Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Catalina Christensen | -- | 3254 | 2023-01-12 21:57:41 | | | |
| 2 | Rita Xu | -54 word(s) | 3200 | 2023-01-13 02:52:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Christensen, C.; Allesø, M.; Rose, M.; Cornett, C. Medicinal Cannabis as Analgesic in Cancer Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/40134 (accessed on 07 February 2026).
Christensen C, Allesø M, Rose M, Cornett C. Medicinal Cannabis as Analgesic in Cancer Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/40134. Accessed February 07, 2026.
Christensen, Catalina, Morten Allesø, Martin Rose, Claus Cornett. "Medicinal Cannabis as Analgesic in Cancer Patients" Encyclopedia, https://encyclopedia.pub/entry/40134 (accessed February 07, 2026).
Christensen, C., Allesø, M., Rose, M., & Cornett, C. (2023, January 12). Medicinal Cannabis as Analgesic in Cancer Patients. In Encyclopedia. https://encyclopedia.pub/entry/40134
Christensen, Catalina, et al. "Medicinal Cannabis as Analgesic in Cancer Patients." Encyclopedia. Web. 12 January, 2023.
Copy Citation
The analgesic potential of Cannabis sativa L.—based medicinal cannabis products for treatment of cancer associated chronic pains has gained increased interest. To ensure a controlled distribution of these products and investigate their therapeutic potential, several countries have established so-called pilot trials.
cancer pain
medicinal cannabis
cannabinoid-based medicine
endocannabinoid system
clinical evidence
1. Introduction
Administration of the cannabis plant (Cannabis sativa L.) as an alternative to conventional medicines in the management of various diseases and disease-related symptoms, including cancer-associated pain, has increased massively during the last decades [1][2]. It has been postulated that researchers are currently experiencing a re-medicalization of cannabis, as more patients express interest to try out treatment with cannabis for medicinal purposes [3]. This tendency is believed to be caused by several factors, among other the discovery of the endocannabinoid system (ECS), which has accelerated the development of cannabis-based medicinal products.
Although documentation is sparse, it is generally recognized that patients suffering from various conditions have been sourcing cannabis illegally for symptom relief, thereby accepting the inherent risks of such an unregulated treatment strategy [3][4][5]. Supported by the rapidly growing understanding of the pharmacology of cannabinoids, this has led to an increased focus on and demand for legal medicinal cannabis, i.e., produced within the frameworks of Good Agricultural Collection Practice (GACP) and Good Manufacturing Practice (GMP) frameworks [6][7]. As a result, several countries have developed policies and pilot programs allowing for the prescription of cannabis for medicinal purposes [3]. Denmark’s pilot program (running from 2018 till the end of 2025) allows Danish doctors to prescribe medicinal cannabis, however only if authorized conventional medicinal products have been tried without satisfactory effects. The medicinal cannabis products have not yet been researched in-depth through high-quality clinical randomized controlled trials (RCTs), which means that evidence regarding their safety and efficacy in different patient populations is scarce and highly needed. This challenges the administration and dosing of the products in a clinical setting [8].
The term “medicinal cannabis” is applied broadly to products containing either extracts from the Cannabis sativa L. plant or isolated active compounds or synthetic analogues hereof. To avoid confusion and provide clarity, the product types are specifically defined based on the existing formal definitions as follows: The medicinal cannabis products that are part of several pilot programs currently running are defined as products containing the full spectrum of naturally occurring compounds that can be extracted from the Cannabis sativa L. plant, thereby referred to as full-spectrum medicinal cannabis [8]. Another product type is the cannabis-based medicines, such as the authorized medicinal product Sativex®, also known as nabiximols, approved for cancer-associated pain in Canada [9]. Sativex® is a natural Cannabis sativa L. plant extract product, composed of a combination of extracts from a high cannabidiol (CBD) cultivar and a high ∆-9-tetrahydrocannabinol (THC) cultivar, combined to a final drug product composed of almost equal amounts of CBD and THC [10].The additional content of unlabeled naturally occurring compounds (e.g., residual cannabinoids and terpenoids) from extract of the Cannabis sativa L. plant defines Sativex® as a so-called broad-spectrum product [11]. Other types of products are based on single cannabinoids, either isolated from the cannabis plant or produced through chemical synthesis. These are considered active pharmaceutical ingredients (APIs) [12].
To the best of the knowledge, clinical evidence of medicinal cannabis as an analgesic in the management of cancer pain is limited both in scope and in quality, while clinical evidence of higher quality, in the form of RCTs, exists in support of the analgesic effects of the cannabis-based product Sativex® in cancer patients. However, the evidence behind it is based on only a few clinical trials [1][13].
Despite the lack of high-quality research evidence, it is not uncommon to administer cannabis within clinical settings to manage chronic pains often associated with cancer [1]. Several studies confirm this tendency, with cannabis consumption observed prevalently for both symptom management, in particular pains, and for the purpose of treating the underlying disease [14][15]. It has been estimated that among 80% of advanced cancer patients, irrespective of cancer type, suffer from disease-related pains, with as many as a third of the survivors reporting to experience symptoms of pain [16]. Because of improved methods to diagnose the patients, the population of cancer patients surviving the disease is believed to increase in the future. Cancer-associated pains are highly heterogenous and complex to treat satisfactorily and completely in all patients. The pain can be caused by the cancer tissue itself or as an adverse effect of the therapeutic management of the cancer, e.g., through chemotherapy. The currently most abundant and primarily used analgesic is opioids. However, opioids often do not provide satisfactory analgesia for all patients and additionally often comes with a line of adverse effects and an increased risk of developing addiction. Thus, frequent opioid usage often requires addition of other medicinal products to manage the adverse effect symptoms [17][18].
Several challenges pertain to performing research within the medicinal cannabis field: (1) the synergistic and complex effects occurring when administering hundreds of molecules into the body all at once, (2) the molecular composition of the herbal material (i.e., either as dried biomass or extract) being affected by the cultivation and processing factors, resulting in inter-supplier product variability even when label claim of primary cannabinoids are identical, (3) the holistic multi-functionality of the ECS itself, where inter-individual and population-based differences exist, and (4) the complex heterogeneity related to cancer pain etiology. These factors, among others, add to the high complexity of medicinal cannabis research, which—combined with the current low-quality clinical evidence of its efficacy—challenges the preparation of evidence-based recommendations to support the administration and dosing guidelines in the clinical settings [19][20]. A study, investigating cancer patient survivors’ perceptions of barriers against using medicinal cannabis, reported the following reasons: lack of research evidence, concerns of dependency and healthcare providers’ criticism towards medicinal cannabis usage [21]. The hesitancy of doctors to prescribe these products, has left many patients without professional medical guidance and as a result have turned to the illegal market to source their cannabis to manage their disease [22][23]. These illegal products do not come with a GMP-compliant certificate of analysis and thus may potentially cause harm to the patient, either due to little or no efficacy or because of harmful components that were not controlled during production (e.g., erroneous cannabinoid content, foreign matter, heavy metals, pesticides, mycotoxins, microbes, etc.) [3][14]. Ultimately, this causes a societal challenge for authorities to tackle.
The rapid increase in product availability calls for evidence-based practical guidelines to support the process of decision-making by physicians. Recently, a review study was published by Gorzo et al. [24], providing an updated status of existing research evidence of relevance to oncologists in their consideration of administrating cannabis-based medicines in the management of cancer pain. The study found that while convincing pre-clinical research exist, the translation of these data into high-quality clinical trials is lacking, which is necessary to determine their clinical relevance and safety. Current selective review contributes to this field through a comprehensive summary of existing clinical research evidence supporting the analgesic effects of medicinal cannabis in cancer patients. A particular focus will be on research that potentially qualifies as a guidance to practitioners for the administration and dosing of medicinal cannabis to patients suffering from cancer-related pains. Only clinical research regarding medicinal cannabis (i.e., full-spectrum) and the, thus far only, cannabis-based product Sativex® will be considered relevant for the purpose of this research. In addition, the clinical findings will be discussed in the context of challenges related to the field of medicinal cannabis research, such as the multi-faceted role of the endocannabinoid system, lack of product standardization and unavailability of a true placebo. It is important to state that this research should not be perceived as a guideline nor a recommendation for treatment with medicinal cannabis as a replacement to standard conventional treatments. It is rather to be considered as an objective, yet critical, overview of published studies that physicians and professionals may consult at their own discretion.
2. The Endocannabinoid System and Its Implication in Cancer Pain
The ECS is an endogenous multifunctional pro-homeostatic signaling system being almost ubiquitously distributed within the body. The system is generally recognized to consist of three main parts (1) receptors: G protein-coupled receptors (GPCRs); cannabinoid receptor 1 (CB1R) and 2 (CB2R), (2) endocannabinoids: the body’s own signaling molecules regulating the ECS through the cannabinoid receptors, including N-arachidonylethanolamine (anandamide, i.e., AEA) and 2-arachidonoylglycerol (2-AG) and (3) the enzymes: responsible for the metabolism and regulation of endocannabinoids available at a given time. In line with the development of research in the field, additional components have been discovered being part of the ECS. This including (1) receptors: GPCRs (e.g., GPR18, GPR55 and GPR119), ion channels (e.g., Transient Receptor Potential Vanilloid 1 (TRPV1) and nuclear receptors (e.g., Peroxisome Proliferator-activated receptor gamma (PPAR-y), (2) endocannabinoid-like compounds: e.g., Palmitoylethanolamide (PEA) and Oleoylethanolamine (OEA) and (3) synthesizing and degradative enzymes and transport proteins of the endocannabinoids and alike ligands. The ECS acts locally at the various body parts it is localized within, where it responds to and is activated by disturbances occurring within these systems, with the aim to recover and maintain homeostasis [25][26].
The ECS has receptors located at various body sites, such as in the central nervous system (CNS), peripheral nervous system (PNS), immune system cells and peripheral tissues, see Figure 1.
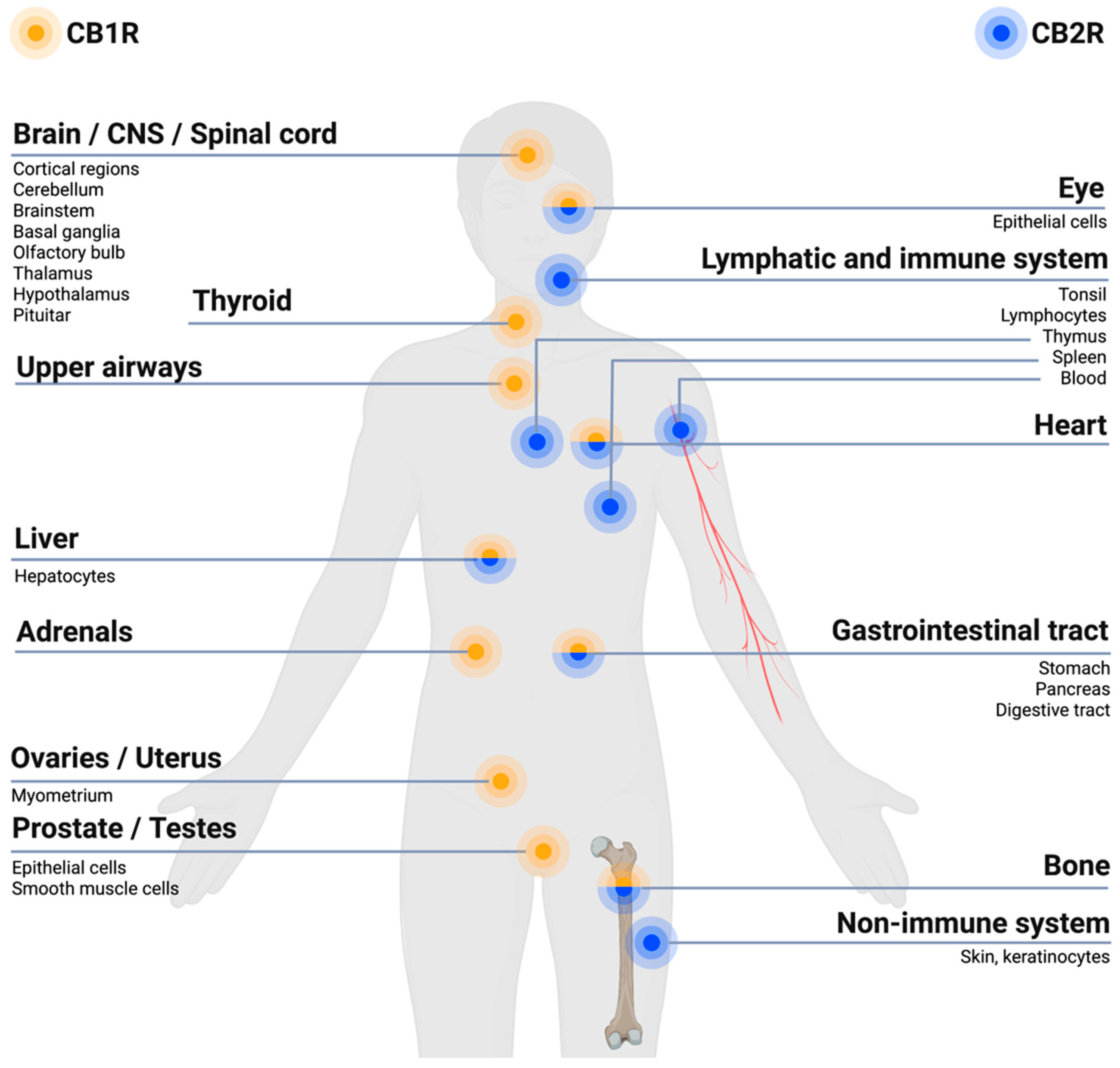
Figure 1. Endocannabinoid system (ECS) receptor distribution in the body. In general,  receptors (CB1Rs) are primarily expressed within brain regions and the nervous system, especially in the central nervous system (CNS), and to a lesser extent at other sites of the body.
receptors (CB1Rs) are primarily expressed within brain regions and the nervous system, especially in the central nervous system (CNS), and to a lesser extent at other sites of the body.  receptors (CB2Rs) are primarily expressed within cells related to the immune system and peripheral body tissues [27][28]. Created with BioRender.com.
receptors (CB2Rs) are primarily expressed within cells related to the immune system and peripheral body tissues [27][28]. Created with BioRender.com.
 receptors (CB1Rs) are primarily expressed within brain regions and the nervous system, especially in the central nervous system (CNS), and to a lesser extent at other sites of the body.
receptors (CB1Rs) are primarily expressed within brain regions and the nervous system, especially in the central nervous system (CNS), and to a lesser extent at other sites of the body.  receptors (CB2Rs) are primarily expressed within cells related to the immune system and peripheral body tissues [27][28]. Created with BioRender.com.
receptors (CB2Rs) are primarily expressed within cells related to the immune system and peripheral body tissues [27][28]. Created with BioRender.com.The ECS is involved in regulating pain within the body due to its expressions of cannabinoid receptors within the different pain pathways involved in the transmission of pain signals throughout the body. Overall, the ECS exerts an inhibitory effect on pain signaling [29]. The CB1Rs and CB2Rs are differently located within the body, with the CB1Rs predominantly localized within brain regions and the nervous system, in particular the CNS, while CB2Rs are predominantly found within cells of the immune system and the PNS, as illustrated in Figure 1 [28][30]. The CB1Rs have been found particularly at presynaptic sites of neurons within the CNS. Upon activation of the CB1R an inhibition of neurotransmitter release occurs, resulting in a dampening of the pain signaling [30], as illustrated in Figure 2. Upon activation of the CB2R, the transduction of pain signaling from the PNS into the CNS is inhibited thus reducing, among other, nociception (pain sensation) and inflammation [29][30], qua., the holistic function of the ECS. A deficiency of endocannabinoids thereby causes a hypofunctional ECS and has been proposed to be linked to a decreased pain threshold because of homeostatic ECS functions that cannot be maintained [31].
The ECS regulates and activates the cannabinoid receptors through the endocannabinoids, where especially the functioning of AEA and 2-AG has been studied and discussed within the research literature [32]. Upon binding of the endocannabinoids to the CB1R, an intracellular signaling cascade is initiated, which ultimately inhibits neurotransmitter release, thereby blocking pain signaling (Figure 2) [32][33]. AEA has been observed to bind as a partial agonist at the CB1R and CB2R, with a higher affinity at the CB1R, where 2-AG has been observed to be an agonist of both the CB1R and CB2R, binding with low to moderate affinity [28][32][34].
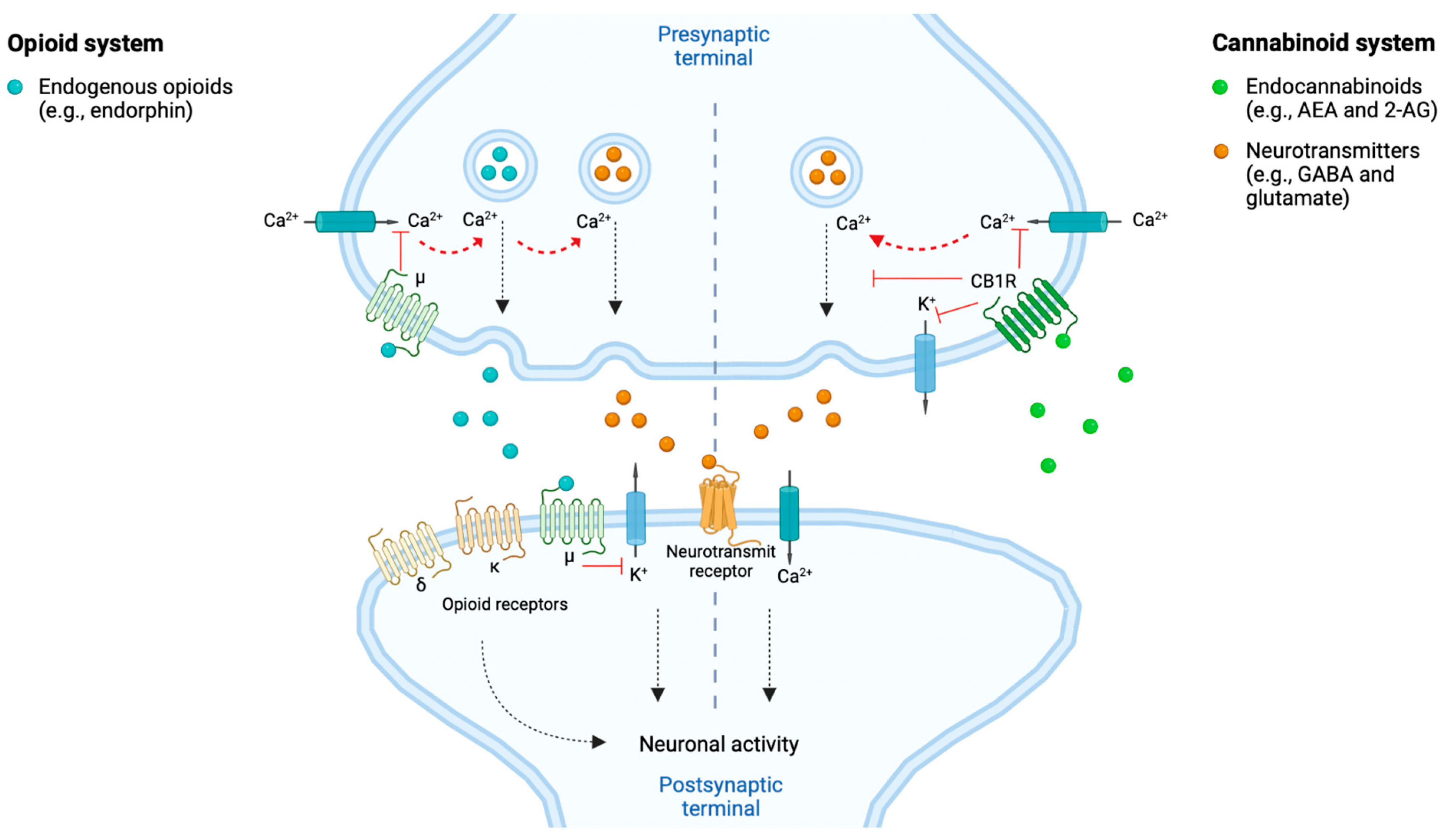

Figure 2. The opioid- and cannabinoid systems implicated in the modulation of neuronal activity and pain signal. Both endogenous opioids (e.g., endorphins) and endocannabinoids (e.g., anandamide, i.e., AEA and 2-arachidonoylglycerol, i.e., 2-AG) are involved in the response to and modulation of pain signaling. This through reducing the neuronal activity and thereby pain signaling. The endogenous opioids can, e.g., act at the postsynaptic neurons’ opioid receptors (i.e., δ, κ and μ) and at the presynaptic neurons’ μ receptors. The endocannabinoids can, e.g., act at the presynaptic neurons’ cannabinoid 1 receptors (CB1Rs). Both pathways, when activated by their respective agonists, modulate the intracellular release of neurotransmitters (e.g., GABA and glutamate) through calcium (Ca2+)-dependent vesicular release blockade, by blocking Ca2+ and kalium (K+) channels. Hereby is the neuronal activity decreased and results in dampening of the pain signaling [28][33][34]. Created with BioRender.com.
Cannabinoid receptors share several characteristics with the opioid receptors, such as their potential to induce analgesic effects through their neuronal presynaptic expressions within neurons whereby they inhibit neurotransmitter release and decrease the neuronal activity and thus, e.g., pain signaling [1][33], as illustrated in Figure 2. Both receptors belong to the GPCR family of the subtype Gi/o, thereby sharing intracellular signaling mechanisms upon stimulation by their respective ligands, such as reduction in activity of calcium voltage-dependent channels [35]. It has been proposed, based on pre-clinical studies, that CB1Rs and μ opioid receptors can interact by forming heterodimers in neurons where they are co-expressed [36]. Furthermore, cannabinoids have been proposed to stimulate synthesis and release of endogenous opioids and vice versa [37]. These characteristics overall support the occurrence of interactions among opioids and cannabinoids in inducing synergistic additive analgesic effects when co-consumed ([2], pp. 2–4, [35]).
3. Cannabis sativa L. Compounds Possessing Analgesic Effects
More than 100 different (phyto)cannabinoids have been discovered among the existing cultivars of Cannabis sativa L. plants. Only some of them have been investigated in-depth, particularly THC and CBD. The cannabinoids possess different receptor affinities at different targets within the body, both within and outside the ECS, explaining partly the diverse therapeutic potential of different cannabis cultivars [38].
Common cannabinoids such as THC, CBD and cannabinol (CBN) possess affinities for the cannabinoid receptors and thereby to some extent mimic the binding of endocannabinoids [30]. CBN binds to and modulates the cannabinoid receptors, with highest affinity at the CB2R and weak agonistic binding at the CB1R [30][38]. THC shows partial agonistic as well as antagonistic binding to both the CB1R and CB2R, depending on factors such as the concentration of THC consumed, cell type specific receptor expressions and presence of other cannabinoids (i.e., endocannabinoids or exocannabinoids, the latter derived from outside the body) [30]. CBD possesses low affinities and no agonistic binding at the CB1Rs and CB2Rs but is on the contrary believed to exert negative allosteric or antagonistic binding at the CB1R [32][39]. It is suggested that CBD can positively modulate THC-mediated mechanism of actions and thus reduce the adverse psychoactive effects related hereto, which allows for higher doses of THC to be administered and an overall increased therapeutical effect [40]. This might aid in explaining the therapeutic benefits of co-administering CBD and THC to cancer pain patients, e.g., as, demonstrated for Sativex® in a clinical study [41]. In addition, CBD indirectly enhances the level of AEA through inhibition of its cellular re-uptake [30]. Another GPCR, the GPR55, was found to be activated by THC, CBD and 2-AG, with THC and 2-AG possessing higher agonistic efficacy and potency, respectively, for this receptor over CB1R and CB2R. Thus, the GPR55 has been proposed to in fact be the third cannabinoid receptor [42]. Minor cannabinoids such as cannabigerol (CBG) and cannabichromene (CBC) have been demonstrated to exert analgesic effects through affecting other targets than the cannabinoid receptors (e.g., inhibition of AEA re-uptake, inhibition of certain serotonin receptors, inhibition of enzymes involved in synthesis of inflammatory mediators and activation of certain adrenergic receptors) [30]. The ability of minor cannabinoids to interact with targets within and outside the ECS has led to the proposal of an expanded endocannabinoid system, or the so-called endocannabidiome [43][44], which is believed to further expand as more research is conducted.
Terpenoids are another group of compounds contained within the cannabis plant. They are responsible for the characteristic aroma associated with cannabis and believed to possess individual bioactive effects in addition to modulatory effects of cannabinoids, however to the best of the knowledge currently mainly based on pre-clinical research. More than 200 different terpenoids have been detected within the Cannabis sativa L. plants, while only the terpenoids β-caryophyllene, β-myrcene and D-limonene have been observed to exert analgesic effects [45]. Much about their mechanism of actions is still unknown, although pre-clinical studies of β-caryophyllene have demonstrated agonistic binding to the CB2R, particularly the peripherally located, thereby exerting synergistic effects with THC [30].
A Pharmacological Perspective on the Entourage Effect Mechanism Theory
The entourage effect mechanism theory has been proposed to be the underlying reason for many patients claiming to experience an overall better effect when consuming full-spectrum cannabis products compared to products containing single cannabinoids [46]. Research aims to establish the pharmacological effects that collectively contribute to the entourage effect as it is currently not supported by solid research evidence [47]. The mechanism of actions are still unknown but are in general believed to be caused by pharmacokinetic as well as pharmacodynamic interactions between the bioactive compounds consumed from cannabis products. Pharmacokinetic interactions affect the absorption, distribution, metabolism, and excretion of the other compounds. This ultimately impacts the bioavailability, while pharmacodynamic interactions affect the efficacy of the bioactive compounds, by targeting receptors or enzymes and thereby enhancing or suppressing the bioactive effects of other compounds. However, both synergistic, additive and antagonistic effects among the compounds of Cannabis sativa L. have been demonstrated [48]. Different mechanisms have been proposed to cause this overall synergistic effect, including effects on the pharmacological properties of the individual compounds, which ultimately affects the clinical efficacy and occurrence of potential adverse effects [45].
Terpenoids have been proposed to act as entourage compounds by for example acting as vasodilators of the alveolar capillaries, thereby increasing the permeability of the blood–brain barrier, whereby the pharmacokinetic effects related to, e.g., THC is increased [49][50]. β-myrcene specifically has been found to possess multiple mechanism of actions, e.g., enhancing CBD’s effects on hepatic cytochrome P450 (CYP) enzymes. CBD has been reported to inhibit the subtype CYP2C9, which is responsible for the metabolism of THC into its more psychoactive breakdown product, 11-OH-THC [30][51]. This potentially contributes to enhance the beneficial effects of THC and CBD while reducing the adverse effects associated with the psychoactivity of THC and its breakdown product. However, this can potentially also affect the metabolism of additional co-consumed medicinal products metabolized by the same hepatic enzymes, whereby the pharmaceutical effects can be increased or decreased, ultimately affecting the clinical outcome [30]. As CBD exerts multiple effects that affect the bioactivity of THC, it has been proposed that CBD is an entourage compound. These findings have led to the proposal of THC being perceived as a “silver bullet” and the additional bioactive cannabis compounds and the cannabis plant itself as a “synergistic shotgun”, with the entourage effect been referred to as the cannabis plant synergy [45][46].
In summary, the ability of cannabinoids to bind to a multitude of receptors, potentiate or even block the effects of one another adds to the complexity of medicinal cannabis research. For full-spectrum products, the presence of additional potentially bioactive compounds, like terpenoids, furthermore adds to the complexity and challenges the production of a truly standardized product for clinical testing, ultimately reducing the statistical power of a clinical study design. The presented complexity of the ECS and the compounds of the Cannabis sativa L. plant should be kept in mind when critically assessing the outcomes of clinical trials investigating full-spectrum medicinal cannabis products for relief of cancer-induced pain.
References
- Chung, M.; Kim, H.K.; Abdi, S. Update on Cannabis and Cannabinoids for Cancer Pain. Curr. Opin. Anaesthesiol. 2020, 33, 825–831.
- Cyr, C.; Davis, M.P.; Schecter, D.; Daeninck, P. Cannabis and Cannabinoid-Based Medicines in Cancer Care: A Comprehensive Guide to Medical Management; Springer Nature: Cham, Switzerland, 2022; pp. 2–145.
- Kvamme, S.L.; Pedersen, M.M.; Alagem-Iversen, S.; Thylstrup, B. Beyond the high: Mapping patterns of use and motives for use of cannabis as medicine. Nord. Stud. Alcohol Drugs 2021, 38, 270–292.
- Swift, W.; Gates, P.; Dillon, P. Survey of Australians using cannabis for medical purposes. Harm Reduct. J. 2005, 2, 18.
- Hawley, P.; Gobbo, M.; Afghari, N. The impact of legalization of access to recreational Cannabis on Canadian medical users with Cancer. BMC Health Serv. Res. 2020, 20, 977.
- World Health Organization. WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants. Available online: https://www.who.int/publications/i/item/9241546271 (accessed on 30 September 2022).
- European Commission. Eudralex—Volume 4—Good Manufacturing Practice (GMP) Guidelines; Annex 4 and 7. Available online: https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en (accessed on 30 September 2022).
- Danish Medicines Agency (DKMA). Medicinal Cannabis Pilot Programme. Available online: https://laegemiddelstyrelsen.dk/en/special/medicinal-cannabis-/medicinal-cannabis-pilot-programme/ (accessed on 30 September 2022).
- FDA News. Health Canada Approves Sativex for Cancer Pain. Available online: https://www.fdanews.com/articles/96888-health-canada-approves-sativex-for-cancer-pain (accessed on 1 October 2022).
- Electronic Medicines Compendium (EMC). Sativex Oromucosal Spray. Available online: https://www.medicines.org.uk/emc/product/602/smpc#gref (accessed on 3 October 2022).
- The National Center for Advancing Translational Sciences (NCATS). Nabiximols. Available online: https://drugs.ncats.io/drug/K4H93P747O#publications (accessed on 3 October 2022).
- Danish Medicines Agency (DKMA). Rules Applicable to Active Substances (APIs). Available online: https://laegemiddelstyrelsen.dk/en/licensing/company-authorisations-and-registrations/registration-as-manufacturer,-importer-and-distributor-ofactive-substances-apis/rules-for-active-substances/ (accessed on 3 October 2022).
- The National Academics of Sciences, Engineering and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. 2017. Available online: https://nap.nationalacademies.org/catalog/24625/the-health-effects-of-cannabis-and-cannabinoids-the-current-state (accessed on 24 November 2022).
- Nielsen, S.W.; Ruhlmann, C.H.; Eckhoff, L.; Brønnum, D.; Herrstedt, J. Cannabis use among Danish patients with cancer: A cross-sectional survey of sociodemographic traits, quality of life, and patient experiences. Support. Care Cancer 2022, 30, 1181–1190.
- Raghunathan, N.J.; Brens, J.; Vemuri, S.; Li, Q.S.; Mao, J.J.; Korenstein, D. In the weeds: A retrospective study of patient interest in and experience with cannabis at a cancer center. Support. Care Cancer 2022, 30, 7491–7497.
- Jiang, C.;Wang, H.;Wang, Q. Prevalence of chronic pain and high-impact chronic pain in cancer survivors in the United States. JAMA Oncol. 2019, 5, 1224–1226.
- Wiffen, P.J.; Wee, B.; Derry, S.; Bell, R.F.; Moore, R.A. Opioids for cancer pain—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2017, 7, CD012592.
- Meng, H.; Dai, T.; Hanlon, J.G.; Downar, J.; Alibhai, A.M.H.; Clarke, H. Cannabis and cannabinoids in cancer pain management. Curr. Opin. Support. Palliat. Care 2020, 14, 87–93.
- Brown, D.;Watson, M.; Schloss, J. Pharmacological evidence of medicinal cannabis in oncology: A systematic review. Support. Care Cancer 2019, 27, 3195–3207.
- Alexander, S.P.H. Barriers to the wider adoption of medicinal Cannabis. British Journal of Pain. SAGE J. 2020, 14, 122–132.
- McTaggart-Cowan, H.; Bentley, C.; Raymakers, A.; Metcalfe, R.; Hawley, P.; Peacock, S. Understanding cancer survivors’ reasons to medicate with cannabis: A qualitative study based on the theory of planned behavior. Cancer Med. 2020, 10, 396–404.
- Buchwald, D.; Winter, K.A.I.; Brønnum, D.; Melgaard, D.; Leutscher, P. Perception of Patients with Cancer Enquiring About Adjuvant Therapy with Cannabis Medicine for Palliation of Symptoms: An Interview Study among Danish Health Care Professionals. Palliat. Med. Rep. 2022, 3, 75–79.
- Buchwald, D.; Brønnum, D.; Melgaard, D.; Leutscher, P. Living with a Hope of Survival Is Challenged by a Lack of Clinical Evidence: An Interview Study among Cancer Patients Using Cannabis-Based Medicine. J. Palliat. Med. 2019, 23, 1090–1093.
- Gorzo, A.; Havasi, A.; Spînu, S.; Oprea, A.; Burz, C.; Sur, D. Practical Considerations for the Use of Cannabis in Cancer Pain Management -What a Medical Oncologist Should Know. J. Clin. Med. 2022, 11, 5036.
- Petrocellis, L.D.; Marzo, V.D. An introduction to the endocannabinoid system: From the early to the latest concepts. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 1–15.
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472.
- Salami, S.A.; Martinelli, F.; Giovino, A.; Bachari, A.; Arad, N.; Mantri, N. It Is Our Turn to Get Cannabis High: Put Cannabinoids in Food and Health Baskets. Molecules 2020, 25, 4036
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833.
- Maldonado, R.; Banos, J.E.; Cabanero, D. The endocannabinoid system and neuropathic pain. Pain 2016, 157, 23–32.
- Maayah, Z.H.; Takahara, S.; Ferdaoussi, M.; Dyck, J.R.B. The molecular mechanisms that underpin the biological benefits of fullspectrum cannabis extract in the treatment of neuropathic pain and inflammation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165771.
- Russo, E.B. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res. 2016, 1, 154–165.
- Rodrigues, R.S.; Lourenco, D.M.; Paulo, S.L. Cannabinoid Actions on Neural Stem Cells: Implications for Pathophysiology. Molecules 2019, 24, 1350.
- Stemmer, K.; Pfluger, P.T.; Tschop, M.H. CNS-targeting pharmacological interventions for the metabolic syndrome. J. Clin. Investig. 2019, 129, 4058–4071.
- Nahtigal, I.; Blake, A.; Florentinus-Mefailoski, A.; Hashemi, H.; Friedberg, J. The pharmacological properties of Cannabis. J. Pain Manag. 2016, 9, 481–491.
- Nielsen, S.; Picco, L.; Murnion, B.; Winters, B.; Matheson, J.; Graham, M.; Campbell, G.; Parvaresh, L.; Khor, K.; Betz-Stablein, B.; et al. Opioid-sparing effect of cannabinoids for analgesia: An updated systematic review and meta-analysis of preclinical and clinical studies. Neuropsychopharmacology 2022, 47, 1315–1330.
- Hojo, M.; Sudo, Y.; Ando, Y.; Minami, K.; Takada, M.; Matsubara, T.; Taniyama, K.; Sumikawa, K.; Uezono, Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: Electrophysiological and FRET assay analysis. J. Pharm. Sci. 2008, 108, 308–319.
- Babalonis, S.;Walsh, S.L. Therapeutic potential of opioid/cannabinoid combinations in humans: Review of the evidence. Eur. Neuropsychopharmacol. 2020, 36, 206–216.
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131.
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805.
- McPartland, J.M.; Russo, E.B. Non-phytocannabinoid constituents of cannabis and herbal synergy. In Handbook of Cannabis; Oxford Univeristy Press: Oxford, UK, 2014; Chapter 15; pp. 280–295.
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M. Multicenter, double-blind, randomized, placebo-controlled, parallel group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom Manag. 2010, 39, 167–179.
- Pertwee, R.G. GPR55: A new member of the cannabinoid receptor clan? Br. J. Pharmacol. 2007, 152, 984–986.
- Grotsch, K.; Fokin, V.V. Between Science and Big Business: Tapping Mary Jane’s Uncharted Potential. ACS Cent. Sci. 2022, 8, 156–168.
- Christino, L.; Bisogno, T.; Marzo, V.D. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29.
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain”, No Gain. Front. Plant Sci. 2018, 9, 969.
- Worth, T. Unpicking the entourage effect. Nature 2019, 527, 12–13.
- McPartland, J.M.; Russo, E.B. Non-phytocannabinoid constituents of cannabis and herbal synergy. In Handbook of Cannabis; Oxford Univeristy Press: Oxford, UK, 2014; Section 15.3; pp. 283–284.
- McPartland, J.M.; Russo, E.B. Non-phytocannabinoid constituents of cannabis and herbal synergy. In Handbook of Cannabis; Oxford Univeristy Press: Oxford, UK, 2014; Section 15.5; pp. 286–288.
- Andre, C.M.; Hausman, J.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19.
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246.
More
Information
Subjects:
Anesthesiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
764
Revisions:
2 times
(View History)
Update Date:
13 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




