Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Małgorzata Józkowiak | -- | 3732 | 2023-01-13 13:10:38 | | | |
| 2 | Dean Liu | Meta information modification | 3732 | 2023-01-16 01:58:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jozkowiak, M.; Piotrowska-Kempisty, H.; Kobylarek, D.; Gorska, N.; Mozdziak, P.; Kempisty, B.; Rachon, D.; Spaczynski, R.Z. Role of Granulosa and Theca Cells in PCOS. Encyclopedia. Available online: https://encyclopedia.pub/entry/40163 (accessed on 07 February 2026).
Jozkowiak M, Piotrowska-Kempisty H, Kobylarek D, Gorska N, Mozdziak P, Kempisty B, et al. Role of Granulosa and Theca Cells in PCOS. Encyclopedia. Available at: https://encyclopedia.pub/entry/40163. Accessed February 07, 2026.
Jozkowiak, Malgorzata, Hanna Piotrowska-Kempisty, Dominik Kobylarek, Natalia Gorska, Paul Mozdziak, Bartosz Kempisty, Dominik Rachon, Robert Z. Spaczynski. "Role of Granulosa and Theca Cells in PCOS" Encyclopedia, https://encyclopedia.pub/entry/40163 (accessed February 07, 2026).
Jozkowiak, M., Piotrowska-Kempisty, H., Kobylarek, D., Gorska, N., Mozdziak, P., Kempisty, B., Rachon, D., & Spaczynski, R.Z. (2023, January 13). Role of Granulosa and Theca Cells in PCOS. In Encyclopedia. https://encyclopedia.pub/entry/40163
Jozkowiak, Malgorzata, et al. "Role of Granulosa and Theca Cells in PCOS." Encyclopedia. Web. 13 January, 2023.
Copy Citation
Polycystic ovary syndrome (PCOS) is the most common heterogeneous endocrine disorder among women of reproductive age. The pathogenesis of PCOS remains elusive and there is evidence suggesting the potential contribution of genetic interactions or predispositions combined with environmental factors.
polycystic ovary syndrome
granulosa cells
theca cells
1. Granulosa and Theca Cells—Two Cell, Two Gonadotropin Theory
GCs are widely considered a critical somatic part of the ovary. GCs surround the oocyte, promote oocyte development, produce sex steroids and growth factors, and overall contribute to normal folliculogenesis and menstrual cycle [1]. GCs can be divided into two types, mural GCs and cumulus cells, which transform from each other at pre-antral to antral follicle transition. Mural GCs consist of the external layer of lining the follicle, whereas cumulus cells adhere to the developing oocyte. Further, GCs aromatize androgens, produced by neighboring theca cells, during folliculogenesis [2]. Theca cells are endocrine cells that differentiate from the interfollicular stroma in response to factors secreted by the growing follicles. Any disturbance in the complex processes in GCs and theca cells may lead to endocrine disorders, such as PCOS, or even cause infertility.
Granulosa and theca cells are known to cooperate in the biosynthesis of ovarian hormones (Figure 1). This cooperation is described by the two-cell, two-gonadotropin theory, which claims that ovarian steroids are synthesized from cholesterol through complex interactions between the granulosa and theca cells [3].
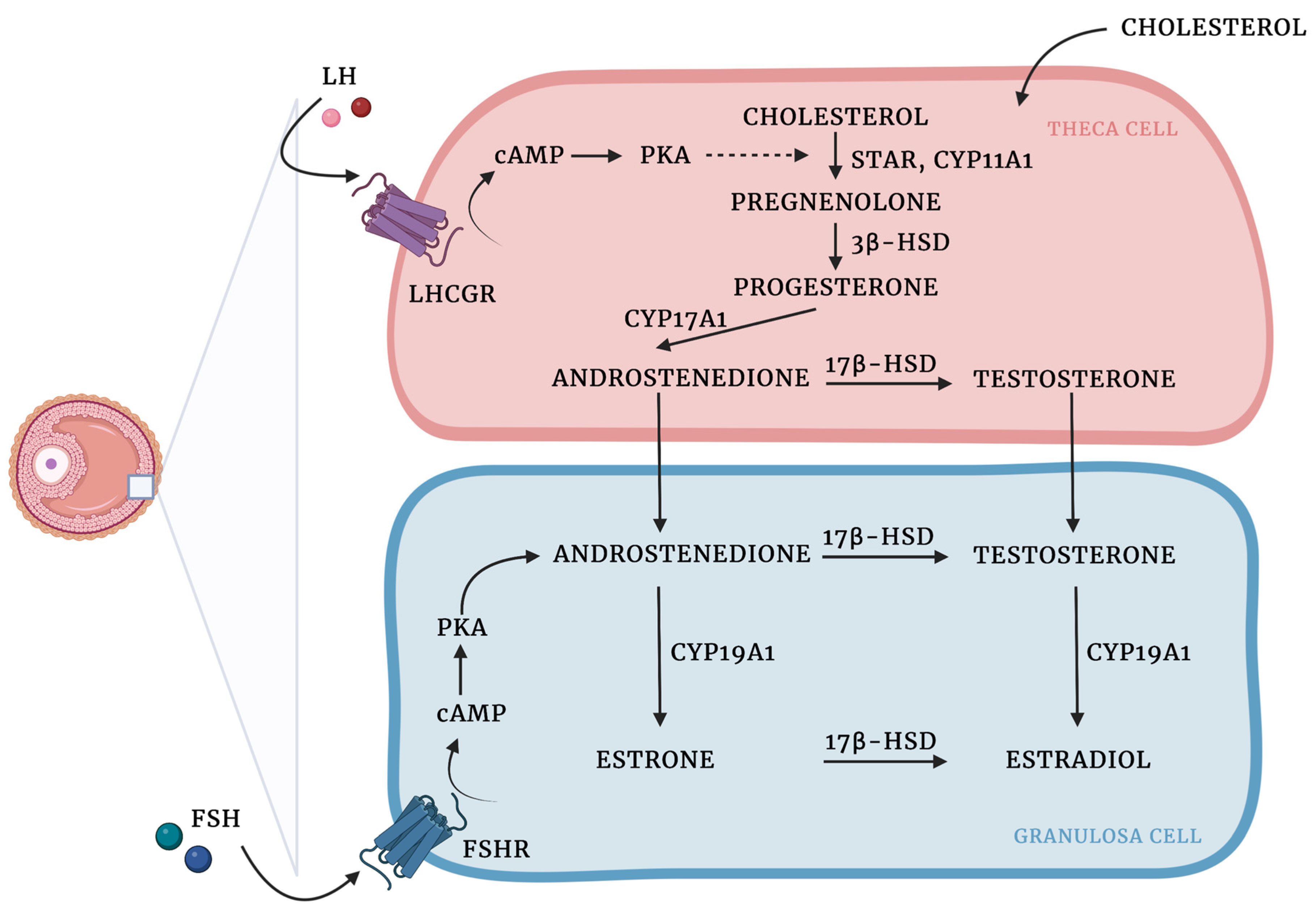
Figure 1. Ovarian steroidogenesis: two cell, two-gonadotropin theory. Ovarian steroids are synthesized from cholesterol, which diffuses from the circulation into theca cells and is mobilized into mitochondria by steroidogenic acute regulatory protein (STAR) activity [4]. LH binds to LHCGR on the cell surface, which results in the increased expression of steroidogenic enzymes involved in androgen production. Cholesterol is then converted into pregnenolone by the cholesterol sidechain cleavage enzyme (CYP11A1). In the smooth endoplasmic reticulum, pregnenolone is transformed into progesterone due to the activity of 3β-hydroxysteroid dehydrogenase (3β-HSD). Then, due to the activity of CYP17A1 progesterone is converted to androstenedione, which in turn might be transformed into testosterone by 17β-hydroxysteroid dehydrogenase (17β-HSD) or translocated into the GCs, where aromatase (CYP450arom; CYP19A1) converts androstenedione to estrone and testosterone to estradiol. 17β-HSD might also produce estradiol using estrone as a substrate [5][6][7][8]. Created with BioRender.com.
There is an ongoing discussion on how various EDCs can alter the complexity of the synthesis and metabolism of ovarian steroid hormones [9]. Thus, disruption of the endocrine system occurs when the hormones do not bind to the receptors, and the way hormones elicit their function is changed.
2. The Role of AMH-Mediated SMAD Signaling Pathway in PCOS
Anti-Müllerian hormone (AMH), a glycoprotein hormone from the TGF-β superfamily, is produced by GCs with the highest expression in the preantral and small antral follicles, and has an important role in folliculogenesis. During the ovary cycle in physiological ovaries, AMH continues to be expressed in growing follicles, playing a crucial role in the arrest of antral follicle development, reducing follicle sensitivity to FSH, and inhibiting recruitment of follicles from the resting pool. When the follicles reach the size at which they are dominant, the production of AMH is timely reduced. AMH is known to be used as a molecular biomarker for the determination of ovarian reserve, but also ovarian dysfunction, such as PCOS [10].
Elevated levels of AMH blood concentration in women with PCOS were recently confirmed by several studies [11][12][13]. Anomalies in follicle growth, resulting in an increased number of small antral follicles, contribute to anovulatory infertility in PCOS women. It has been revealed that serum AMH levels are two to five times higher in PCOS women, and relatively elevated in women presenting anovulatory cycles compared to the ovulatory PCOS phenotype [14][15][16]. Therefore, there is increasing evidence that this derangement in ovarian physiology is associated with unsatisfactory pregnancy outcomes.
Multiple molecular mechanisms have been proposed to explain the impact of AMH on human ovarian GCs. AMH has been shown to reduce follicle responsiveness to FSH due to the downregulation of FSH receptor expression in vitro in human GCs [17] and the expression of aromatase [18]. Interestingly, gonadotropins are also involved in the regulation of AMH expression; FSH has been indicated as a suppressor, and LH has been shown to stimulate AMH expression in the GCs of PCOS women [19][20]. Furthermore, Pierre et al. have revealed that the mRNA expression of AMH receptor II (AMHRII) is downregulated by LH in GCs from women with regular ovaries, but not those suffering from PCOS [21].
In GCs derived from polycystic ovaries, hyperandrogenism inhibits AMH down-expression through elevated 5α-dihydrotestosterone (5α-DHT) levels, or indirectly through the conversion of testosterone to estradiol and increased expression of ERα [22]. The studies of Dilaver et al. have pointed out for the first time differences in the AMH/AMHRII signaling, associated with the intracellular SMAD signaling pathway, in regular and polycystic ovaries. Prolonged exposure of GCs derived from polycystic ovaries to high levels of AMH has been revealed to affect the expression patterns of aromatase and FSHR and disrupt SMAD signaling by increasing the level of I-SMAD-6, -7, and diminishing activation of SMAD-1/5/8 and co-SMAD-4 [22].
AMH-mediated SMAD signaling is a complex downstream of events, beginning with AMH binding to the AMHRII transmembrane serine/threonine kinase receptor and activating the Type 1 receptor, which contributes to the phosphorylation of SMAD-1/5/8 proteins. Then, a tetrameric complex of two AMHRII and two Type I receptors (probably ALK 2,3 or 6) is formed, and SMADs-1/5/8 are joined to the common SMAD-4 (co-SMAD-4). The mentioned complexes are translocated to the nucleus, where they alter various genes’ expression due to transcriptional factors, coactivators, and corepressors [23]. In PCOS, the cascade contributing to SMAD signaling is disrupted by high AMH concentration, leading to increased protein levels of the inhibitory SMADs (I-SMAD), associated with negative regulation of intracellular SMAD signaling (Figure 2). SMAD-6 has been revealed to inhibit activation of bone morphogenetic protein (BMP) pathways, altering pSMAD-1/5/8 binding to co-SMAD-4 in the mechanism of competitive inhibition. Furthermore, SMAD-7 is known to inhibit BMP signaling by binding to the type I receptor [24]. Moreover, follicle growth may also be disrupted by reduced expression of AMHRII [22].
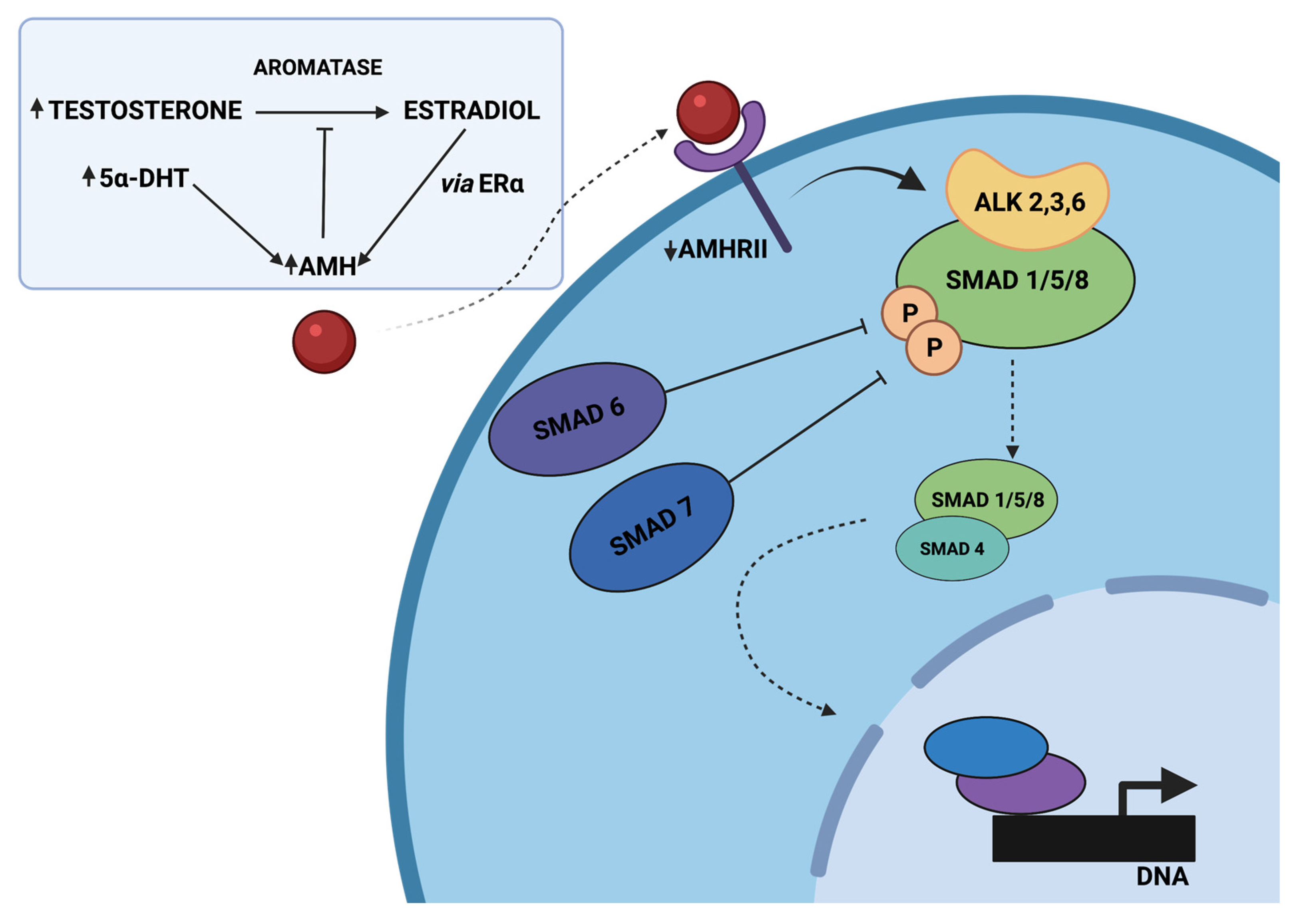
Figure 2. The proposed model of disrupted AMH signaling in women with PCOS, adapted from Dilaver et al. [22]. Hyperandrogenism inhibits the decrease in AMH levels directly by elevated 5α-dihydrotestosterone (5α-DHT) levels or indirectly through the conversion of testosterone to estradiol and the increased expression of ERα. Elevated AMH levels might diminish the expression of aromatase and increase the protein levels of the inhibitory SMADs (SMAD-6, SMAD-7), associated with negative regulation of intracellular SMAD signaling. It might disrupt pSMAD-1/5/8 binding to SMAD-4 and, as a consequence, alter the expression of various genes. Created with BioRender.com.
3. The Role of the PI3K/AKT/FOXO Signaling Pathway in PCOS
Subsequent studies have confirmed that insulin resistance and impaired glucose metabolism in PCOS are related to the promotion of ovarian GCs apoptosis and follicular development dysfunctions [25][26]. The mechanism of this pro-apoptotic activity is not yet fully understood; however, the role of SH2B adaptor protein 3 (LNK), an important regulator of the insulin signaling pathway, has been suggested.
LNK is a member of the Src homology 2B (SH2B) family of intracellular adaptor proteins and is known to play an important role in the insulin signaling pathway in the ovary, glucose homeostasis, and reproduction [27]. Furthermore, several studies have also indicated the participation of LNK in the pathogenesis of type 1 diabetes, hypertension, and cardiovascular disease, but also in malignant tumors [28][29][30][31]. In patients with insulin resistance, LNK levels have been revealed to be significantly increased as compared to the control group [32]. The authors suggested that LNK negatively regulates the insulin-activated PI3K/AKT/FOXO3 signaling pathway in GCs and, consequently, promotes GCs derangements and apoptosis, leading to ovulation disorders in PCOS [33]. Phosphatidylinositol 3-kinase (PI3K) signaling is one of the main pathways involved in the regulation of cell proliferation, survival, migration, and metabolism in physiological and pathological processes. Subsequent studies in humans and mice have confirmed that PI3K/AKT signaling and the downstream pro-apoptotic genes (e.g., FOXO1, Bax, caspase-9, caspase-3) participate in the regulation of GC growth and apoptosis during follicular development [34][35]. FOXO transcription factors are members of the Fork-head family of proteins and the main direct substrates of the protein kinase AKT following insulin or growth factors stimulation [36]. Among the FOXO subgroup, four members (FOXO1, FOXO3, FOXO4, FOXO6) have been identified in humans [36]. The FOXO family is known to be a key downstream target of PI3K/AKT.
Normally, insulin binds to the receptor, leading to activation of the PI3K/AKT/FOXO3 signaling pathway, promotes FOXO3 export from the nucleus to the cytoplasm, contributes to inhibition of the expression of pro-apoptotic genes, increasing cell survival, growth, and proliferation [33] [37]. Increased LNK levels alter insulin-mediated phosphorylation of AKT and FOXO3, promoting nuclear localization of FOXO3, and consequently leading to enhanced apoptosis in GCs [33]. In vitro studies have also revealed that LNK knockout moderately restores the estrous cycle and improves glucose metabolism in the PCOS mouse model, compared to wild-type PCOS mice [33].
To date, several studies have confirmed derangements in the PI3K/AKT signaling pathway in PCOS patients and animal models of PCOS [38][39]. Gong et al. have suggested that derangements in PI3K/AKT signaling alter the balance between pro- and anti-apoptotic events in GCs. The increased expression of pro-apoptotic FOXO1, Bax, caspase-9, caspase-3, and decreased levels of PI3K, AKT, and Bcl-2 have been observed [40]. Moreover, the intracellular ROS level in PCOS GCs was three times higher compared to the control. Interestingly, the study has revealed that growth hormone (GH) significantly decreased ROS production by more than 50%, and decreased the apoptotic rate in PCOS GCs, probably through the activation of PI3K/AKT signaling [40]. In contrast to these findings, several studies have shown enhanced activity of the PI3K/AKT signaling pathway in some PCOS patients [41][42], which might be associated with ethnic differences. Therefore, considering the conflicting results, further research is needed.
4. The Role of the HMGA2/ IGF2BP2 Signaling Pathway in PCOS
The HMGA2/IGF2BP2 signaling pathway has been indicated to play a critical role in cell proliferation and differentiation [43][44]. HMGA2 belongs to a family of HMGA genes that consist of three DNA-binding domains and an acidic C-terminal tail [45]. An increase in HMGA2 expression has been observed not only during embryonic development but also in various cancers, suggesting its role in controlling cell proliferation [46]. Insulin-like Growth Factor 2 mRNA Binding Protein (IGF2BP2) plays a vital role in metabolism, and the variants in this gene have been associated with susceptibility to T2DM [47].
Recent studies have revealed that mRNA levels of HMGA2, a proposed GWAS susceptibility locus, and IGF2BP2 expression were significantly increased in GCs derived from women with PCOS compared with controls [48]. In KGN and SVOG cell lines, the HMGA2/IGF2BP2 signaling pathway has been shown to regulate the expression of the CCND2 and SERBP1 genes, which are involved in promoting cell proliferation. Interestingly, the mRNA, as well as protein levels of CCND2 and SERBP1 were also elevated in the GCs of PCOS women, leading to enhanced proliferation and decreased apoptosis. Taken together, the studies suggest that overexpression of HMGA2 and increased activity of the HMGA2/IGF2BP2 signaling pathway in ovarian GCs promote cell proliferation and, consequently, the PCOM [48].
5. The Role of Theca Cells in PCOS Development
Studies conducted in the past decade have built a convincing argument that ovarian theca cells are the main source of excess androgen secretion in women suffering from PCOS [49][50][51]. Therefore, it has been revealed that thecal tissue or theca cell cultures derived from women with PCOS secrete significantly higher amounts of androgens compared to cultures derived from healthy women [50][52][53].
In vitro studies have revealed that derangements in theca cell functions are associated with androgen excess and abnormal steroid secretion in response to gonadotropin stimulation. It has been shown that progesterone, 17-hydroxyprogesterone, and testosterone secretion were significantly increased in theca cell cultures derived from PCOS patients [53][54]. Furthermore, studies have revealed a remarkably enhanced metabolism of precursors (basal and cyclic AMP-stimulated pregnenolone, progesterone, and dehydroepiandrosterone) into testosterone, associated with increased androgenic 17β-HSD activity. Moreover, increased mRNA expression of CYP11A, CYP17A1, P450c17, 3β-HSD, and 17β-HSD enzyme activities were noted in PCOS theca cells compared to normal cells [54]. CYP17A1 and CYP11A1 genes encode the pivotal enzymes associated with androgen biosynthesis in theca cells: steroid-17-α-hydroxylase/17,20 lyase and cholesterol side-chain cleavage enzyme, respectively [53][55][56][57]. Thus, increased expression of the mentioned enzymes in women with PCOS enhances androgen biosynthesis by theca cells. Recently, increased activity of P450c17 and 3β-HSD has also been revealed to play a crucial role in the increased synthesis of testosterone precursors, and consequently increased androgen secretion in PCOS by theca cells [55].
DENND1A is a member of the family of 18 human genes called “connecdenns” and encodes a protein that has been identified as a guanine nucleotide exchange factor converting inactive GDP-bound Rab35 into its active GTP-bound form. Genetic alterations within the DENND1A gene have been noted in PCOS. Furthermore, the DENND1A locus at 9q22.32 has been identified in both Asian and European populations [58][59][60][61]. Thus, DENND1A might be considered a strong PCOS susceptibility gene [62]. McAlisster et al. have revealed that DENND1A.V2, a splice variant derived from the DENND1A gene, plays a pivotal role in theca cell steroidogenesis. Overexpression of DENND1A.V2 results in the expression of the CYP17A1 and CYP11A1 genes and, consequently, increased androgen secretion. Moreover, recent studies have indicated that knock-down of the DENND1A.V2 gene in PCOS theca cells diminished androgen secretion due to decreased CYP17A1 and CYP11A1 genes transcription, restoring the normal phenotype of theca cells, which confirmed the role of DENND1A in hyperandrogenism associated with PCOS [54].
However, the mechanism of DENND1A.V2 steroidogenic activity is not fully understood. Since DENND1A is one of the proteins involved in protein trafficking, clathrin-mediated endocytosis, and receptor recycling, it might be suggested that DENND1A alters LH action due to LH receptor signaling upregulation [63][64].
Moreover, according to the genotype-phenotype assessment performed by Tian et al., PCOS susceptibility variants in the THADA and INSR genes are associated with a higher risk of metabolic syndrome in women suffering from PCOS, while variants in DENND1A and TOX3 increase the risk of insulin resistance [65].
6. The Role of Circadian Rhythm in PCOS Development
In recent years, several studies have confirmed that light exposure and sleep disturbance are associated with acute circadian misalignment, which consequently contributes to the development of metabolic diseases and fertility impairment [66][67]. Interestingly, it has been suggested that circadian rhythm, which orchestrates the physiological functions of the body, could be one of the contributing factors to androgen excess in patients with PCOS [67]. Therefore, Wang et al. have observed a significant association between long-term night shift work and PCOS [67].
Recently, Johnson et al. have suggested that circadian rhythm is one of the factors contributing to androgen excess in PCOS due to its role in altering peripheral androgen metabolism [68]. In fact, the study demonstrated increased mRNA levels of steroidogenic enzymes: STAR, CYP17A1, and aldo-keto reductase family 1 member C3 (AKR1C3). The AKR1C3 is known to encode 17β-hydroxysteroid dehydrogenase type 5 that converts androstenedione to testosterone. Furthermore, different expression patterns of steroid 5-alpha-reductase 1 and 2 (SRD5A1 and SRD5A2) were observed in patients with PCOS [68]. The androgen receptor (AR) transcript level was also elevated in the peripheral blood mononuclear cells (PBMCs) of women with PCOS. In contrast, the authors found a decrease in CYP19A1, a key factor responsible for estrogen synthesis, in women with PCOS compared to healthy women [68].
Interestingly, the expression of the steroidogenesis genes was shown to vary between PCOS phenotypes. The most significant differences in transcript levels were observed in phenotype A (hyperandrogenism, ovulatory dysfunction, polycystic ovaries), while in phenotype D (ovulatory dysfunction, polycystic ovaries), the changes were less pronounced. It might be a result of heterogeneity as well as a different presentation of the clinical and biochemical characteristics of PCOS cases [68].
Circadian rhythm is known to be modulated through several transcriptional and post-translational autoregulatory feedback loops. The study has shown downregulation of transcript levels of circadian locomotor output cycles kaput (CLOCK), brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1), and neuronal PAS domain protein 2 (NPAS2) in PBMCs, as well as significantly decreased CLOCK protein expression in women with PCOS [68]. The mRNA expression profiles of the circadian genes BMAL1 and PER1 were also altered after darkness treatment in rats [69].
Heterodimers of CLOCK, BMAL1, and NPAS2 act as transcriptional factors that activate the promoter sequences of the repressor genes-cryptochrome circadian regulators (CRY1 and CRY2) and period circadian regulators (PER1, PER2, and PER3). Once the PER/CRY heterodimer reaches a critical level, the proteins are translocated to the nucleus where CRYs repress CLOCK-BMAL1-induced transcription. CRYs and PER are therefore negative regulators, while CLOCK-BMAL1 is the positive arm of the feedback loop [68]. In the GCs of PCOS patients, it has been shown that there is decreased expression of BMAL1, which contributes to aromatase expression, and consequently there is reduced estrogen synthesis [70] . The study of Johnson et al. has revealed increased expression of mRNA levels of negative regulators of circadian pathway genes (PER1, PER2, CRY1, CRY2, as well as DEC1 and DEC2) in the PCOS group compared to controls [68].
Retinoic acid receptor-related orphan receptor α (RORα) and the nuclear orphan receptor α (REV-ERBα) are other key regulators of BMAL1, the secondary feedback loop in the circadian cycle [71]. On the one hand, the transcription of REV-ERBα is activated by the BMAL1/CLOCK heterodimer; on the other hand, it is repressed by CRY/PER which results in circadian oscillations of REV-ERBα. Moreover, REV-ERBα and REV-ERBβ are known to repress the transcription of BMAL1/CLOCK and BMAL1, respectively [72].
The study of Sun et al. has shown that the expression of REV-ERBα and REV-ERBβ is significantly downregulated in the GCs derived from PCOS patients compared to healthy women [73]. REV-ERBs have been revealed to play an important role in various metabolic, neuronal, and inflammatory processes, as well as in lipid homeostasis [73]. Genetic knock-out experiments have, in turn, explained the meaning of these proteins in the circadian cycle; the expression of BMAL1 and CLOCK in Rev-erbα-deficient mice was significantly increased when compared with wild-type mice [74], and Rorα– and Rorβ-deficient mice were found to display an abnormal circadian rhythm [71].
Until now, some studies have suggested that long-term environmental exposure to darkness might induce hyperandrogenism via melatonin receptor 1 and reduced expression of aromatase [69]. Melatonin receptors belong to transmembrane G-protein-coupled receptors, and two subtypes in humans and other mammals can be distinguished: melatonin receptor 1 (MT1; MTNR1A) and melatonin receptor 2 (MT2; MTNR1B) [75]. In vitro experiments on the KGN cell line have demonstrated that long-term darkness leads to estrous cycle disorder, PCOM, increased LH levels as well as the LH:FSH ratio, hyperandrogenism, and glucose intolerance [69]. Furthermore, decreased expression of MTNR1A in rat ovarian GCs was also noted in darkness-treated cells [69]. The decrease in MTNR1A inhibited the androgen receptor (AR) and the expression of CYP19A1 (aromatase). The authors suggested that altered expressions of MTNR1A and AR play a crucial role in the pathological development of hyperandrogenisms [69]. These findings were in accordance with changes in hGCs collected during the oocyte retrieval process from women with PCOS, who underwent in vitro fertilization and embryo transfer [69]. On the other hand, rescue treatment with a melatonin receptor agonist and restoration of the normal light/dark circadian rhythm has partially alleviated reproductive abnormalities, such as estrous cycle disturbance and PCOM, and endocrinal hormone balance in rats treated with long-term darkness [69].
Furthermore, recent studies have revealed the association between common genetic variations of the melatonin receptor, such as single nucleotide polymorphisms (SNPs) rs2119882 as well as rs10830963, and the prevalence of PCOS [76][77]. In addition, Wang et al. have described a significant association between the rs10830963 SNP and concentrations of testosterone in women with PCOS [78].
The master pacemaker of the circadian clock in hypothalamic suprachiasmatic nucleus (SCN), modulates the circadian cycle through a rhythmic secretion of regulatory hormones such as melatonin and corticotropin-releasing hormone (CRH)/adrenocorticotropic hormone (ACTH) [79][80]. In fact, the central circadian clock regulates pineal melatonin secretion. The levels of melatonin are modulated by photoperiod; the secretion is enhanced at night in response to darkness, while bright light directly inhibits its production [81].
Nevertheless, melatonin is also produced in other tissues and organs such as the skin, gastrointestinal tract, retina, bone marrow, and lymphocytes [82][83]. Interestingly, there is emerging evidence that melatonin synthetic enzymes such as arylalkylamine N-acetyl-transferase and hydroxyindole-O-methyltransferase are present in most tissues, including ovaries and follicular cells, oocytes, and cytotrophoblasts [82][84].
Until now, several studies have noted an altered melatonin rhythm in women with PCOS [85][86]. It has been revealed that levels of melatonin and its metabolites, such as 6-sulphatoxymelatonin (aMT6s), are significantly elevated in the serum and urine of PCOS patients, particularly at night [87][88][89]. aMT6s is one of the major metabolites of melatonin, which can serve as an accurate marker for melatonin production [90]. On the contrary, a reduction in melatonin levels was reported in follicular fluid from women with PCOS [91][92]. Due to its antioxidant properties, melatonin is known to protect the follicles against oxidative stress and atresia; thus, melatonin plays an important role during ovulation [88]. It has been revealed that deficiency of melatonin leads to disturbance of gonadotropin secretion and alteration of the LH:FSH ratio, the remarkable features in women with PCOS [93].
Another study has revealed that increased serum concentrations of melatonin in PCOS patients were associated with testosterone levels [88]. Furthermore, it has also been highlighted that the night-time urine levels of aMT6s and 8-hydroxy-2′-deoxyguanosine (8-OHdG) were significantly elevated in women with PCOS compared to those in the control group. In contrast, the day-time urine levels of aMT6s and 8-OHdG were comparable to healthy women [89]. 8-OHdG is a product of free radical-induced oxidative damage to 2′-deoxyguanosine. It has been widely used as a marker for assessing oxidative stress and carcinogenesis, since it can be detected in urine [89]. Higher levels of aMT6s at night are suggested to be a result of increased melatonin secretion in response to increased oxidative stress in women with PCOS [94]. Furthermore, melatonin levels have also been shown to be inversely correlated with the serum LH:FSH ratio in PCOS patients [88]. There is emerging evidence that supplementation with melatonin can improve the oocyte and embryo quality in PCOS women, and could be a good strategy in the management of hormonal aberrations as well as insulin resistance associated with PCOS.
References
- Kranc, W.; Brązert, M.; Celichowski, P.; Bryja, A.; Nawrocki, M.; Ożegowska, K.; Jankowski, M.; Jeseta, M.; Pawelczyk, L.; Bręborowicz, A.; et al.et al. ‘Heart Development and Morphogenesis’ Is a Novel Pathway for Human Ovarian Granulosa Cell Differentiation during Long-term in Vitro Cultivation-a Microarray Approach.. Mol. Med. Rep. 2019, 19, 1705-1715.
- Kossowska-Tomaszczuk, K.; de Geyter, C.; Cells with Stem Cell Characteristics in Somatic Compartments of the Ovary. Biomed. Res. Int. 2013, 2013, 310859.
- Richards, J.S.; Maturation of Ovarian Follicles: Actions and Interactions of Pituitary and Ovarian Hormones on Follicular Cell Differentiation. Physiol. Rev. 1980, 60, 51-89.
- Clark, B.J.; Stocco, D.M.. The Steroidogenic Acute Regulatory Protein (StAR). In Cholesterol Transporters of the START Domain Protein Family in Health and Disease: START Proteins-Structure and Function; Clark, B.J.; Stocco, D.M., Eds.; Springer: New York, USA, 2014; pp. 15-47.
- Hanukoglu, I.; Steroidogenic Enzymes: Structure, Function, and Role in Regulation of Steroid Hormone Biosynthesis.. J. Steroid. Biochem. Mol. Biol. 1992, 43, 779–804.
- Penning, T.M.; Molecular Endocrinology of Hydroxysteroid Dehydrogenases. Endocr. Rev. 1997, 18, 281–305.
- Hayes, C.L.; Spink, D.C.; Spink, B.C.; Cao, J.Q.; Walker, N.J.; Sutter, T.R.; 17 Beta-Estradiol Hydroxylation Catalyzed by Human Cytochrome P450 1B1. Proc. Natl. Acad. Sci. USA 1996, 93, 9776–9781.
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T.; Cytochrome P450-Mediated Metabolism of Estrogens and Its Regulation in Human. Cancer Lett. 2005, 227, 115–124.
- Guarnotta, V.; Amodei, R.; Frasca, F.; Aversa, A.; Giordano, C.; Impact of Chemical Endocrine Disruptors and Hormone Modulators on the Endocrine System. Int. J. Mol. Sci. 2022, 23, 5710.
- Moolhuijsen, L.M.E.; Visser, J.A.; Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020 , 105, 3361–3373.
- Parco, S.; Serum Anti-Müllerian Hormone as a Predictive Marker of Polycystic Ovarian Syndrome. Int. J. Gen. Med. 2011, 4, 759.
- Pellatt, L.; Rice, S.; Mason, H.D.; Anti-Müllerian Hormone and Polycystic Ovary Syndrome: A Mountain Too High? . Reproduction 2010, 139, 825–833.
- Catteau-Jonard, S.; Pigny, P.; Reyss, A.-C.; Decanter, C.; Poncelet, E.; Dewailly, D.; Changes in Serum Anti-Müllerian Hormone Level during Low-Dose Recombinant Follicular-Stimulating Hormone Therapy for Anovulation in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 4138–4143.
- Pigny, P.; Merlen, E.; Robert, Y.; Cortet-Rudelli, C.; Decanter, C.; Jonard, S.; Dewailly, D.; Elevated Serum Level of Anti-Mullerian Hormone in Patients with Polycystic Ovary Syndrome: Relationship to the Ovarian Follicle Excess and to the Follicular Arrest. J. Clin. Endocrinol. Metab. 2003, 88, 5957–5962.
- Laven, J.S.E.; Mulders, A.G.M.G.J.; Visser, J.A.; Themmen, A.P.; de Jong, F.H.; Fauser, B.C.J.M.; Anti-Müllerian Hormone Serum Concentrations in Normoovulatory and Anovulatory Women of Reproductive Age. J. Clin. Endocrinol. Metab. 2004, 89, 318–323.
- Park, A.S.; Lawson, M.A.; Chuan, S.S.; Oberfield, S.E.; Hoeger, K.M.; Witchel, S.F.; Chang, R.J.; Serum Anti-Müllerian Hormone Concentrations Are Elevated in Oligomenorrheic Girls without Evidence of Hyperandrogenism. J. Clin. Endocrinol. Metab. 2010, 95, 1786–1792.
- Pellatt, L.; Rice, S.; Dilaver, N.; Heshri, A.; Galea, R.; Brincat, M.; Brown, K.; Simpson, E.R.; Mason, H.D.; Anti-Müllerian Hormone Reduces Follicle Sensitivity to Follicle-Stimulating Hormone in Human Granulosa Cells. Fertil. Steril. 2011, 96, 1246–1251.e1.
- Sacchi, S.; D’Ippolito, G.; Sena, P.; Marsella, T.; Tagliasacchi, D.; Maggi, E.; Argento, C.; Tirelli, A.; Giulini, S.; la Marca, A.; et al. The Anti-Müllerian Hormone (AMH) Acts as a Gatekeeper of Ovarian Steroidogenesis Inhibiting the Granulosa Cell Response to Both FSH and LH. J. Assist. Reprod. Genet. 2016, 33, 95–100.
- Roy, S.; Gandra, D.; Seger, C.; Biswas, A.; Kushnir, V.A.; Gleicher, N.; Kumar, T.R.; Sen, A; Oocyte-Derived Factors (GDF9 and BMP15) and FSH Regulate AMH Expression Via Modulation of H3K27AC in Granulosa Cells. Endocrinology 2018, 159, 3433–3445.
- Pellatt, L.; Hanna, L.; Brincat, M.; Galea, R.; Brain, H.; Whitehead, S.; Mason, H.; Granulosa Cell Production of Anti-Müllerian Hormone Is Increased in Polycystic Ovaries. J. Clin. Endocrinol. Metab. 2007, 92, 240–245.
- Pierre, A.; Peigne, M.; Grynberg, M.; Arouche, N.; Taieb, J.; Hesters, L.; Gonzales, J.; Picard, J.-Y.; Dewailly, D.; Fanchin, R.; et al.et al. Loss of LH-Induced down-Regulation of Anti-Mullerian Hormone Receptor Expression May Contribute to Anovulation in Women with Polycystic Ovary Syndrome. Hum. Reprod. 2013, 28, 762–769.
- Dilaver, N.; Pellatt, L.; Jameson, E.; Ogunjimi, M.; Bano, G.; Homburg, R.; D Mason, H.; Rice, S.; The Regulation and Signalling of Anti-Müllerian Hormone in Human Granulosa Cells: Relevance to Polycystic Ovary Syndrome. Hum. Reprod. 2019, 34, 2467–2479.
- Josso, N.; di Clemente, N.; Gouédard, L.; Anti-Müllerian Hormone and Its Receptors. Mol. Cell. Endocrinol. 2001, 179, 25-32.
- Attisano, L.; Wrana, J.L.; Signal Transduction by the TGF-β Superfamily. Science 2002, 296, 1646–1647.
- Ni, X.-R.; Sun, Z.-J.; Hu, G.-H.; Wang, R.-H.; High Concentration of Insulin Promotes Apoptosis of Primary Cultured Rat Ovarian Granulosa Cells Via Its Increase in Extracellular HMGB1. Reprod. Sci. 2015, 22, 271–277.
- Wang, M.; Sun, J.; Xu, B.; Chrusciel, M.; Gao, J.; Bazert, M.; Stelmaszewska, J.; Xu, Y.; Zhang, H.; Pawelczyk, L.; et al.et al. Functional Characterization of MicroRNA-27a-3p Expression in Human Polycystic Ovary Syndrome. Endocrinology 2018, 159, 297–309.
- Slack, C.; Werz, C.; Wieser, D.; Alic, N.; Foley, A.; Stocker, H.; Withers, D.J.; Thornton, J.M.; Hafen, E.; Partridge, L.; et al. Regulation of Lifespan, Metabolism, and Stress Responses by the Drosophila SH2B Protein, Lnk. PLoS Genet. 2010, 6, e1000881.
- Devallière, J.; Charreau, B.; The Adaptor Lnk (SH2B3): An Emerging Regulator in Vascular Cells and a Link between Immune and Inflammatory Signaling. Biochem. Pharmacol. 2011, 82, 1391–1402.
- Fox, E.R.; Young, J.H.; Li, Y.; Dreisbach, A.W.; Keating, B.J.; Musani, S.K.; Liu, K.; Morrison, A.C.; Ganesh, S.; Kutlar, A.; et al.et al. Association of Genetic Variation with Systolic and Diastolic Blood Pressure among African Americans: The Candidate Gene Association Resource Study. Hum. Mol. Genet. 2011, 20, 2273–2284.
- Jiang, J.; Balcerek, J.; Rozenova, K.; Cheng, Y.; Bersenev, A.; Wu, C.; Song, Y.; Tong, W.; 14-3-3 Regulates the LNK/JAK2 Pathway in Mouse Hematopoietic Stem and Progenitor Cells. J. Clin. Investig. 2012, 122, 2079–2091.
- Bersenev, A.; Wu, C.; Balcerek, J.; Jing, J.; Kundu, M.; Blobel, G.A.; Chikwava, K.R.; Tong, W.; Lnk Constrains Myeloproliferative Diseases in Mice. J. Clin. Investig. 2010 2010, 120, 2058–2069.
- Hao, M.; Yuan, F.; Jin, C.; Zhou, Z.; Cao, Q.; Xu, L.; Wang, G.; Huang, H.; Yang, D.; Xie, M.; et al.et al. Overexpression of Lnk in the Ovaries Is Involved in Insulin Resistance in Women With Polycystic Ovary Syndrome. Endocrinology 2016, 157, 3709–3718.
- Tan, M.; Cheng, Y.; Zhong, X.; Yang, D.; Jiang, S.; Ye, Y.; Ding, M.; Guan, G.; Yang, D.; Zhao, X.; et al. LNK Promotes Granulosa Cell Apoptosis in PCOS via Negatively Regulating Insulin-Stimulated AKT-FOXO3 Pathway. Aging (Albany N.Y.) 2021, 13, 4617–4633.
- Hu, C.-L.; Cowan, R.G.; Harman, R.M.; Quirk, S.M.; Cell Cycle Progression and Activation of Akt Kinase Are Required for Insulin-Like Growth Factor I-Mediated Suppression of Apoptosis in Granulosa Cells. Mol. Endocrinol. 2004, 18, 326–338.
- John, G.B.; Shidler, M.J.; Besmer, P.; Castrillon, D.H; Kit Signaling via PI3K Promotes Ovarian Follicle Maturation but Is Dispensable for Primordial Follicle Activation. Dev. Biol. 2009, 331, 292–299.
- Greer, E.L.; Brunet, A.; FOXO Transcription Factors at the Interface between Longevity and Tumor Suppression. Oncogene 2005, 24, 7410-7425.
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K.; Akt, FoxO and Regulation of Apoptosis. . Biochim. Biophys. 2011, 1813, 1978–1986.
- Zheng, W.; Nagaraju, G.; Liu, Z.; Liu, K.; Functional Roles of the Phosphatidylinositol 3-Kinases (PI3Ks) Signaling in the Mammalian Ovary. Mol. Cell. Endocrinol. 2012, 356, 24-30.
- Li, T.; Mo, H.; Chen, W.; Li, L.; Xiao, Y.; Zhang, J.; Li, X.; Lu, Y.; Role of the PI3K-Akt Signaling Pathway in the Pathogenesis of Polycystic Ovary Syndrome. Reprod. Sci. 2017, 24, 646–655.
- Gong, Y.; Luo, S.; Fan, P.; Zhu, H.; Li, Y.; Huang, W.; Growth Hormone Activates PI3K/Akt Signaling and Inhibits ROS Accumulation and Apoptosis in Granulosa Cells of Patients with Polycystic Ovary Syndrome. Reprod. Biol. Endocrinol. 2020, 18, 121.
- Nekoonam, S.; Naji, M.; Nashtaei, M.S.; Mortezaee, K.; Koruji, M.; Safdarian, L.; Amidi, F.; Expression of AKT1 along with AKT2 in Granulosa-Lutein Cells of Hyperandrogenic PCOS Patients. Arch. Gynecol. Obstet. 2017, 295, 1041–1050.
- Villavicencio, A.; Goyeneche, A.; Telleria, C.; Bacallao, K.; Gabler, F.; Fuentes, A.; Vega, M.; Involvement of Akt, Ras and Cell Cycle Regulators in the Potential Development of Endometrial Hyperplasia in Women with Polycystic Ovarian Syndrome. Gynecol. Oncol. 2009, 115, 102-107.
- Brants, J.R.; Ayoubi, T.A.Y.; Chada, K.; Marchal, K.; van de Ven, W.J.M.; Petit, M.M.R.; Differential Regulation of the Insulin-like Growth Factor II MRNA-Binding Protein Genes by Architectural Transcription Factor HMGA2. FEBS Lett. 2004, 569, 277-283.
- Cleynen, I.; Brants, J.R.; Peeters, K.; Deckers, R.; Debiec-Rychter, M.; Sciot, R.; van de Ven, W.J.M.; Petit, M.M.R.; HMGA2 Regulates Transcription of the Imp2 Gene via an Intronic Regulatory Element in Cooperation with Nuclear Factor-ΚB. Mol. Cancer. Res. 2007, 5, 363-372.
- Reeves, R.; Nissen, M.S.; The A.T-DNA-Binding Domain of Mammalian High Mobility Group I Chromosomal Proteins. A Novel Peptide Motif for Recognizing DNA Structure. J. Biol. Chem. 1990, 265, 8573–8582.
- Fedele, M.; Visone, R.; de Martino, I.; Troncone, G.; Palmieri, D.; Battista, S.; Ciarmiello, A.; Pallante, P.; Arra, C.; Melillo, R.M.; et al.et al. HMGA2 Induces Pituitary Tumorigenesis by Enhancing E2F1 Activity. Cancer Cell 2006, 9, 459–471.
- Saxena, R.; Voight, B.F.; Lyssenko, V.; Burtt, N.P.; de Bakker, P.I.W.; Chen, H.; Roix, J.J.; Kathiresan, S.; Hirschhorn, J.N.; Daly, M.J.; et al.et al. Genome-Wide Association Analysis Identifies Loci for Type 2 Diabetes and Triglyceride Levels. Science 2007, 316, 1331–1336.
- Li, M.; Zhao, H.; Zhao, S.-G.; Wei, D.-M.; Zhao, Y.-R.; Huang, T.; Muhammad, T.; Yan, L.; Gao, F.; Li, L.; et al.et al The HMGA2-IMP2 Pathway Promotes Granulosa Cell Proliferation in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 1049–1059.
- Jakubowicz, D.J.; Nestler, J.E.; 17α-Hydroxyprogesterone Responses to Leuprolide and Serum Androgens in Obese Women with and without Polycystic Ovary Syndrome after Dietary Weight Loss. J. Clin. Endocrinol. Metab. 1997, 82, 556-560.
- Gilling-Smith, C.; Story, H.; Rogers, V.; Franks, S.; Evidence for a Primary Abnormality of Thecal Cell Steroidogenesis in the Polycystic Ovary Syndrome. Clin. Endocrinol. 1997, 47, 93-99.
- Nestler, J.E.; Jakubowicz, D.J.; Falcon de Vargas, A.; Brik, C.; Quintero, N.; Medina, F.; Insulin Stimulates Testosterone Biosynthesis by Human Thecal Cells from Women with Polycystic Ovary Syndrome by Activating Its Own Receptor and Using Inositolglycan Mediators as the Signal Transduction System. J. Clin. Endocrinol. Metab. 1998, 83, 2001-2005.
- Gilling-Smith, C.; Willis, D.S.; Beard, R.W.; Franks, S.; Hypersecretion of Androstenedione by Isolated Thecal Cells from Polycystic Ovaries. J. Clin. Endocrinol. Metab. 1994, 79, 1158-1165.
- Nelson, V.L.; Legro, R.S.; Strauss, J.F.; McAllister, J.M.; Augmented Androgen Production Is a Stable Steroidogenic Phenotype of Propagated Theca Cells from Polycystic Ovaries. Mol. Endocrinol. 1999, 13, 946-957.
- McAllister, J.M.; Modi, B.; Miller, B.A.; Biegler, J.; Bruggeman, R.; Legro, R.S.; Strauss, J.F; Overexpression of a DENND1A Isoform Produces a Polycystic Ovary Syndrome Theca Phenotype. Proc. Natl. Acad. Sci. USA 2014, 111, E1519–E1527.
- Nelson, V.L.; Qin, K.; Rosenfield, R.L.; Wood, J.R.; Penning, T.M.; Legro, R.S.; Strauss, J.F.; McAllister, J.M; The Biochemical Basis for Increased Testosterone Production in Theca Cells Propagated from Patients with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2001, 86, 5925–5933.
- Wickenheisser, J.K.; Biegler, J.M.; Nelson-DeGrave, V.L.; Legro, R.S.; Strauss, J.F.; McAllister, J.M.; Cholesterol Side-Chain Cleavage Gene Expression in Theca Cells: Augmented Transcriptional Regulation and MRNA Stability in Polycystic Ovary Syndrome. PLoS ONE 2012, 7, e48963.
- Wickenheisser, J.K.; Quinn, P.G.; Nelson, V.L.; Legro, R.S.; Strauss, J.F.; McAllister, J.M.; Differential Activity of the Cytochrome P450 17α-Hydroxylase and Steroidogenic Acute Regulatory Protein Gene Promoters in Normal and Polycystic Ovary Syndrome Theca Cells. J. Clin. Endocrinol. Metab. 2000, 85, 2304–2311.
- Goodarzi, M.O.; Jones, M.R.; Li, X.; Chua, A.K.; Garcia, O.A.; Chen, Y.-D.I.; Krauss, R.M.; Rotter, J.I.; Ankener, W.; Legro, R.S.; et al.et al. Replication of Association of DENND1A and THADA Variants with Polycystic Ovary Syndrome in European Cohorts. J. Med. Genet. 2012, 49, 90–95.
- Welt, C.K.; Styrkarsdottir, U.; Ehrmann, D.A.; Thorleifsson, G.; Arason, G.; Gudmundsson, J.A.; Ober, C.; Rosenfield, R.L.; Saxena, R.; Thorsteinsdottir, U.; et al.et al. Variants in DENND1A Are Associated with Polycystic Ovary Syndrome in Women of European Ancestry. J. Clin. Endocrinol. Metab. 2012, 97, E1342–E1347.
- Lerchbaum, E.; Trummer, O.; Giuliani, A.; Gruber, H.-J.; Pieber, T.; Obermayer-Pietsch, B.; Susceptibility Loci for Polycystic Ovary Syndrome on Chromosome 2p16.3, 2p21, and 9q33.3 in a Cohort of Caucasian Women. Horm. Metab. Res. 2011, 43, 743–747.
- Eriksen, M.B.; Brusgaard, K.; Andersen, M.; Tan, Q.; Altinok, M.L.; Gaster, M.; Glintborg, D.; Association of Polycystic Ovary Syndrome Susceptibility Single Nucleotide Polymorphism Rs2479106 and PCOS in Caucasian Patients with PCOS or Hirsutism as Referral Diagnosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 163, 39-42.
- Kosova, G.; Urbanek, M.; Genetics of the Polycystic Ovary Syndrome. Mol. Cell. Endocrinol. 2013, 373, 29-38.
- McAllister, J.M.; Legro, R.S.; Modi, B.P.; Strauss, J.F.; Functional Genomics of PCOS: From GWAS to Molecular Mechanisms. Trends. Endocrinol. Metab. 2015, 26, 118-124.
- Rosenfield, R.L.; Ehrmann, D.A.; The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520.
- Tian, Y.; Li, J.; Su, S.; Cao, Y.; Wang, Z.; Zhao, S.; Zhao, H.; PCOS-GWAS Susceptibility Variants in THADA, INSR, TOX3, and DENND1A Are Associated With Metabolic Syndrome or Insulin Resistance in Women With PCOS. Front. Endocrinol. 2020, 11, 274.
- Khan, S.; Duan, P.; Yao, L.; Hou, H.; Shiftwork-Mediated Disruptions of Circadian Rhythms and Sleep Homeostasis Cause Serious Health Problems. Int. J. Genom. 2018, 2018, 8576890..
- Wang, F.; Xie, N.; Wu, Y.; Zhang, Q.; Zhu, Y.; Dai, M.; Zhou, J.; Pan, J.; Tang, M.; Cheng, Q.; et al.et al. Association between Circadian Rhythm Disruption and Polycystic Ovary Syndrome. Fertil. Steril. 2021, 115, 771–781.
- Johnson, B.S.; Krishna, M.B.; Padmanabhan, R.A.; Pillai, S.M.; Jayakrishnan, K.; Laloraya, M.; Derailed Peripheral Circadian Genes in Polycystic Ovary Syndrome Patients Alters Peripheral Conversion of Androgens Synthesis. Hum. Reprod. 2022, 37, 835–1855.
- Chu, W.; Li, S.; Geng, X.; Wang, D.; Zhai, J.; Lu, G.; Chan, W.-Y.; Chen, Z.-J.; Du, Y.; Long-Term Environmental Exposure of Darkness Induces Hyperandrogenism in PCOS via Melatonin Receptor 1A and Aromatase Reduction. Front. Cell Dev. Biol. 2022, 10, 954186.
- Zhang, L.; Hirano, A.; Hsu, P.-K.; Jones, C.R.; Sakai, N.; Okuro, M.; McMahon, T.; Yamazaki, M.; Xu, Y.; Saigoh, N.; et al.et al. A PERIOD3 Variant Causes a Circadian Phenotype and Is Associated with a Seasonal Mood Trait. Proc. Natl. Acad. Sci. USA 2016, 113, E1536–E1544.
- Solt, L.A.; Kojetin, D.J.; Burris, T.P.; The REV-ERBs and RORs: Molecular Links between Circadian Rhythms and Lipid Homeostasis. Future Med. Chem. 2011, 3, 623–638.
- Guillaumond, F.; Dardente, H.; Giguère, V.; Cermakian, N.; Differential Control of Bmal1 Circadian Transcription by REV-ERB and ROR Nuclear Receptors. J. Biol. Rhythm. 2005, 20, 391–403.
- Sun, L.; Tian, H.; Xue, S.; Ye, H.; Xue, X.; Wang, R.; Liu, Y.; Zhang, C.; Chen, Q.; Gao, S.; et al. Circadian Clock Genes REV-ERBs Inhibits Granulosa Cells Apoptosis by Regulating Mitochondrial Biogenesis and Autophagy in Polycystic Ovary Syndrome. Front. Cell Dev. Biol. 2021, 9, 2079.
- Preitner, N.; Damiola, F.; Luis-Lopez-Molina; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U.; The Orphan Nuclear Receptor REV-ERBα Controls Circadian Transcription within the Positive Limb of the Mammalian Circadian Oscillator. Cell 2002, 110, 251–260.
- Kohsaka, A.; Bass, J. A.; Sense of Time: How Molecular Clocks Organize Metabolism. Trends Endocrinol. Metab. 2007, 18, 4-11.
- Li, C.; Shi, Y.; You, L.; Wang, L.; Chen, Z.-J.; Melatonin Receptor 1A Gene Polymorphism Associated with Polycystic Ovary Syndrome. Gynecol. Obstet. 2011, 72, 130-134.
- Li, C.; Shi, Y.; You, L.; Wang, L.; Chen, Z.-J.; Association of Rs10830963 and Rs10830962 SNPs in the Melatonin Receptor (MTNR1B) Gene among Han Chinese Women with Polycystic Ovary Syndrome. Mol. Hum. Reprod. 2011, 17, 193–198.
- Wang, L.; Wang, Y.; Zhang, X.; Shi, J.; Wang, M.; Wei, Z.; Zhao, A.; Li, B.; Zhao, X.; Xing, Q.; et al.et al. Common Genetic Variation in MTNR1B Is Associated with Serum Testosterone, Glucose Tolerance, and Insulin Secretion in Polycystic Ovary Syndrome Patients. Fertil. Steril. 2010, 94, 2486–2489.e2.
- Moore, R.Y.; Lenn, N.J. J.; A Retinohypothalamic Projection in the Rat. Comp. Neurol. 1972, 146, 1-14.
- Chan, S.; Debono, M.; Review: Replication of Cortisol Circadian Rhythm: New Advances in Hydrocortisone Replacement Therapy. Ther. Adv. Endocrinol. Metab. 2010, 1, 129-138.
- Asghari, M.H.; Moloudizargari, M.; Bahadar, H.; Abdollahi, M. Expert. Opin.; A Review of the Protective Effect of Melatonin in Pesticide-Induced Toxicity. Drug Metab. Toxicol. 2017, 13, 545–554.
- Asghari, M.H.; Moloudizargari, M.; Ghobadi, E.; Fallah, M.; Abdollahi, M.; Melatonin as a Multifunctional Anti-Cancer Molecule: Implications in Gastric Cancer. Life Sci. 2017, 185, 38–45.
- Asghari, M.H.; Moloudizargari, M.; Baeeri, M.; Baghaei, A.; Rahimifard, M.; Solgi, R.; Jafari, A.; Aminjan, H. H.; Hassani, S.; Moghadamnia, A.A.; et al.et al. On the Mechanisms of Melatonin in Protection of Aluminum Phosphide Cardiotoxicity. Arch. Toxicol. 2017, 91, 3109–3120.
- Reiter, R.J.; Tan, D.-X.; Tamura, H.; Cruz, M.H.C.; Fuentes-Broto, L.; Clinical Relevance of Melatonin in Ovarian and Placental Physiology: A Review. Gynecol. Endocrinol. 2014, 30, 83-89.
- Sack, R.L.; Blood, M.L.; Lewy, A.J.; Melatonin Rhythms in Night Shift Workers. Sleep 1992, 15, 434-441.
- Fernandez, R.; Moore, V.; van Ryswyk, E.; Varcoe, T.; Rodgers, R.; March, W.; Moran, L.; Avery, J.; McEvoy, D.; Davies, M.; et al. Sleep Disturbances in Women with Polycystic Ovary Syndrome: Prevalence, Pathophysiology, Impact and Management Strategies. Nat. Sci. Sleep 2018, 10, 45-64.
- Terzieva, D.D.; Orbetzova, M.M.; Mitkov, M.D.; Mateva, N.G.; Serum Melatonin in Women with Polycystic Ovary Syndrome. Folia Med. 2013, 55, 10-15.
- Jain, M.; Jain, S.; Singh, T.; Haldar, C.; Jain, P.; Melatonin and Its Correlation with Testosterone in Polycystic Ovarian Syndrome. J. Hum. Reprod. Sci. 2013, 6, 253.
- Shreeve, N.; Cagampang, F.; Sadek, K.; Tolhurst, M.; Houldey, A.; Hill, C.M.; Brook, N.; Macklon, N.; Cheong, Y.; Poor Sleep in PCOS; Is Melatonin the Culprit?. Hum. Reprod. 2013, 28, 1348–1353.
- Luboshitzky, R.; Qupti, G.; Ishay, A.; Shen-Orr, Z.; Futerman, B.; Linn, S.; Increased 6-Sulfatoxymelatonin Excretion in Women with Polycystic Ovary Syndrome. Fertil. Steril. 2001, 76, 506-510.
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.-X.; Sugino, N.; Reiter, R.J.; Melatonin and the Ovary: Physiological and Pathophysiological Implications. Fertil. Steril. 2009, 92, 328–343.
- Kim, M.K.; Park, E.A.; Kim, H.J.; Choi, W.Y.; Cho, J.H.; Lee, W.S.; Cha, K.Y.; Kim, Y.S.; Lee, D.R.; Yoon, T.K.; et al. Does Supplementation of In-Vitro Culture Medium with Melatonin Improve IVF Outcome in PCOS?. Reprod. Biomed. Online 2013, 26, 22-29.
- Polson, D.W.; Wadsworth, J.; Adams, J.; Franks, S.; Polycystic ovaries—A common finding in normal women.. Lancet 1988 1988, 331, 870-872.
- Mojaverrostami, S.; Asghari, N.; Khamisabadi, M.; Heidari Khoei, H.; The Role of Melatonin in Polycystic Ovary Syndrome: A Review. Int. J. Reprod. Biomed. 2019, 2019, 17, 865-882.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
5.6K
Revisions:
2 times
(View History)
Update Date:
16 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




