Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hai-Xue Pan | -- | 1675 | 2023-01-14 04:34:00 | | | |
| 2 | Rita Xu | + 1 word(s) | 1676 | 2023-01-16 03:02:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, X.; Pan, H.; Tang, G. Antibiotic Self-Resistance in Natural Product Biosynthesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/40171 (accessed on 08 February 2026).
Chen X, Pan H, Tang G. Antibiotic Self-Resistance in Natural Product Biosynthesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/40171. Accessed February 08, 2026.
Chen, Xiaorong, Hai-Xue Pan, Gong-Li Tang. "Antibiotic Self-Resistance in Natural Product Biosynthesis" Encyclopedia, https://encyclopedia.pub/entry/40171 (accessed February 08, 2026).
Chen, X., Pan, H., & Tang, G. (2023, January 14). Antibiotic Self-Resistance in Natural Product Biosynthesis. In Encyclopedia. https://encyclopedia.pub/entry/40171
Chen, Xiaorong, et al. "Antibiotic Self-Resistance in Natural Product Biosynthesis." Encyclopedia. Web. 14 January, 2023.
Copy Citation
Self-resistance determinants are essential for the biosynthesis of bioactive natural products and are closely related to drug resistance in clinical settings. The study of self-resistance mechanisms has long moved forward on the discovery of new resistance genes and the characterization of enzymatic reactions catalyzed by these proteins.
self-resistance
natural products
biosynthetic gene clusters
1. Introduction
Natural products are small chemical compounds derived from secondary metabolites of animals, plants, and microorganisms with an array of biological activities. Since the discovery and clinical use of penicillin and streptomycin, natural products have been an important source of drugs and play a critical role in modern medicine and agricultural industries. However, widespread use and misuse of these small chemical compounds has led to the rising emergence of antibiotic resistance, resulting in the gradual loss of efficacy of antibiotics in clinic and natural environment. Antibiotic resistance has become one of the greatest public health threats that humans will have to face in the coming decades. According to a report from the UK government, the death toll caused by antibiotic resistance is estimated to be up to 10 million per year by 2050, with a cost of drug resistance to $100 trillion. Therefore, there is an urgent need for uncovering the resistance mechanisms in pathogens and developing new compounds with novel modes of action.Generally, the clinical antibiotic resistance in human pathogens belongs to acquired resistance, with only a small fraction under the category of innate resistance. The origins of these acquired resistance genes have been traced to antibiotic-producers in natural environments. In antibiotic-producing microbes, self-resistance is a prerequisite for the synthesis of antibiotics. Antibiotic biosynthetic gene clusters (BGCs) contain one or more resistance genes to achieve self-protection, and these genes are considered to be the reservoirs of resistance genes, which may transfer to human pathogens by conjugation, transformation or transduction. Consequently, the elucidation of self-resistance mechanisms from antibiotic-producing microbes will not only reveal the action model of antibiotics and guide the discovery of new natural products, but also provide key clues for the studies of clinical antibiotic resistance.
To avoid suicide, antibiotic-producers have developed several mechanisms, including efflux pumps, chemical modification, prodrugs, compound sequestration, (sub)cellular location, target modification, and damage repair to shield the toxicity of antibiotics, thereby achieving self-protection. Different strategies are adapted depending on the structure of antibiotics, molecular target and producer species. For instance, within the enediyne producers, apoproteins are known to afford self-protection to the producers by binding the nine-membered enediyne chromophore, whereas the strategies of chemical modification guided by self-sacrifice proteins and sequestration mediated by drug binding proteins are utilized for the detoxification of ten-membered enediynes. To prevent self-harm from bleomycin, its producer Steptomyces verticillus employs bleomycin N-acetylation and sequestration mechanisms to protect itself. The antitumor agent mitomycin C producer, Steptomyces lavendulae, has developed several mechanisms, including prodrug, efflux pump, drug sequestration, and reoxidation of the active reduced mitomycin C to ensure self-resistance.
2. Resistance Widespread in Nature
Self-resistance determinants are not confined to the antibiotic producers. Instead, some of them are widely prevalent in the clinical pathogens and environmental bacteria [1]. Recent studies of self-resistance mechanisms against enediyne antitumor antibiotics revealed that an unprecedented sequestration mechanism for the anthraquinone-fused enediynes has been evolved in their producers and the homologs of these resistance elements are widely distributed in nature [2]. Within the gene cluster of tiancimycin (Figure 1), resistance genes tnmS1, tnmS2, and tnmS3 play a role in the sequestration of tiancimycin. The homologs of TnmS1, TnmS2, and TnmS3 are widespread in anthraquinone-fused enediynes producers and other bacteria, from different body sites, including the human microbiome [2]. The expression of homologous genes from the gene clusters encoding enediyne biosynthesis has been reported to endow E. coli BL21(DE3) with cross-resistance to anthraquinone-fused enediynes, while the homologs from human microbiome confer specific resistance to tiancimycin A [2]. These results further highlight that the resistance elements responsible for anthraquinone-fused enediynes sequestration are widely distributed in nature, although little is known about how these resistance genes disseminate in the environment. Similarly, homologous resistance genes encoding following enzymes are widespread in nature and perform conserved biological functions, such as GyrI-like cyclopropane hydrolases that mediate cyclopropyl moiety opening of DNA-alkylating agents YTM/CC-1065 [3], AlbA-like drug-binding proteins that guide resistance to albicidin [4][5], and NapW-like short-chain dehydrogenase/reductase that catalyze hemiaminal pharmacophore inactivation for tetrahydroisoquinoline antibiotics (Figure 1) [6]. In addition to antibiotic resistance, non-antibiotic drug resistance is also widespread. As direct evidence for this conception, Acbk-like kinase, inactivating a clinically used non-antibiotic antidiabetic drug acarbose by phosphorylation, is widely distributed in the human gut and oral microbiome (Figure 1) [7]. The specific kinase AcbK derived from Actinoplanes sp. SE50/110, is located within the gene cluster for acarbose synthesis. It phosphorylates acarbose at the O6A hydroxyl and serves as the self-resistance mechanism for acarbose production [8]. Recently, Donia et al. performed a metagenomics-based investigation of the human microbiome and found that homologues of AcbK are widespread in the bacteria from the human gut and oral microbiome and provide acarbose resistance, indicating the phosphorylation strategy of acarbose has disseminated in the human microbiome as a resistance mechanism [7]. Therefore, research on these widely distributed resistance elements will contribute to predicting and combating clinical drug resistance.
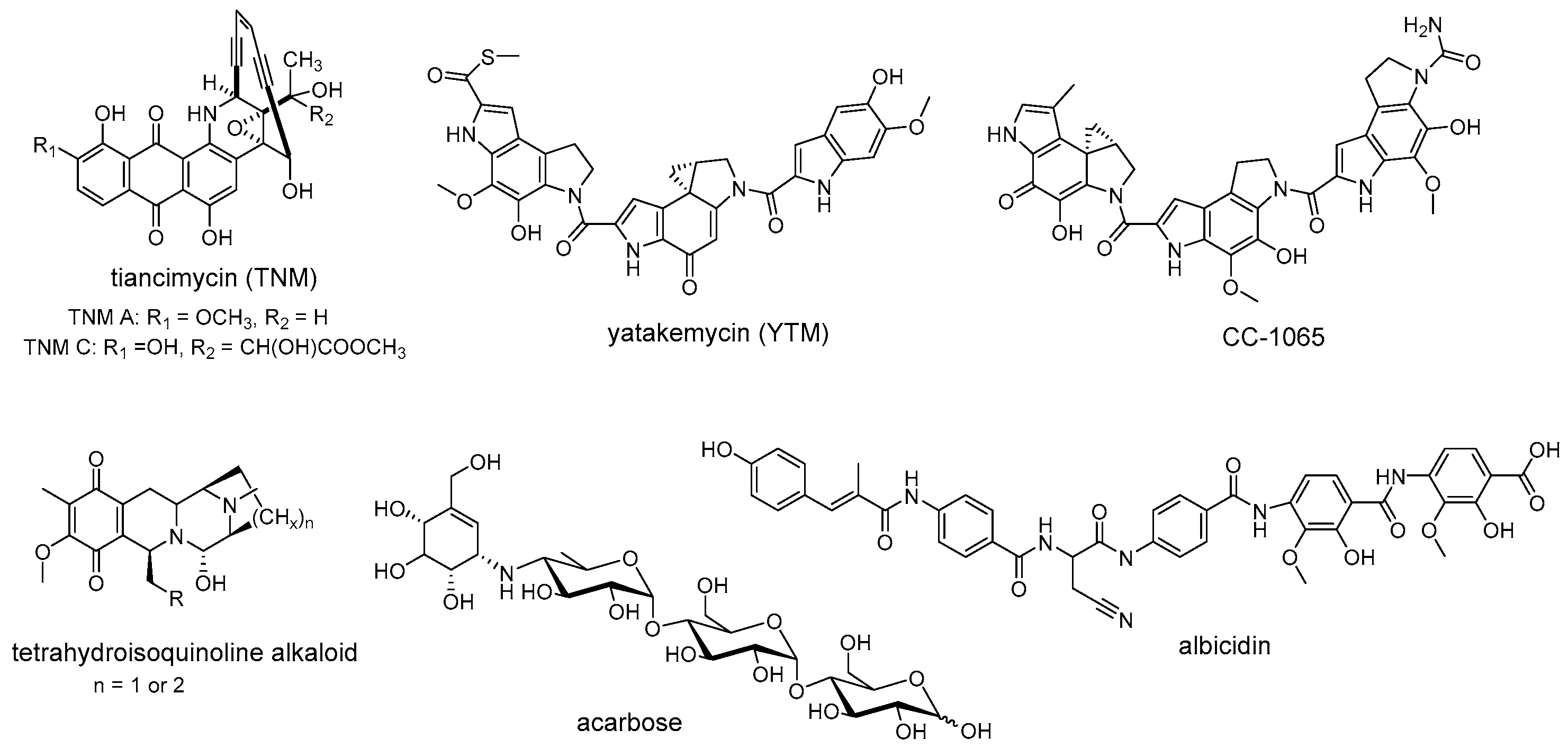
Figure 1. Representative natural products that resistance determinants are widespread in nature.
3. Resistance-Guided Natural Products Discovery
The rapid development of bioinformatics tools and genome sequencing technologies has brought a revolution in the discovery of natural products, leading to a transformation from traditional bioactivity-guided fractionation to modern genome-based target mining [9][10]. The enormous amount of genome data that is now available has revealed that microorganisms harbor more natural product BGCs than those observed under laboratory cultivation conditions, and most of them gene clusters encoding unknown products [11][12]. However, how to deal with the increasing BGCs and how to mine the desired products from the huge resources have become a major focus. Recently, researchers found that the self-resistance genes co-localized with BGCs can be used as a potential tool to link BGCs with molecular targets for mining natural products with desired activity (Figure 2) [13][14][15]. For example, Tang and coworkers successfully discovered a natural herbicide with a new mode of action from Aspergillus terreus by a putative self-resistance gene, astD, encoding a dihydroxyacid dehydratase (DHAD) homolog [16]. The DHAD is an essential enzyme that catalyzes the last step of branched-chain amino acid biosynthesis, and is therefore effectively targeted for herbicide development [16]. However, no compounds that target this enzyme have been reported in planta. Fungal genomes scanning of a DHAD homologue revealed that a BGC encoding a sesquiterpene cyclase homologue and a DHAD homologue was present in the genome of Aspergillus terreus. Subsequent experiments demonstrated that the aspterric acid encoded by this BGC is indeed a competitive inhibitor of DHAD and effectively functions as a herbicide, and the DHAD variant AstD functions as a self-resistance enzyme in the BGC for aspterric acid [16]. Similarly, the Müller group discovered a novel group of topoisomerase inhibitors, including pyxidicycline A and B, by putative self-resistance genes encoding topoisomerase-targeting pentapeptide repeat protein [17]. Wright et al. identified the caseinolytic protease (ClpP) inhibitor clipibicyclene from Streptomyces cattleya using ClpP as putative antibiotic resistance gene [18]. Ge et al. discovered a novel tetracycline, hainancycline, by using the common tetracycline antibiotics resistance enzyme TetR/MarR-transporter as probe [19].
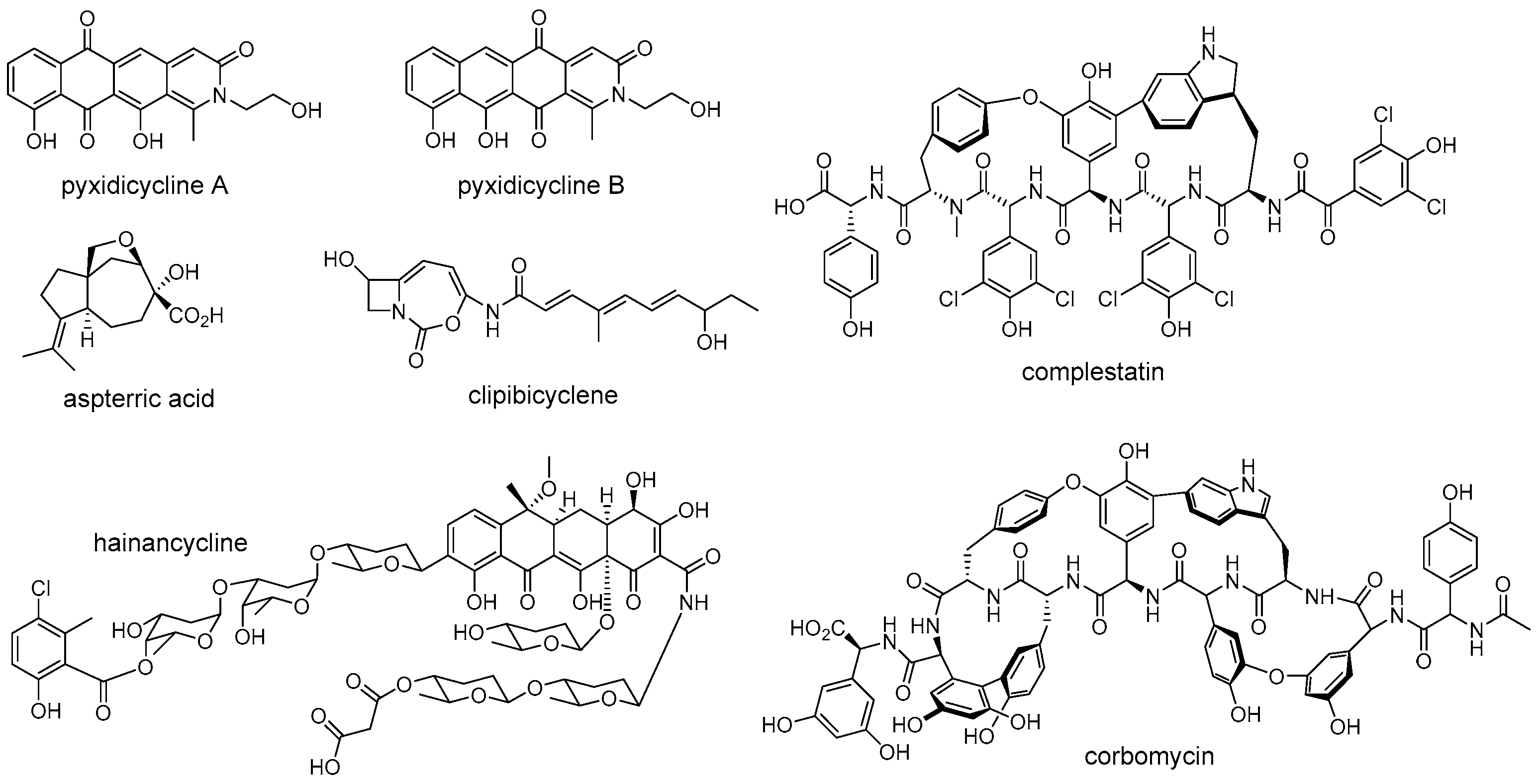
Figure 2. Structures of representative natural products that discovered by using antibiotic resistance gene as a probe.
In addition to discovering natural products with desired activity, self-resistance genes can also be used in determining the biomolecular target of known antibiotics (Figure 3). For instance, the β-lactone obafluorin isolated from Pseudomonas fluorescens ATCC 39502 shows potent antibacterial activity against both Gram-positive and Gram-negative pathogens [20]. The mechanism of action of obafluorin, however, was unknown as this molecule was reported to cause an unusual cell-elongation phenotype compared to other β-lactone antibiotics. During comparative genomic analysis of obafluorin BGCs, an open reading frame, obaO, was identified and speculated to be an immunity gene [20]. ObaO was shown to be a homologue of threonyl-tRNA synthetase and conferred resistance to obafluorin-sensitive strains and obafluorin producer when expressed. Subsequently, in vitro enzyme assays demonstrated that the obafluorin did indeed fully inhibit E. coli threonyl-tRNA synthetase with an IC50 of 92 ± 21 nM, thus indicating the target of this compound [20]. In another example, harzianic acid is a N-methylated tetramic acid isolated from Trichoderma harzianum in 1994. Although it displays excellent antifungal activity, including against plant pathogens Sclerotinia sclerotiorum and Rhizoctonia solani, the molecular target of harzianic acid remains unknown [21]. Recently, Tang et al. discovered that the harzianic acid is an inhibitor of acetohydroxyacid synthase (AHAS, the first enzyme on branched-chain amino acid biosynthesis pathway), which was guided by a truncated AHAS homolog resided within the BGC that was demonstrated to be the self-resistance enzyme [22]. A similar biomolecular target discovery scenario is also observed in determining the mode of action of polyketide rumbrins, which further revealed their promising potential to be HIV inhibitors [23].
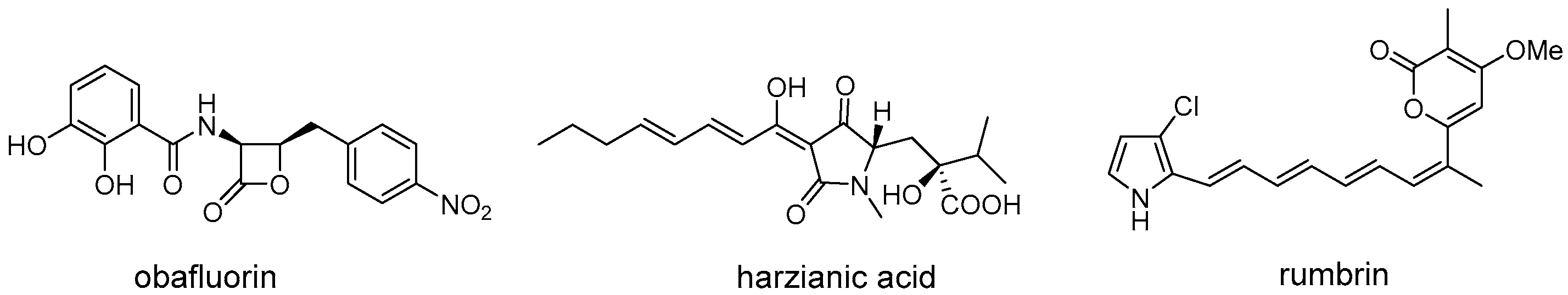
Figure 3. Structures of representative natural products that determine the biomolecular target through antibiotic resistance genes.
Although the above examples have successfully confirmed the potential of self-resistance genes in directed genome mining for natural products with known or predicted biomolecular targets, the development of compounds with novel mechanisms of action is also an urgent need to solve the ongoing antibiotic crisis. Recently, Wright et al. reported that the method of combining the absence of known self-resistance genes with phylogenetic analysis of biosynthetic genes could be effective in finding natural products with new modes of action [24]. They applied this approach to the glycopeptide family of antibiotics and successfully discovered a novel functional class of glycopeptide antibiotics composed of complestatin and corbomycin (Figure 2), which have a new mechanism of action that inhibits peptidoglycan re-modelling. This research outcome again indicated that self-resistance determinants are useful for prioritizing BGCs than just function in the self-protection. Other examples of employing a self-resistance determinant in natural products discovery are reviewed elsewhere [13][25][26]. Taken together, self-resistance genes can be a bridge between the bioactivity-guided and genome-based methods for natural products discovery. Studying the complex self-resistance strategies from a temporal-spatial shielding perspective will allow researchers to further understanding the evolutionary relationship between natural product biosynthesis and resistance, thereby facilitating discovery of new drug candidates with high activity.
References
- Jiang, X.; Ellabaan, M.M.H.; Charusanti, P.; Munck, C.; Blin, K.; Tong, Y.; Weber, T.; Sommer, M.O.A.; Lee, S.Y. Dissemination of antibiotic resistance genes from antibiotic producers to pathogens. Nat. Commun. 2017, 8, 15784.
- Chang, C.Y.; Yan, X.; Crnovcic, I.; Annaval, T.; Chang, C.; Nocek, B.; Rudolf, J.D.; Yang, D.; Hindra; Babnigg, G.; et al. Resistance to enediyne antitumor antibiotics by sequestration. Cell Chem. Biol. 2018, 25, 1075–1085.
- Yuan, H.; Zhang, J.; Cai, Y.; Wu, S.; Yang, K.; Chan, H.C.S.; Huang, W.; Jin, W.-B.; Li, Y.; Yin, Y.; et al. GyrI-like proteins catalyze cyclopropanoid hydrolysis to confer cellular protection. Nat. Commun. 2017, 8, 1485.
- Sikandar, A.; Cirnski, K.; Testolin, G.; Volz, C.; Bronstrup, M.; Kalinina, O.V.; Muller, R.; Koehnke, J. Adaptation of a bacterial multidrug resistance system revealed by the structure and function of AlbA. J. Am. Chem. Soc. 2018, 140, 16641–16649.
- Rostock, L.; Driller, R.; Gratz, S.; Kerwat, D.; von Eckardstein, L.; Petras, D.; Kunert, M.; Alings, C.; Schmitt, F.J.; Friedrich, T.; et al. Molecular insights into antibiotic resistance—How a binding protein traps albicidin. Nat. Commun. 2018, 9, 3095.
- Wen, W.H.; Zhang, Y.; Zhang, Y.Y.; Yu, Q.; Jiang, C.C.; Tang, M.C.; Pu, J.Y.; Wu, L.; Zhao, Y.L.; Shi, T.; et al. Reductive inactivation of the hemiaminal pharmacophore for resistance against tetrahydroisoquinoline antibiotics. Nat. Commun. 2021, 12, 7085.
- Balaich, J.; Estrella, M.; Wu, G.; Jeffrey, P.D.; Biswas, A.; Zhao, L.; Korennykh, A.; Donia, M.S. The human microbiome encodes resistance to the antidiabetic drug acarbose. Nature 2021, 600, 110–115.
- Wehmeier, U.F. The biosynthesis and metabolism of acarbose in Actinoplanes sp. SE 50/110: A progress report. Biocatal. Biotransform. 2003, 21, 279–284.
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug. Discov. 2015, 14, 111–129.
- Van Lanen, S.G.; Shen, B. Microbial genomics for the improvement of natural product discovery. Curr. Opin. Microbiol. 2006, 9, 252–260.
- Scherlach, K.; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760.
- Walsh, C.T.; Fischbach, M.A. Natural products version 2.0: Connecting genes to molecules. J. Am. Chem. Soc. 2010, 132, 2469–2493.
- Yan, Y.; Liu, N.; Tang, Y. Recent developments in self-resistance gene directed natural product discovery. Nat. Prod. Rep. 2020, 37, 879–892.
- Thaker, M.N.; Wang, W.; Spanogiannopoulos, P.; Waglechner, N.; King, A.M.; Medina, R.; Wright, G.D. Identifying producers of antibacterial compounds by screening for antibiotic resistance. Nat. Biotechnol. 2013, 31, 922–927.
- Alanjary, M.; Kronmiller, B.; Adamek, M.; Blin, K.; Weber, T.; Huson, D.; Philmus, B.; Ziemert, N. The antibiotic resistant target seeker (ARTS), an exploration engine for antibiotic cluster prioritization and novel drug target discovery. Nucleic Acids Res. 2017, 45, W42–W48.
- Yan, Y.; Liu, Q.; Zang, X.; Yuan, S.; Bat-Erdene, U.; Nguyen, C.; Gan, J.; Zhou, J.; Jacobsen, S.E.; Tang, Y. Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature 2018, 559, 415–418.
- Panter, F.; Krug, D.; Baumann, S.; Muller, R. Self-resistance guided genome mining uncovers new topoisomerase inhibitors from myxobacteria. Chem. Sci. 2018, 9, 4898–4908.
- Culp, E.J.; Sychantha, D.; Hobson, C.; Pawlowski, A.C.; Prehna, G.; Wright, G.D. ClpP inhibitors are produced by a widespread family of bacterial gene clusters. Nat. Microbiol. 2022, 7, 451–462.
- Li, L.Y.; Hu, Y.L.; Sun, J.L.; Yu, L.B.; Shi, J.; Wang, Z.R.; Guo, Z.K.; Zhang, B.; Guo, W.; Tan, R.; et al. Resistance and phylogeny guided discovery reveals structural novelty of tetracycline antibiotics. Chem. Sci. 2022, 13, 12892–12898.
- Scott, T.A.; Batey, S.F.D.; Wiencek, P.; Chandra, G.; Alt, S.; Francklyn, C.S.; Wilkinson, B. Immunity-guided identification of threonyl-tRNA synthetase as the molecular target of obafluorin, a beta-lactone antibiotic. ACS Chem. Biol. 2019, 14, 2663–2671.
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035.
- Xie, L.; Zang, X.; Cheng, W.; Zhang, Z.; Zhou, J.; Chen, M.; Tang, Y. Harzianic acid from trichoderma afroharzianum is a natural product inhibitor of acetohydroxyacid synthase. J. Am. Chem. Soc. 2021, 143, 9575–9584.
- Zhong, B.; Wan, J.; Shang, C.; Wen, J.; Wang, Y.; Bai, J.; Cen, S.; Hu, Y. Biosynthesis of rumbrins and inspiration for discovery of HIV inhibitors. Acta Pharm. Sin. B. 2022, 12, 4193–4203.
- Culp, E.J.; Waglechner, N.; Wang, W.; Fiebig-Comyn, A.A.; Hsu, Y.P.; Koteva, K.; Sychantha, D.; Coombes, B.K.; Van Nieuwenhze, M.S.; Brun, Y.V.; et al. Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature 2020, 578, 582–587.
- Almabruk, K.H.; Dinh, L.K.; Philmus, B. Self-resistance of natural product producers: Past, present, and future focusing on self-resistant protein variants. ACS Chem. Biol. 2018, 13, 1426–1437.
- Hobson, C.; Chan, A.N.; Wright, G.D. The antibiotic resistome: A guide for the discovery of natural products as antimicrobial agents. Chem. Rev. 2021, 121, 3464–3494.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
790
Revisions:
2 times
(View History)
Update Date:
16 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




