound healing is a complex process, relying on many cell types and mediators interacting in a very precise temporal sequence. The repair process includes intercellular interactions, growth factors, and cytokines involved in the restoration process. In general, spontaneous regeneration of the skin requires 28 days, but when the loss is greater than 4 cm in diameter, recovering full-thickness skin is impractical, especially at an older age [23][24]. In addition, deep skin damage and burns cannot be fully recovered, and often leave scars due to changes in the cellular microenvironment, which leads to abnormal cell growth and matrix formation, with a reduction of hair follicles and sweat glands [25][26][27].
3. 3D Bioprinting Methods
3D bioprinting is the process of printing biomaterials and cells to produce biological constructs that mimic the characteristics of natural tissues and organs. This is a technology applied by expanding additive manufacturing (AM) technology, to print bio-functional materials in a layer-by-layer (LbL) manner on substrates, after loading cytocompatible biomaterials. 3D bioprinting technology is considered a way to create an ideal 3D structure during in vitro experiments because it can deposit a tailored dose of various cells with spatial manipulation. Using an appropriate 3D bioprinting process, to suit the properties of the material to be used, allows for the proper distribution and positioning of biomaterials, signaling factors, and heterogeneous cells in high densities to form tissue scaffolds. To achieve high cell viability and 3D printability, novel 3D bioprinting technologies such as extrusion-based bioprinting (EBB), laser-assisted bioprinting (LAB), and droplet-based bioprinting (DBB) have been most widely used (Figure 3) [28][29][30].
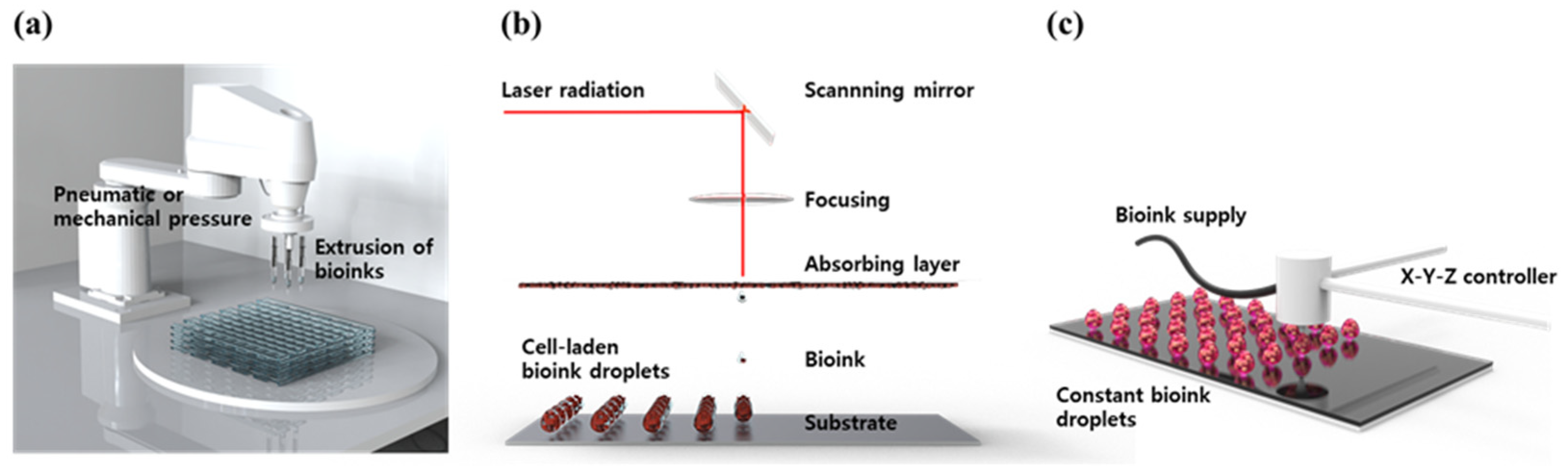
Figure 3. Schematic diagram of common 3D bioprinting methods. (a) EBB, (b) LAB, and (c) DBB.
EBB technology enables finely controlled printing based on automated machine systems and fluid distribution systems [31]. The cell-containing bioink passes through a continuous filament-type micro nozzle in a pneumatic, piston- or screw-driven manner, under computer control. After printing layer by layer, a complete 3D construct is formed. By endowing shear stress, the fine filament structure can be achieved by the EBB method. Recently, EBB has been developed to mount multiple printer heads, which can minimize cross-contamination, while simultaneously depositing various bioink [32][33][34]. Furthermore, they allow better control over shape, porosity, and cell distribution in the printed structure. The advantages of EBB are a wide range of printable bioink types including high-viscosity hydrogel, cell clumps, acellular matrix components, and microcarriers. The most important advantage of EBB is that it can print porous grid structures. The grid structure can promote the circulation of nutrients and metabolites [35][36]. However, there is the disadvantage of low resolution, of which the minimum resolution typically exceeds 100 μm [37][38].
The LAB system consists of four parts: a pulsed laser source, a laser focusing tool, a laser energy-absorbing metal ribbon film, and a receiving substrate [39]. The LAB technology emits laser light through a pulsed laser source, which collects on a metal film on the back of a silicate glass and causes it to be locally heated. At this time, the bioink deposited on the substrate quickly evaporates and is sprayed on the substrate in the form of droplets. As the main energy source, ultraviolet (UV) or near UV wavelengths of nanosecond lasers are used, and the printing resolution is excellent at the level of picogram [40]. In addition, 3D structures printed using these technologies exhibit excellent cell survival rates of 90% or more. Although it has the advantage of being able to print non-contact, showing high cell activity, and printing with high resolution [41][42], it has the disadvantage of not being able to print at high speed, due to the lack of an appropriate fast gelation mechanism.
DBB technology mainly includes Inkjet bioprinting (IJB) and Electrohydrodynamic (EHD) jetting bioprinting. IJB can be divided into continuous inkjet (CIJ) printing and drop-on-demand (DOD) printing. CIJ printing relies on a unique tendency of liquid flow to continuously disperse conductive ink droplets. A CIJ-based bioprinter produces drops at a fast rate. DOD printing generates bioink droplets on the substrate when needed. Thus, it is more suitable for material deposition and patterning with higher precision and minimal waste of bioink [43]. DOD produces droplets, mainly using piezoelectric, thermal, or electrostatic forces, which can flexibly and accurately deposit various biomaterials to construct spatially heterogeneous tissues. However, there are limitations to DOD. First, because the inkjet aperture is extremely small (10–150 μm), it is easily blocked by biomaterials. Therefore, the solvents that can be applied to DOD are only low-viscosity hydrogel or other low-concentration biological agents. Second, since a porous structure cannot be produced, there is a limit to issue perfusion or substance exchange, making it difficult to apply it clinically. EHD jetting bioprinting uses an electric field to pull the bioink droplet out [44]. Therefore, applied voltage, and the physical properties of bioink such as viscosity, and flow rate, have a great influence on cell viability. The EHD method is especially suitable for using highly concentrated bioink [45].
References
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 615–623.
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574–20417314211028601.
- Park, W.; Gao, G.; Cho, D.-W. Tissue-specific decellularized extracellular matrix bioinks for musculoskeletal tissue regeneration and modeling using 3D bioprinting technology. Int. J. Mol. Sci. 2021, 22, 7837.
- Daikuara, L.Y.; Chen, X.; Yue, Z.; Skropeta, D.; Wood, F.M.; Fear, M.W.; Wallace, G.G. 3D bioprinting constructs to facilitate skin regeneration. Adv. Funct. Mater. 2022, 32, 2105080–2105103.
- Wang, R.; Wang, Y.; Yao, B.; Hu, T.; Li, Z.; Huang, S.; Fu, X. Beyond 2D: 3D bioprinting for skin regeneration. Int. Wound J. 2019, 16, 134–138.
- Arampatzis, A.S.; Kontogiannopoulos, K.N.; Theodoridis, K.; Aggelidou, E.; Rat, A.; Willems, A.; Tsivintzelis, I.; Papageorgiou, V.P.; Kritis, A.; Assimopoulou, A.N. Electrospun wound dressings containing bioactive natural products: Physico-chemical characterization and biological assessment. Biomater. Res. 2021, 25, 23–43.
- Shin, Y.C.; Kang, S.H.; Lee, J.H.; Kim, B.; Hong, S.W.; Han, D.-W. Three-dimensional graphene oxide-coated polyurethane foams beneficial to myogenesis. J. Biomater. Sci. Polym. Ed. 2017, 29, 762–774.
- Biswas, D.; Tran, P.; Tallon, C.; O’connor, A. Combining mechanical foaming and thermally induced phase separation to generate chitosan scaffolds for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 207–226.
- Khoshnood, N.; Zamanian, A. Decellularized extracellular matrix bioinks and their application in skin tissue engineering. Bioprinting 2020, 20, e00095–e00103.
- Kang, M.S.; Kwon, M.; Lee, S.Y.; Lee, S.H.; Jo, H.J.; Kim, B.; Kim, K.S.; Han, D.-W. In situ crosslinkable collagen-based hydrogels for 3d printing of dermis-mimetic constructs. ECS J. Solid State Sci. Technol. 2022, 11, 045014–045021.
- Cha, M.; Jin, Y.-Z.; Park, J.W.; Lee, K.M.; Han, S.H.; Choi, B.S.; Lee, J.H. Three-dimensional printed polylactic acid scaffold integrated with BMP-2 laden hydrogel for precise bone regeneration. Biomater. Res. 2021, 25, 35–45.
- Zhang, W.; Du, A.; Liu, S.; Lv, M.; Chen, S. Research progress in decellularized extracellular matrix-derived hydrogels. Regen. Ther. 2021, 18, 88–96.
- Abaci, A.; Guvendiren, M. Designing decellularized extracellular matrix-based bioinks for 3D bioprinting. Adv. Healthc. Mater. 2020, 9, 2000734–2000751.
- Garreta, E.; Oria, R.; Tarantino, C.; Pla-Roca, M.; Prado, P.; Fernandez-Aviles, F.; Campistol, J.M.; Samitier, J.; Montserrat, N. Tissue Engineering by decellularization and 3D bioprinting. Mater. Today 2017, 20, 166–178.
- Vijayavenkataraman, S.; Lu, W.; Fuh, J. 3D bioprinting of skin: A state-of-the-art review on modelling, materials, and processes. Biofabrication 2016, 8, 032001–032032.
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30.
- McLafferty, E.; Hendry, C.; Farley, A. The integumentary system: Anatomy, physiology and function of skin. Nurs. Stand. 2012, 27, 35–42.
- James, W.; Berger, T.; Elston, D. Andrews’ diseases of the skin: Clinical dermatology. Postgrad. Med. J. 1990, 66, 984.
- White, S.D.; Yager, J.A. Resident dendritic cells in the epidermis: Langerhans cells, Merkel cells and melanocytes. Vet. Dermatol. 1995, 6, 1–8.
- Yussof, S.J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228.
- Park, S.G.; Shin, H.; Shin, Y.K.; Lee, Y.; Choi, E.-C.; Park, B.-J.; Kim, S. The novel cytokine p43 stimulates dermal fibroblast proliferation and wound repair. Am. J. Pathol. 2005, 166, 387–398.
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101.
- Kang, M.S.; Kwon, M.; Lee, S.H.; Kim, W.H.; Lee, G.W.; Jo, H.J.; Kim, B.; Yang, S.Y.; Kim, K.S.; Han, D.-W. 3D printing of skin equivalents with hair follicle structures and epidermal-papillary-dermal layers using gelatin/hyaluronic acid hydrogels. Chem. Asian J. 2022, 17, e202200620–e202200628.
- Langton, A.K.; Graham, H.K.; Griffiths, C.E.; Watson, R.E. Ageing significantly impacts the biomechanical function and structural composition of skin. Exp. Dermatol. 2019, 28, 981–984.
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 1–16.
- Nuutila, K. Hair follicle transplantation for wound repair. Adv. Wound Care 2021, 10, 153–163.
- Castano, O.; Pérez-Amodio, S.; Navarro-Requena, C.; Mateos-Timoneda, M.Á.; Engel, E. Instructive microenvironments in skin wound healing: Biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018, 129, 95–117.
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343.
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256.
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42.
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161.
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319.
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 2017, 112, 264–274.
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935.
- Singh, Y.P.; Bandyopadhyay, A.; Mandal, B.B. 3D bioprinting using cross-linker-free silk–gelatin bioink for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2019, 11, 33684–33696.
- Liu, N.; Huang, S.; Yao, B.; Xie, J.; Wu, X.; Fu, X. 3D bioprinting matrices with controlled pore structure and release function guide in vitro self-organization of sweat gland. Sci. Rep. 2016, 6, 34410–34417.
- Liu, W.; Zhang, Y.S.; Heinrich, M.A.; De Ferrari, F.; Jang, H.L.; Bakht, S.M.; Alvarez, M.M.; Yang, J.; Li, Y.C.; Trujillo-de Santiago, G. Rapid continuous multimaterial extrusion bioprinting. Adv. Mater. 2017, 29, 1604630–1604637.
- Munaz, A.; Vadivelu, R.K.; John, J.S.; Barton, M.; Kamble, H.; Nguyen, N.-T. Three-dimensional printing of biological matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17.
- Koch, L.; Gruene, M.; Unger, C.; Chichkov, B. Laser assisted cell printing. Curr. Pharm. Biotechnol. 2013, 14, 91–97.
- Raja, I.S.; Kang, M.S.; Hong, S.W.; Bae, H.; Kim, B.; Hwang, Y.-S.; Cha, J.M.; Han, D.-W. State-of-the-art techniques for promoting tissue regeneration: Combination of three-dimensional bioprinting and carbon nanomaterials. Int. J. Bioprinting 2023, 9, 181–198.
- Ali, M.; Pages, E.; Ducom, A.; Fontaine, A.; Guillemot, F. Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution. Biofabrication 2014, 6, 045001–045010.
- Serra, P.; Duocastella, M.; Fernández-Pradas, J.; Morenza, J. Liquids microprinting through laser-induced forward transfer. Appl. Surf. Sci. 2009, 255, 5342–5345.
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Ann. Rev. Mater. Res. 2010, 40, 395–414.
- Onses, M.S.; Sutanto, E.; Ferreira, P.M.; Alleyne, A.G.; Rogers, J.A. Mechanisms, capabilities, and applications of high-resolution electrohydrodynamic jet printing. Small 2015, 11, 4237–4266.
- Jayasinghe, S.N.; Qureshi, A.N.; Eagles, P.A. Electrohydrodynamic jet processing: An advanced electric-field-driven jetting phenomenon for processing living cells. Small 2006, 2, 216–219.
More
Information
Subjects:
Engineering, Biomedical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
959
Revisions:
2 times
(View History)
Update Date:
16 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





