Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zeyidan Jiapaer | -- | 3046 | 2023-01-14 15:35:23 | | | |
| 2 | Camila Xu | Meta information modification | 3046 | 2023-01-16 02:56:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jiapaer, Z.; Li, C.; Yang, X.; Sun, L.; Chatterjee, E.; Zhang, L.; Lei, J.; Li, G. Extracellular Non-Coding RNAs as Biomarkers in Cardiovascular Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/40173 (accessed on 08 February 2026).
Jiapaer Z, Li C, Yang X, Sun L, Chatterjee E, Zhang L, et al. Extracellular Non-Coding RNAs as Biomarkers in Cardiovascular Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/40173. Accessed February 08, 2026.
Jiapaer, Zeyidan, Chengyu Li, Xinyu Yang, Lingfei Sun, Emeli Chatterjee, Lingying Zhang, Ji Lei, Guoping Li. "Extracellular Non-Coding RNAs as Biomarkers in Cardiovascular Diseases" Encyclopedia, https://encyclopedia.pub/entry/40173 (accessed February 08, 2026).
Jiapaer, Z., Li, C., Yang, X., Sun, L., Chatterjee, E., Zhang, L., Lei, J., & Li, G. (2023, January 14). Extracellular Non-Coding RNAs as Biomarkers in Cardiovascular Diseases. In Encyclopedia. https://encyclopedia.pub/entry/40173
Jiapaer, Zeyidan, et al. "Extracellular Non-Coding RNAs as Biomarkers in Cardiovascular Diseases." Encyclopedia. Web. 14 January, 2023.
Copy Citation
Cardiovascular diseases (CVDs) remain the world’s leading cause of death despite the best available healthcare and therapy. Extracellular non-coding RNAs (Ex-ncRNAs) are a heterogeneous group of RNAs, including small ncRNAs, long ncRNAs (lncRNAs), and circular RNAs (circRNAs), which encompass the majority of the extracellular transcriptome.
extracellular RNAs
non-coding RNAs
cardiovascular diseases
1. Introduction
Cardiovascular diseases (CVDs) are widely recognized as the leading cause of death worldwide. Despite the considerable advances in both healthcare and therapies over the past decades, CVD has contributed to 17.9 million deaths in 2019 and is estimated to result in 22.2 million deaths in 2030 [1][2]. There is, therefore, an unmet medical need to develop novel diagnostics and therapeutics targeting CVDs. Emerging evidence suggests that extracellular non-coding RNAs (Ex-ncRNAs), particularly non-coding RNAs (ncRNAs) encapsulated in extracellular vesicles (EVs), function as key mediators of intercellular and inter-organ communication and play versatile roles in both homeostasis and disease [3]. EVs are continuously secreted by cells into circulation and are found in all biological fluids. The encapsulated molecular cargo inside EVs, especially RNAs, are increasingly recognized as promising biomarkers and therapeutic targets for various diseases, including CVDs [4][5][6].
Ex-ncRNAs are a heterogeneous group of RNAs, including small ncRNAs, long ncRNAs (lncRNAs), and circular RNAs (circRNAs), which encompass the majority of the extracellular transcriptome [7][8]. Ex-ncRNAs can be translocated into receipt cells after being secreted into the extracellular space through a variety of methods, thereby regulating various biological processes in targeted cells under both physiological and pathological conditions [9] (Figure 1).
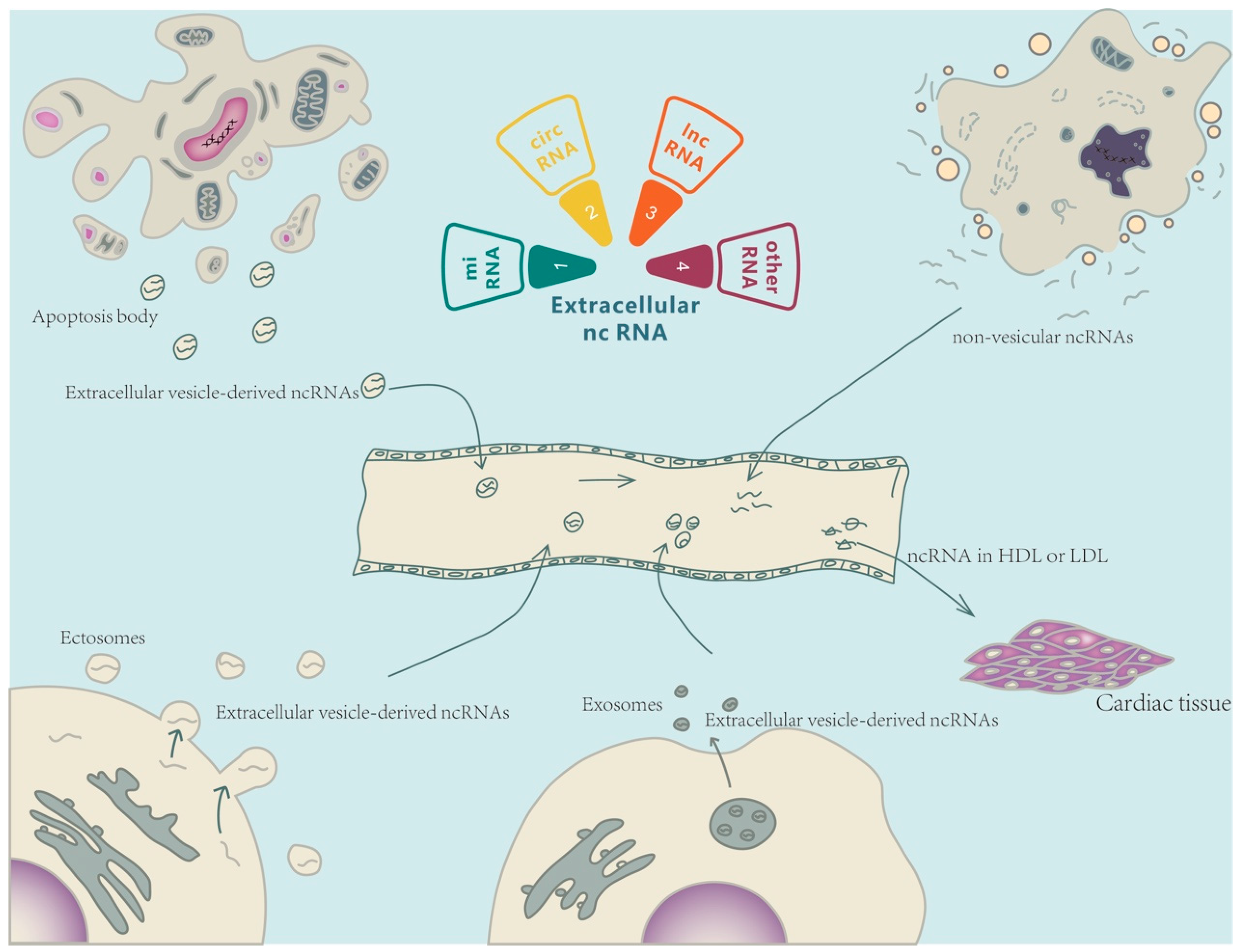
Figure 1. Extracellular non-coding RNA-mediated intercellular crosstalk. Extracellular non-coding RNAs (Ex-ncRNAs) are a heterogeneous group of RNAs and mainly include small ncRNAs, lncRNAs, and circRNAs. Ex-ncRNAs can be translocated into receipt cells and modulate the cellular functions of targeted cells (heart or other cardiovascular systems). Ex-ncRNAs can be secreted into the extracellular space through a variety of pathways, including embedded into exosomes, ectosomes, and apoptotic bodies, or partnered with ribonucleoproteins and lipoproteins.
2. Ex-ncRNAs
2.1. Extracellular Small ncRNAs
The main classes of small ncRNAs are miRNAs, small interfering RNAs (siRNAs), piwi-interacting RNAs (piRNAs), Y-RNAs, and tRNA-derived small RNAs (tDRs) [10][11]. Most small ncRNAs are capable of regulating gene expression either transcriptionally or epitranscriptionally [10][12]. Recent evidence suggests that small ncRNAs are encapsulated into EVs and modulate the transcriptome of recipient cells following release through cellular autocrine or paracrine pathways. In addition, small ncRNAs, embedded in ribonucleoproteins and lipoproteins, are found to be secreted into extracellular spaces [13]. An increasing number of studies have detected extracellular small ncRNAs in a variety of biological fluids, such as serum or plasma, and indicated that extracellular small ncRNA profiles in various body fluids could serve as novel biomarkers for different pathological conditions [14][15][16][17]. As one of the most abundant RNA species in circulation, extracellular small ncRNAs are actively involved in a variety of pathological and physiological processes. Therefore, the detection of specific circulating small ncRNAs, based on the small ncRNA profiles, could be a promising approach to diagnosing diseases.
2.2. Extracellular LncRNAs
LncRNAs are a class of transcripts with a length of more than 200 nucleotides and lack protein-coding potential [18]. There are many categories and sub-categories of lncRNAs, with major classifications including antisense, bi-directional, enhancer-associated, intergenic lncRNAs (lincRNAs), and pseudogenes [19]. They function primarily in two fashions: (1) regulating the expression of miRNA target genes by mimicking miRNA sponges via competitive endogenous RNA (ceRNA) inhibition, and (2) regulating the post-translational modification of specific proteins, thereby affecting the activity of downstream signaling pathways [20]. Recent studies have noted that EVs are the primary means through which lncRNAs are transferred outside cells [21]. Extracellular lncRNAs can affect a broad range of downstream effects. In general, lncRNAs act as heterologous RNAs that can be transferred to target cells by EVs and regulate the cellular functions of receipt cells [22]. The membrane-bound nature of EVs shields ncRNA cargos from degradation. Due to the lack of evolutionary conservation and high specificity in different cells/organs, lncRNAs continue to be investigated as potential mediators of intercellular communication, exemplifying a valuable class of therapeutic targets for disorders as well as potential biomarkers [23].
2.3. Extracellular CircRNAs
CircRNAs are single-stranded RNAs that are linked end-to-end by a back-splicing mechanism. According to their splicing sequence, circRNAs can be categorized into the following groups: exonic circular RNAs (ecircRNAs), circular intronic RNAs (ciRNAs), exon-intron circular RNAs (EIciRNAs), intergenic circRNAs, anti-sense circRNAs, and tRNA intronic circRNAs (tricRNAs). Until now, the functionalities of circRNAs have been mainly classified into four categories: (1) acting as ceRNAs or sponges; (2) regulation of pre-RNA cleavage; (3) regulation of gene expression; and (4) as a potential source for translation of proteins and peptides [24]. Similar to the aforementioned ncRNAs, circRNAs can also be loaded into EVs to mediate cell-cell communication [13]. CircRNAs demonstrate a notable advantage over other ncRNAs. Without a linear terminal, circRNAs have a longer half-life, allowing for accumulation in tissues with a low proliferation rate. Additionally, the lack of a linear terminal impedes RNase degradation and improves stability and integrity in the extracellular environment, thus elevating their utility as biomarkers of disease [25]. Recent evidence has identified a crucial role of several extracellular circRNAs in alleviating damage due to cardiomyocyte hypertrophy, heart failure (HF), myocardial infarction (MI), and dysfunction caused by ischemia-reperfusion (I/R) [26]. Furthermore, several studies have also reported their association with proliferation, apoptosis, and inflammatory responses, thus influencing physiological and pathological phenomena in various tissues [7][27]. Investigation of circRNAs continues to elucidate their role in the pathogenesis of CVDs and provides a potential avenue for therapeutic development.
3. Ex-ncRNAs as Biomarkers in CVDs
In recent decades, several molecular mechanisms have been identified to be associated with the induction and progression of CVDs, especially in coronary atherosclerosis and HF [28]. Although clinical management of HF is improving, incidence rates remain above 20% in the adult population, with mortality rates hovering at 50% within 5 years of diagnosis, which makes HF a leading cause of morbidity and mortality in developed countries [29]. Consequently, it is necessary to delve into the disease’s underlying mechanisms and subsequent release of ncRNAs to develop more effective diagnostic and prognostic tools to reduce mortality (Table 1).
3.1. Atherosclerosis
Atherosclerosis is a chronic inflammatory artery disease characterized by the deposition of atherosclerotic plaque in the arteries, which leads to the hardening and narrowing of the artery lumen and subsequent obstruction of blood flow [30]. Atherosclerotic plaque results from the accumulation of circulating low-density lipoprotein (LDL) cholesterol alongside fibrous materials and inflammatory cells on the inner layers of arteries [31]. On the contrary, other cholesterol classes, namely high-density lipoproteins (HDLs), hold a significant anti-atherosclerotic role, transporting cholesterol from peripheral tissues to the liver for metabolism [32].
3.1.1. Extracellular Small ncRNAs
In 2011, a study found that circulating HDLs and LDLs can transport miRNAs, such as HDL-transported miR-223, to regulate different intercellular signaling pathways in atherosclerosis [33][34]. In addition to cholesterol-driven miRNA transport, circulating miRNAs have also been identified as potential biomarkers of atherosclerosis in hypertensive patients. By comparing the expression levels of miR-92a in plasma of different levels of carotid intima-media thickness (CIMT) and hypertensive patients, a recent study found that the increased level of miR-92a expression was positively correlated with the measured value of CIMT, ambulatory blood pressure monitoring results and carotid-femoral pulse wave velocity, all symptoms of atherosclerosis [35]. Given that lipoproteins play an important role in the progression of atherosclerosis, differential expression of HDL-miRNAs, in addition to circulating miRNAs, can help track disease progression and enable more accurate diagnosis and treatment.
3.1.2. Extracellular LncRNAs
Extracellular lncRNAs such as lncRNA Sox2 Overlapping Transcript (SOX2-OT) have also been shown to be potentially involved in the development of vascular disease [36]. Serum SOX2-OT levels were found to be significantly elevated in atherosclerotic patients. The results revealed that it could be used as a diagnostic biomarker and an excellent modality to assess patient prognosis [37].
3.1.3. Extracellular CircRNAs
Another study quantified circRNA in the plasma of patients with coronary artery disease. It was reported that the plasma level of hsa_circ_0001445 was reduced in patients with coronary artery sclerosis, and the condition remained stable in patients with reduced expression of hsa_circ_0001445. The expression level of hsa_circ_0001445 was inversely correlated with the degree of coronary atherosclerosis. Combined with coronary computed tomography, its quantification significantly improved the diagnosis of the degree of disease [38].
3.2. Ischemic Heart Disease
Ischemic cardiomyopathy is caused by an imbalance in the myocardial oxygen demand and available oxygen supply, most often induced by coronary artery stenosis. Based on the pathophysiology, ischemic cardiomyopathy includes coronary heart disease and myocardial infarction (MI) [39].
Although stenting is a crucial method to relieve myocardial ischemia, it may result in long-term cardiac damage when blood flow is suddenly restored, also known as ischemia-reperfusion (I/R) injury [40][41]. I/R injury after reperfusion often occurs due to cardiomyocytes undergoing anaerobic metabolism, sodium-potassium pump dysfunction, and ribosome shedding. Various intracellular ATP-dependent ion pumps become inactive, leading to ion accumulation, pH down-regulation, and up-regulation of intracellular osmolarity. Ultimately, ischemic cardiomyocytes experience cell swelling, impaired enzyme activities, and clumped nuclear chromatin. Due to the low concentration of antioxidants in ischemic cardiomyocytes, the increased reactive oxygen species after reperfusion causes oxidative stress, which further causes cellular dysfunction, DNA damage, and local inflammatory responses in cardiomyocytes. Thus, the persistence of inflammation and oxidative stress may trigger a cytokine storm that exposes cells to severe damage [42].
3.2.1. Extracellular Small ncRNAs
Currently, the primary diagnosis of MI is mainly based on the detection of MI markers like cardiac troponin [43][44]. However, there is evidence showing that elevated cardiac troponins are present outside cardiac injury and can compromise the diagnosis of MI [45]. This opens an avenue to pursue Ex-ncRNA levels as a circulating biomarker for acute myocardial infarction (AMI) [46]. A previous study observed a 1600-fold elevated expression of miR-208b in AMI compared to the control group. Further, miR-208b identified earlier detection than troponin T regarding MI progression. In addition, another study found that miR-204 was down-regulated, and lncRNA-NEAT1 and matrix metalloproteinase-9 (MMP-9) were upregulated in serum EVs from patients with acute ST-segment elevation MI [47]. Serum-derived miRNA signatures have been used to distinguish healthy volunteers from patients with coronary atherosclerosis through the expression levels of miR-370-3p and miR-409-3p, which can serve as a fingerprint for coronary heart disease [48]. By exploring the expression levels of EV-derived miRNAs in the plasma and serum of CS and AMI patients, EV miRNA landscapes facilitated disease differentiation [49].
Novel miRNAs have been found to identify the evolution of cardiac I/R injury [50]. MiRNAs with diagnostic or therapeutic potential have been identified in the context of early cardiac I/R injury with miRNA arrays conducted to screen for differential expression in a mouse model of cardiac I/R injury. A total of 1882 miRNAs were screened, among which 11 were observably down-regulated and 41 were markedly up-regulated 3 h after reperfusion. miR-3113-5P and miR-223-3p were among the most differentially expressed miRNAs and have been confirmed to be up-regulated in the early stage of cardiac tissue I/R injury [51][52]. This data confirms that cardiac miRNAs, such as miRNA-3113-5p, might be a valuable target for therapeutic purposes, and circulating miRNAs such as miRNA-3113-5p might serve as a stable marker for the early diagnosis of cardiac I/R injury.
Extracellular tDR is a newly identified small regulatory RNA species. A recent study has demonstrated that extracellular tDRs are much more dynamically regulated than intracellular tDRs and extracellular miRNAs in both cardiomyocytes and cardiac fibroblasts upon the treatments of ischemia/reperfusion-related stressors [11]. Notably, more than 3000 extracellular tDRs are significantly regulated by nutritional deprivation or ischemia, and approximately 2000 extracellular tDRs are differentially expressed upon ischemia/reoxygenation treatment mimicking ischemia/reperfusion injury in both cardiomyocytes and cardiac fibroblasts [11]. Detailedly, extracellular tDR-1:32-His-GTG-1, tDR-37:72-Val-TAC-1, tDR-1:32-Pro-AGG-1-M4, tDR-2:30-Glu-CTC-1, tDR-2:30-Glu-CTC-1-D4G, tDR-1:31-Glu-TTC-4, tDR-3:31-Gly-GCC-2-M2, and tDR-40:72-Asn-GTT-1-M2 were significantly induced by ischemia/reoxygenation from both cardiomyocytes and cardiac fibroblasts, and extracellular tDR-1:36-Glu-CTC-1, tDR-1:36-Glu-CTC-1-D5G, tDR-1:36-Asp-GTC-2-M2, and tDR-42:75-Ser-GCT-3 were downregulated considerably upon cardiac ischemia/reoxygenation [11]. Although this research is a preliminary cell culture model, the findings clearly suggest that the extracellular tDR may be a promising biomarker for diagnostic and prognostic assessments of ischemic heart diseases.
3.2.2. Extracellular LncRNAs
A previous study has shown that lncRNAs can also serve as potential biomarkers for AMI [53]. It was found that the level of circulating lncRNA ENST000005566899.1 and lncRNA ENST00000575985.1 were significantly elevated in the plasma-derived EVs of AMI patients compared to healthy individuals. Further study of these lncRNAs in combination with mature biomarkers will enhance the understanding of disease progression which is fundamental for developing lncRNA therapeutics treating MI. Owing to the similarities of disease presentation and the vast differences in management, it becomes imperative to pursue avenues that allow for identifying the types and stages of diseases using noninvasive biomarkers.
3.2.3. Extracellular CircRNAs
Previous studies have shown that the downregulation of circRNA MICRA in peripheral blood is associated with the risk of left ventricular remodeling and dysfunction after MI. Additionally, the upregulation of hsa-circ-0098964 and circRNA-284 in the serum has been found to be associated with an increased risk of hypertension and acute ischemia [54].
3.3. Hypertension
Hypertension is a significant cause of heart disease and death worldwide [55]. As the heart pumps blood, the blood applies outward pressure on the arterial wall. The maximum pressure exerted while beating is defined as systolic pressure. Conversely, blood pressure on the arterial wall during relaxation and dilatation of the heart muscles is known as diastolic pressure. Hypertension is diagnosed when systolic pressure exceeds 140 mmHg, or diastolic pressure exceeds 90 mmHg [56]. One of the leading causes of hypertension is vascular dysfunction which manifests as endothelial dysfunction, vascular inflammation, arterial remodeling, and lowered vascular elasticity [57].
Extracellular Small ncRNAs
Extracellular miR-133a, miR-21, and miR-27a have previously been reported to play an essential role in the development of hypertension [58][59][60]. Based on these findings, a study was conducted on a general population over five years, measuring the serum levels of miR-133a, miR-21, and miR-27a using RT-qPCR. It was found that the serum expression levels of miR-133a and miR-27a were negatively correlated with the incidence of hypertension and, as a result, could be used as diagnostic biomarkers and preventive and predictive biomarkers of hypertension [61]. Recently, a study using serum from hypertensive patients quantified miR-92a and found that its expression was significantly increased in the serum of hypertensive patients, verifying its potential as a biomarker [62]. An in vitro study further supports the potential of extracellular miR-92a as a biomarker of hypertension as it targets vascular smooth muscle cells to regulate vascular SMC phenotype, thereby causing arterial stiffness and participating in the occurrence and development of hypertension [62].
Hypertensive disorder during pregnancy is a common type of hypertension [63][64]. Commonly, severe pregnancy-induced hypertension causes irreversible damage to the mother and offspring. Previous studies have revealed that miR-200a-3p can coordinate the function of trophoblast [65]. Based on this conclusion, one study quantified miR-200a-3p in the serum of hypertensive patients during pregnancy by RT-qPCR and found that the expression level of miR-200a-3p in the serum of hypertensive patients during pregnancy was up-regulated. Moreover, its expression level was positively correlated with the development of the disease. As a result, the level of miR-200a-3p bears prognostic potential for gestational hypertension [66].
3.4. Heart Failure
HF is a clinical syndrome of structural or functional abnormalities of the heart [67]. As HF may not have symptoms at its early stage, early screening using biomarkers can significantly reduce the risk of HF. The current standard for diagnosis utilizes the N-terminal-proB-type Natriuretic Peptide (NT-proBNP) test. However, increased concentrations of BNPs are not always associated with the onset of HF and may impede accurate and earlier diagnosis of HF.
3.4.1. Extracellular Small ncRNAs
A previous study has examined the expression of Ex-ncRNAs in plasma in relation to the progression of HF [68], and found altered expression of miR-192, miR-194, and miR-134a in the plasma from patients with HF, implying the capacity of small ncRNAs to identify cardiomyocyte death and HF [68]. Similarly, another study showed that paracrine signaling via cardiomyocyte-derived EVs containing miR-30d could improve cardiac function by reducing myocardial fibrosis and cardiomyocyte apoptosis [69]. Small ncRNAs have also been assessed in the serum of acute HF patients following dilated cardiomyopathy. In this cohort, miR-92b-5p expression was up-regulated in the EVs of the patients, suggesting its use as a potential biomarker in diagnosing acute AHF caused by dilated cardiomyopathy [70].
3.4.2. Extracellular LncRNAs
Ischemic cardiomyopathy (ICM) caused by MI is a major cause of HF. In the context of circulating biomarkers, lncRNAs in ICM have been widely explored. Genome-wide transcriptome analysis has verified that a number of protein-coding genes previously reported to be associated with HF demonstrated altered expression following ICM [71][72]. Among 145 differentially expressed lncRNAs screened in ICM, 35 lncRNAs showed strong positive correlations. Expression correlation coefficient analysis of differentially expressed lncRNAs and the protein-coding genes yielded a strong correlation between lncRNAs and ECM protein-coding genes. The overexpression or knockdown of selected lncRNAs in the cardiac fibroblasts indicated that lncRNAs were significant regulators of fibrosis and ECM synthesis gene expression [73][74]. In addition, lncRNAs were found to be involved in the TGF-β pathway to regulate ECM gene expression and myofibroblast differentiation [75]. This data suggests that lncRNAs might be novel modulators of heart function and HF.
Table 1. Ex-ncRNAs as biomarkers in CVDs.
| Reference | Ex-ncRNA | Carriers | Expression (↑) (↓) |
Type of CVDs | |
|---|---|---|---|---|---|
| Kasey et al. [33] | miR-223 | HDL | ↑ | - | Atherosclerosis |
| Huang et al. [35] | miR-92a | - | ↑ | - | Atherosclerosis |
| Tao et al. [37] | LncRNA SOX2-OT | - | ↑ | - | Atherosclerosis |
| Vilades et al. [38] | hsa_circ_0001445 | - | - | ↓ | Atherosclerosis |
| Corsten et al. [46] | miR-208b | - | ↑ | - | Acute myocardial infarction |
| Li et al. [11] | tDR-1:32-His-GTG-1, tDR-37:72-Val-TAC-1, tDR-1:32-Pro-AGG-1-M4, etc. | - | ↑ | Cardiac ischemia/reperfusion | |
| tDR-1:36-Glu-CTC-1, tDR-1:36-Glu-CTC-1-D5G, tDR-1:36-Asp-GTC-2-M2, etc. | - | ↓ | |||
| Chen et al. [47] | LncRNA-NEAT1, miR-204 | EV | ↑ | ↓ | Acute ST-segment elevation myocardial infarction |
| Hildebrandt et al. [48] | miR-370-3p, miR-409-3p | EV | ↑ | - | Coronary heart disease |
| Chen et al. [50] | microRNA 3113-5P | - | ↑ | - | Cardiac ischemia/Reperfusion injury |
| Zheng et al. [53] | lncRNA ENST00000556899.1, lncRNA ENST00000575985.1 |
EV | ↑ | - | Acute myocardial infarction |
| Kishore et al. [54] | circRNA MICRA, circRNA-284, hsa-circ-0098964 | - | ↑ | ↓ | Myocardial infarction, Ischemic heart disease, Hypertension |
| Suzuki et al. [61] | miR-133a, miR-27a | - | - | ↓ | Hypertension |
| Wang et al. [62] | miR-92a | - | ↑ | - | Hypertension |
| He et al. [66] | miR-200a-3p | - | ↑ | - | Hypertension |
| Janjusevic et al. [68] | lncRNA LIPCAR, miR-192, miR-134a, miR-194, miR-30d | EV | ↑ | ↓ | Heart failure |
| Li et al. [69] | miR-30d | EV | - | ↓ | Heart failure |
| Wu et al. [70] | miR-92b-5p | EV | ↑ | - | Heart failure |
References
- Campbell, N.R.C.; Ordunez, P.; Giraldo, G.; Rodriguez Morales, Y.A.; Lombardi, C.; Khan, T.; Padwal, R.; Tsuyuki, R.T.; Varghese, C. WHO HEARTS: A Global Program to Reduce Cardiovascular Disease Burden: Experience Implementing in the Americas and Opportunities in Canada. Can. J. Cardiol. 2021, 37, 744–755.
- Şahin, B.; İlgün, G. Risk factors of deaths related to cardiovascular diseases in World Health Organization (WHO) member countries. Health Soc. Care Community 2022, 30, 73–80.
- Fang, Y.; Dai, X. Emerging Roles of Extracellular Non-Coding RNAs in Vascular Diseases. J. Cardiovasc. Transl. Res. 2022, 15, 492–499.
- Thompson, A.G.; Gray, E.; Heman-Ackah, S.M.; Mäger, I.; Talbot, K.; Andaloussi, S.E.; Wood, M.J.; Turner, M.R. Extracellular vesicles in neurodegenerative disease—Pathogenesis to biomarkers. Nat. Rev. Neurol. 2016, 12, 346–357.
- Zijlstra, C.; Stoorvogel, W. Prostasomes as a source of diagnostic biomarkers for prostate cancer. J. Clin. Investig. 2016, 126, 1144–1151.
- Gruner, H.N.; McManus, M.T. Examining the evidence for extracellular RNA function in mammals. Nat. Rev. Genet. 2021, 22, 448–458.
- Lin, Z.; Lu, F.; Ma, X.; Xia, X.; Zou, F.; Jiang, J. Roles of circular RNAs in the pathogenesis of intervertebral disc degeneration (Review). Exp. Ther. Med. 2021, 22, 1221.
- Sato-Kuwabara, Y.; Melo, S.A.; Soares, F.A.; Calin, G.A. The fusion of two worlds: Non-coding RNAs and extracellular vesicles--diagnostic and therapeutic implications (Review). Int. J. Oncol. 2015, 46, 17–27.
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 2018, 39, 2704–2716.
- Hu, Y.Y.; Cheng, X.M.; Wu, N.; Tao, Y.; Wang, X.N. Non-coding RNAs Regulate the Pathogenesis of Aortic Dissection. Front. Cardiovasc. Med. 2022, 9, 890607.
- Li, G.; Manning, A.C.; Bagi, A.; Yang, X.; Gokulnath, P.; Spanos, M.; Howard, J.; Chan, P.P.; Sweeney, T.; Kitchen, R.; et al. Distinct Stress-Dependent Signatures of Cellular and Extracellular tRNA-Derived Small RNAs. Adv. Sci. 2022, 9, e2200829.
- Brandão, B.B.; Guerra, B.A.; Mori, M.A. Shortcuts to a functional adipose tissue: The role of small non-coding RNAs. Redox Biol. 2017, 12, 82–102.
- Videira, R.F.; da Costa Martins, P.A. Non-coding RNAs in Cardiac Intercellular Communication. Front. Physiol. 2020, 11, 738.
- Kondkar, A.A.; Abu-Amero, K.K. Utility of circulating microRNAs as clinical biomarkers for cardiovascular diseases. Biomed. Res. Int. 2015, 2015, 821823.
- Peters, L.J.F.; Biessen, E.A.L.; Hohl, M.; Weber, C.; van der Vorst, E.P.C.; Santovito, D. Small Things Matter: Relevance of MicroRNAs in Cardiovascular Disease. Front. Physiol. 2020, 11, 793.
- Shang, X.; Fang, Y.; Xin, W.; You, H. The Application of Extracellular Vesicles Mediated miRNAs in Osteoarthritis: Current Knowledge and Perspective. J. Inflamm. Res. 2022, 15, 2583–2599.
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta 2010, 1803, 1231–1243.
- Xia, W.; Zhu, X.-W.; Mo, X.-B.; Wu, L.-F.; Wu, J.; Guo, Y.-F.; Zeng, K.-Q.; Wang, M.-J.; Lin, X.; Qiu, Y.-H.; et al. Integrative multi-omics analysis revealed SNP-lncRNA-mRNA (SLM) networks in human peripheral blood mononuclear cells. Human Genet. 2017, 136, 451–462.
- Robinson, E.K.; Covarrubias, S.; Carpenter, S. The how and why of lncRNA function: An innate immune perspective. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194419.
- Sun, R.; He, X.Y.; Mei, C.; Ou, C.L. Role of exosomal long non-coding RNAs in colorectal cancer. World J. Gastrointest. Oncol. 2021, 13, 867–878.
- Dragomir, M.; Chen, B.; Calin, G.A. Exosomal lncRNAs as new players in cell-to-cell communication. Transl. Cancer Res. 2018, 7, S243–S252.
- Xue, M.; Chen, W.; Li, X. Extracellular vesicle-transferred long noncoding RNAs in bladder cancer. Clin. Chim. Acta 2021, 516, 34–45.
- Ye, M.; Wang, J.; Pan, S.; Zheng, L.; Wang, Z.-W.; Zhu, X. Nucleic acids and proteins carried by exosomes of different origins as potential biomarkers for gynecologic cancers. Mol. Ther.-Oncolytics 2021, 24, 101–113.
- Zhang, Y.; Zhang, X.; Xu, Y.; Fang, S.; Ji, Y.; Lu, L.; Xu, W.; Qian, H.; Liang, Z.F. Circular RNA and Its Roles in the Occurrence, Development, Diagnosis of Cancer. Front. Oncol. 2022, 12, 845703.
- Tang, X.; Ren, H.; Guo, M.; Qian, J.; Yang, Y.; Gu, C. Review on circular RNAs and new insights into their roles in cancer. Comput. Struct. Biotechnol. J. 2021, 19, 910–928.
- Jiang, L.; Wang, X.; Zhan, X.; Kang, S.; Liu, H.; Luo, Y.; Lin, L. Advance in circular RNA modulation effects of heart failure. Gene X 2020, 5, 100036.
- Yan, F.; Xie, X.; Huo, Q.; Zhang, W.; Wu, T.; Daniyaer, D.; Shi, L. circ-CCND1 regulates the CCND1/P53/P21 pathway through sponging miR-138-5p in valve interstitial cells to aggravate aortic valve calcification. J. Physiol. Biochem. 2022, 78, 845–854.
- E, S.; Costa, M.C.; Kurc, S.; Drożdż, A.; Cortez-Dias, N.; Enguita, F.J. The circulating non-coding RNA landscape for biomarker research: Lessons and prospects from cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1085–1099.
- Shen, S.; Jiang, H.; Bei, Y.; Xiao, J.; Li, X. Long Non-Coding RNAs in Cardiac Remodeling. Cell. Physiol. Biochem. 2017, 41, 1830–1837.
- Zhang, Y.; Ying, F.; Tian, X.; Lei, Z.; Li, X.; Lo, C.-Y.; Li, J.; Jiang, L.; Yao, X. TRPM2 Promotes Atherosclerotic Progression in a Mouse Model of Atherosclerosis. Cells 2022, 11, 1423.
- Aryal, B.; Suárez, Y. Non-coding RNA regulation of endothelial and macrophage functions during atherosclerosis. Vascul. Pharmacol. 2019, 114, 64–75.
- Lund-Katz, S.; Phillips, M.C. High density lipoprotein structure-function and role in reverse cholesterol transport. Subcell. Biochem. 2010, 51, 183–227.
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433.
- Lu, H.; Buchan, R.J.; Cook, S.A. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc. Res. 2010, 86, 410–420.
- Huang, Y.; Tang, S.; Ji-Yan, C.; Huang, C.; Li, J.; Cai, A.P.; Feng, Y.Q. Circulating miR-92a expression level in patients with essential hypertension: A potential marker of atherosclerosis. J. Hum. Hypertens. 2017, 31, 200–205.
- Yang, G.; Lin, C. Long Noncoding RNA SOX2-OT Exacerbates Hypoxia-Induced Cardiomyocytes Injury by Regulating miR-27a-3p/TGFβR1 Axis. Cardiovasc. Ther. 2020, 2020, 2016259.
- Tao, J.; Hu, Y. Diagnostic and prognostic significance of lncRNA SOX2-OT in patients with carotid atherosclerosis. BMC Cardiovasc. Disord. 2022, 22, 211.
- Vilades, D.; Martinez-Camblor, P.; Ferrero-Gregori, A.; Bar, C.; Lu, D.; Xiao, K.; Vea, A.; Nasarre, L.; Sanchez Vega, J.; Leta, R.; et al. Plasma circular RNA hsa_circ_0001445 and coronary artery disease: Performance as a biomarker. FASEB J. 2020, 34, 4403–4414.
- Moroni, F.; Gertz, Z.; Azzalini, L. Relief of Ischemia in Ischemic Cardiomyopathy. Curr. Cardiol. Rep. 2021, 23, 80.
- Han, D.; Kang, S.-H.; Yoon, C.-H.; Youn, T.-J.; Chae, I.-H. Attenuation of ischemia–reperfusion injury by intracoronary chelating agent administration. Sci. Rep. 2022, 12, 2050.
- Rios-Navarro, C.; Daghbouche-Rubio, N.; Gavara, J.; de Dios, E.; Perez, N.; Vila, J.M.; Chorro, F.J.; Ruiz-Sauri, A.; Bodi, V. Ischemia-reperfusion injury to coronary arteries: Comprehensive microscopic study after reperfused myocardial infarction. Ann. Anat. 2021, 238, 151785.
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667.
- Kuster, D.W.; Cardenas-Ospina, A.; Miller, L.; Liebetrau, C.; Troidl, C.; Nef, H.M.; Möllmann, H.; Hamm, C.W.; Pieper, K.S.; Mahaffey, K.W.; et al. Release kinetics of circulating cardiac myosin binding protein-C following cardiac injury. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H547–H556.
- Okamoto, R.; Hirashiki, A.; Cheng, X.W.; Yamada, T.; Shimazu, S.; Shinoda, N.; Okumura, T.; Takeshita, K.; Bando, Y.; Kondo, T.; et al. Usefulness of serum cardiac troponins T and I to predict cardiac molecular changes and cardiac damage in patients with hypertrophic cardiomyopathy. Int. Heart J. 2013, 54, 202–206.
- Mannu, G.S. The non-cardiac use and significance of cardiac troponins. Scott. Med. J. 2014, 59, 172–178.
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506.
- Chen, Z.; Yan, Y.; Wu, J.; Qi, C.; Liu, J.; Wang, J. Expression level and diagnostic value of exosomal NEAT1/miR-204/MMP-9 in acute ST-segment elevation myocardial infarction. IUBMB Life 2020, 72, 2499–2507.
- Hildebrandt, A.; Kirchner, B.; Meidert, A.S.; Brandes, F.; Lindemann, A.; Doose, G.; Doege, A.; Weidenhagen, R.; Reithmair, M.; Schelling, G.; et al. Detection of Atherosclerosis by Small RNA-Sequencing Analysis of Extracellular Vesicle Enriched Serum Samples. Front. Cell. Dev. Biol. 2021, 9, 729061.
- Crouser, E.D.; Julian, M.W.; Bicer, S.; Ghai, V.; Kim, T.K.; Maier, L.A.; Gillespie, M.; Hamzeh, N.Y.; Wang, K. Circulating exosomal microRNA expression patterns distinguish cardiac sarcoidosis from myocardial ischemia. PLoS ONE 2021, 16, e0246083.
- Chen, Y.; Ye, X.; Yan, F. MicroRNA 3113-5p is a novel marker for early cardiac ischemia/reperfusion injury. Diagn. Pathol. 2019, 14, 121.
- Liu, X.; Xu, Y.; Deng, Y.; Li, H. MicroRNA-223 Regulates Cardiac Fibrosis After Myocardial Infarction by Targeting RASA1. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 1439–1454.
- Yang, F.; You, X.; Xu, T.; Liu, Y.; Ren, Y.; Liu, S.; Wu, F.; Xu, Z.; Zou, L.; Wang, G. Screening and Function Analysis of MicroRNAs Involved in Exercise Preconditioning-Attenuating Pathological Cardiac Hypertrophy. Int. Heart J. 2018, 59, 1069–1076.
- Zheng, M.L.; Liu, X.Y.; Han, R.J.; Yuan, W.; Sun, K.; Zhong, J.C.; Yang, X.C. Circulating exosomal long non-coding RNAs in patients with acute myocardial infarction. J. Cell. Mol. Med. 2020, 24, 9388–9396.
- Kishore, R.; Garikipati, V.N.S.; Gonzalez, C. Role of Circular RNAs in Cardiovascular Disease. J. Cardiovasc. Pharmacol. 2020, 76, 128–137.
- DeGuire, J.; Clarke, J.; Rouleau, K.; Roy, J.; Bushnik, T. Blood pressure and hypertension. Health Rep. 2019, 30, 14–21.
- Desai, A.N. High Blood Pressure. JAMA 2020, 324, 1254–1255.
- Cameron, A.C.; Lang, N.N.; Touyz, R.M. Drug Treatment of Hypertension: Focus on Vascular Health. Drugs 2016, 76, 1529–1550.
- Besler, C.; Urban, D.; Watzka, S.; Lang, D.; Rommel, K.P.; Kandolf, R.; Klingel, K.; Thiele, H.; Linke, A.; Schuler, G.; et al. Endomyocardial miR-133a levels correlate with myocardial inflammation, improved left ventricular function, and clinical outcome in patients with inflammatory cardiomyopathy. Eur. J. Heart Fail. 2016, 18, 1442–1451.
- Cengiz, M.; Yavuzer, S.; Kılıçkıran Avcı, B.; Yürüyen, M.; Yavuzer, H.; Dikici, S.A.; Karataş, Ö.F.; Özen, M.; Özen, M.; Uzun, H.; et al. Circulating miR-21 and eNOS in subclinical atherosclerosis in patients with hypertension. Clin. Exp. Hypertens. 2015, 37, 643–649.
- Zou, X.; Wang, J.; Chen, C.; Tan, X.; Huang, Y.; Jose, P.A.; Yang, J.; Zeng, C. Secreted Monocyte miR-27a, via Mesenteric Arterial Mas Receptor-eNOS Pathway, Causes Hypertension. Am. J. Hypertens. 2020, 33, 31–42.
- Suzuki, K.; Yamada, H.; Fujii, R.; Munetsuna, E.; Yamazaki, M.; Ando, Y.; Ohashi, K.; Ishikawa, H.; Mizuno, G.; Tsuboi, Y.; et al. Circulating microRNA-27a and -133a are negatively associated with incident hypertension: A five-year longitudinal population-based study. Biomarkers 2022, 27, 496–502.
- Wang, C.; Wu, H.; Xing, Y.; Ye, Y.; He, F.; Yin, Q.; Li, Y.; Shang, F.; Shyy, J.Y.; Yuan, Z.Y. Endothelial-derived extracellular microRNA-92a promotes arterial stiffness by regulating phenotype changes of vascular smooth muscle cells. Sci. Rep. 2022, 12, 344.
- Corsello, S.M.; Paragliola, R.M. Evaluation and Management of Endocrine Hypertension During Pregnancy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 829–842.
- Shah, S.; Gupta, A. Hypertensive Disorders of Pregnancy. Cardiol. Clin. 2019, 37, 345–354.
- Cao, G.; Cui, R.; Liu, C.; Zhang, Z. MicroRNA regulation of transthyretin in trophoblast biofunction and preeclampsia. Arch. Biochem. Biophys. 2019, 676, 108129.
- He, X.; Ding, D. High miR-200a-3p expression has high diagnostic values for hypertensive disorders complicating pregnancy and predicts adverse pregnancy outcomes. BMC Pregnancy Childbirth 2022, 22, 490.
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Eur. J. Heart Fail. 2021, 23, 352–380.
- Janjusevic, M.; Fluca, A.L.; Ferro, F.; Gagno, G.; D’Alessandra, Y.; Beltrami, A.P.; Sinagra, G.; Aleksova, A. Traditional and Emerging Biomarkers in Asymptomatic Left Ventricular Dysfunction-Promising Non-Coding RNAs and Exosomes as Biomarkers in Early Phases of Cardiac Damage. Int. J. Mol. Sci. 2021, 22, 4937.
- Li, J.; Salvador, A.M.; Li, G.; Valkov, N.; Ziegler, O.; Yeri, A.; Yang Xiao, C.; Meechoovet, B.; Alsop, E.; Rodosthenous, R.S.; et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ. Res. 2021, 128, e1–e23.
- Wu, T.; Chen, Y.; Du, Y.; Tao, J.; Zhou, Z.; Yang, Z. Serum Exosomal MiR-92b-5p as a Potential Biomarker for Acute Heart Failure Caused by Dilated Cardiomyopathy. Cell. Physiol. Biochem. 2018, 46, 1939–1950.
- Huang, Z.P.; Ding, Y.; Chen, J.; Wu, G.; Kataoka, M.; Hu, Y.; Yang, J.H.; Liu, J.; Drakos, S.G.; Selzman, C.H.; et al. Long non-coding RNAs link extracellular matrix gene expression to ischemic cardiomyopathy. Cardiovasc. Res. 2016, 112, 543–554.
- Ounzain, S.; Micheletti, R.; Beckmann, T.; Schroen, B.; Alexanian, M.; Pezzuto, I.; Crippa, S.; Nemir, M.; Sarre, A.; Johnson, R.; et al. Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur. Heart J. 2015, 36, 353–368.
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 2014, 114, 1569–1575.
- Han, P.; Li, W.; Lin, C.H.; Yang, J.; Shang, C.; Nuernberg, S.T.; Jin, K.K.; Xu, W.; Lin, C.Y.; Lin, C.J.; et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature 2014, 514, 102–106.
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-β, WNT, and YAP/TAZ Converge. Front. Med. 2015, 2, 59.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
665
Revisions:
2 times
(View History)
Update Date:
16 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




