Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adalberto Benavides-Mendoza | -- | 5848 | 2023-01-12 18:48:04 | | | |
| 2 | Peter Tang | Meta information modification | 5848 | 2023-01-13 03:27:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Garza-Alonso, C.A.; Olivares-Sáenz, E.; González-Morales, S.; Fuente, M.C.L.; Juárez-Maldonado, A.; González-Fuentes, J.A.; Tortella, G.; Valdés-Caballero, M.V.; Benavides-Mendoza, A. Strawberry Biostimulation. Encyclopedia. Available online: https://encyclopedia.pub/entry/40132 (accessed on 08 February 2026).
Garza-Alonso CA, Olivares-Sáenz E, González-Morales S, Fuente MCL, Juárez-Maldonado A, González-Fuentes JA, et al. Strawberry Biostimulation. Encyclopedia. Available at: https://encyclopedia.pub/entry/40132. Accessed February 08, 2026.
Garza-Alonso, Carlos Alberto, Emilio Olivares-Sáenz, Susana González-Morales, Marcelino Cabrera-De La Fuente, Antonio Juárez-Maldonado, José Antonio González-Fuentes, Gonzalo Tortella, Marin Virgilio Valdés-Caballero, Adalberto Benavides-Mendoza. "Strawberry Biostimulation" Encyclopedia, https://encyclopedia.pub/entry/40132 (accessed February 08, 2026).
Garza-Alonso, C.A., Olivares-Sáenz, E., González-Morales, S., Fuente, M.C.L., Juárez-Maldonado, A., González-Fuentes, J.A., Tortella, G., Valdés-Caballero, M.V., & Benavides-Mendoza, A. (2023, January 12). Strawberry Biostimulation. In Encyclopedia. https://encyclopedia.pub/entry/40132
Garza-Alonso, Carlos Alberto, et al. "Strawberry Biostimulation." Encyclopedia. Web. 12 January, 2023.
Copy Citation
Plant biostimulation consists of using or applying physical, chemical, or biological stimuli that trigger a response—called induction or elicitation—with a positive effect on crop growth, development, and quality. Biostimulation provides tolerance to biotic and abiotic stress, and more absorption and accumulation of nutrients, favoring the metabolism of the plants. The strawberry is a highly appreciated fruit for its high organoleptic and nutraceutical qualities since it is rich in phenolic compounds, vitamins, and minerals, in addition to being a product with high commercial value.
Fragaria
defense inducers
eustressors
elicitors
hormesis
plant stress
phytochemicals
nutraceutics
nutraceutical quality
1. Introduction
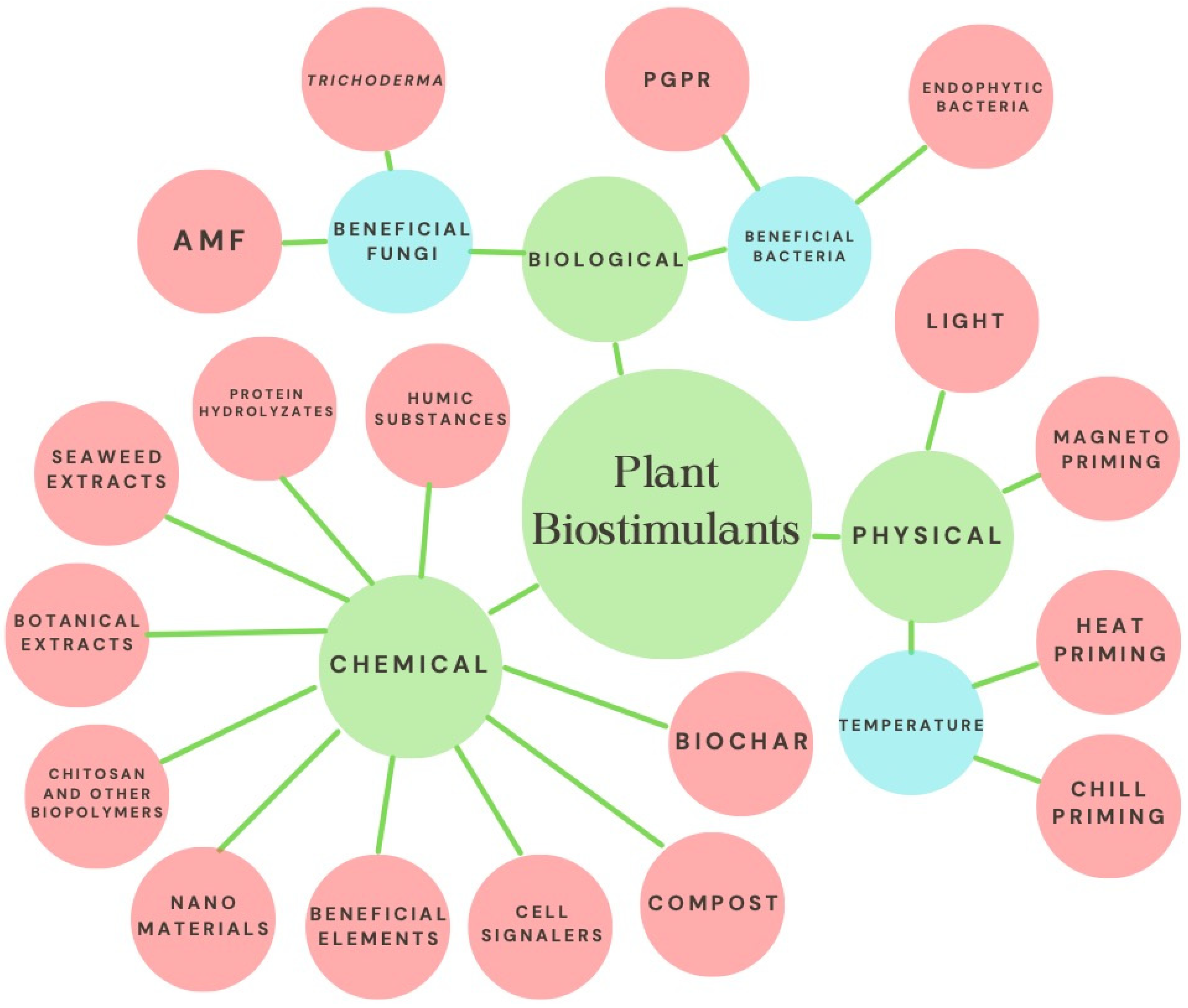
Biostimulation has gained relevance due to its positive effects on the growth and development of diverse crops. However, in the specific case of strawberries, there are currently no reports encompassing the various forms and techniques of application of biostimulants, as well as their mechanisms of action and positive effects on characteristics such as yield and nutraceutical quality of the fruits. In addition to the above, the constant increase in the population forces us to look for alternatives to achieve food security, since some projections estimate that food needs will be up to 70% higher by 2050 [1]. On the other hand, climate change has altered the conditions for agriculture, forcing growers to look for alternatives with new production systems and genotypes better adapted to increasing biotic and abiotic stresses [2]. The strawberry is a plant highly appreciated for its fruits of high organoleptic quality and significant commercial value; the worldwide harvested area exceeds 380,000 ha, with a production close to 9 million tons [3]. Plant biostimulation is a biological response known empirically since ancient times, but its definition is recent. Plant biostimulation has been defined as applying any substance or microorganism to promote nutritional efficiency, tolerance to abiotic stress, and obtain higher quality crops, regardless of nutrient content [4]. Another definition refers to any material that can promote growth by being applied in small amounts to plants [5]. One of the most accepted categorizations includes the following groups of biostimulants: humic substances (humic and fulvic acids), protein hydrolysates, seaweed-botanical extracts, chitosan and other biopolymers, beneficial elements (Si, Se, I, Ti), beneficial fungi (arbuscular mycorrhizal fungi, Trichoderma) and beneficial bacteria (plant growth-promoting rhizobacteria and endophytic bacteria) [4]. However, other materials or stimuli that are not categorized in the above list can induce biostimulation in plants; these include compost, biochar, nanomaterials, as well as the exogenous application of signalers (H2O2, H2S, NO), and physical stimuli such as light (LED, UV), magnetism and high-low temperature (Figure 1).
2. General Mechanism of Plant Biostimulation
2.1. Plant Cell Receptors
The first step in the biostimulation process is the reception of environmental stimuli. When biostimulant agents (physical, chemical, biological) interact with plant cells, the signal is perceived through various types of receptors or physiochemical changes in cell walls or membranes. The mechanisms of cellular reception to the stimulus perceived by biostimulants are not yet well known. However, they are likely related to the mechanism of perception of molecular damage by abiotic or biotic factors. The receptors are known as plant pattern-recognition receptors (PPRs) and are responsible for recognizing pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) [8]. One of the main groups of receptors is receptor-like cytoplasmic kinases (RLCKs), within which there are specific proteins that perceive different stimuli depending on their nature; one example is the chitin elicitor receptor kinase 1 (CERK1), which is responsible for the perception of chitin [9]. Another group of membrane receptors is the wall-associated kinases (WAKs), of which 26 genes related to Arabidopsis have been identified; these receptors perceive the stimuli to provide pathogen resistance, heavy-metal tolerance, and plant development [10]. Another critical group of receptors is the G Protein-Coupled Receptors (GPCRs), which perceive various extracellular stimuli and trigger signaling cascades to respond [11].
2.2. From Perception to Transduction and Signaling
Once specific receptors perceive the stimulus, transduction of the signals immediately occurs, with various molecules or ions playing an important role [12]. Mitogen-activated protein kinases (MAPKs) are an example of proteins responsible for initiating a cascade of signaling that ranges from the perception of the stimulus to the arrival of information to other sites of the cell [6]. Usually, the process begins with the mitogen-activated protein kinase kinase kinases (MAPKKKs), following downstream toward the mitogen-activated protein kinase kinases (MAPKKs) and finally to the MAPKs. Protein phosphorylation is a posttranslational modification (PTM) [13] that alters the cell medium's proteostasis (protein homeostasis). Proteostasis alteration is possibly recognized by cells and is partially responsible for inducing a biostimulation response in plants [14]. On the other hand, MAPKs can phosphorylate transcription factors that directly modify gene expression [6]. An essential element in signaling is Ca2+, which is a secondary messenger in plant cells. When the cell walls perceive a stimulus, the subsequent transduction response activates Ca2+ channels and the cytoplasmic Ca2+ (Ca2+cyt) concentration increases. The change in Ca2+ is detected by various intracellular receptors, among which calmodulin (CaMs), calmodulin-like proteins, calcium-dependent protein kinases (CDPKs), and calcineurin B-like proteins stand out [15]. On the other hand, the high concentration of Ca2+cyt induces Ca-binding proteins (CaBPs) production, modifying cells' proteostasis. Likewise, the increase in Ca2+cyt is fundamental for the phosphorylation of transcription factors by CDPKs [11]. Another compound that fulfills the role of a signaler is extracellular ATP (eATP), which is extruded from the cytoplasm to the apoplast when plants perceive some stimulus. This eATP is perceived by the membrane receptor called Does not Respond to Nucleotides 1 (DORN1), producing a response similar to that caused by DAMPs [16]. Some phytohormones, such as abscisic acid (ABA) and salicylic acid (SA), also play an important role in cell signaling. For example, when the membranes perceive some external stimulus, the cytoplasmic concentration of ABA increases, regulating genes related to resistance to salinity, drought, and cold stress [17]. Likewise, an elevation in the concentration of SA is detected by specific receptors, which favors the interaction with several transcription factors that modify the expression of genes mainly related to the defense system against biotic and abiotic stress [18]. On the other hand, when biostimulants first encounter cell walls and membranes, groups of important signalers arise. These signalers include reactive oxygen species (ROS), like H2O2, O2−, OH−; reactive nitrogen species (RNS), specifically NO and NO2; and reactive sulfur species (RNS), such as H2S, which can commonly be grouped together as reactive oxygen, hydrogen, and sulfur species (RONSS). One of the main biostimulation pathways is related to changes in the redox balance of cells when the RONSS:antioxidant ratio is increased in cells [19]. RONSS function as cell signalers due to their high reactivity and capacity to modify molecules by oxidation, nitrosation, nitration, or persulfidation. For example, ROS induces the oxidation of cysteine and methionine residues, which causes inactivation or changes in protein structures [20] (Figure 2).
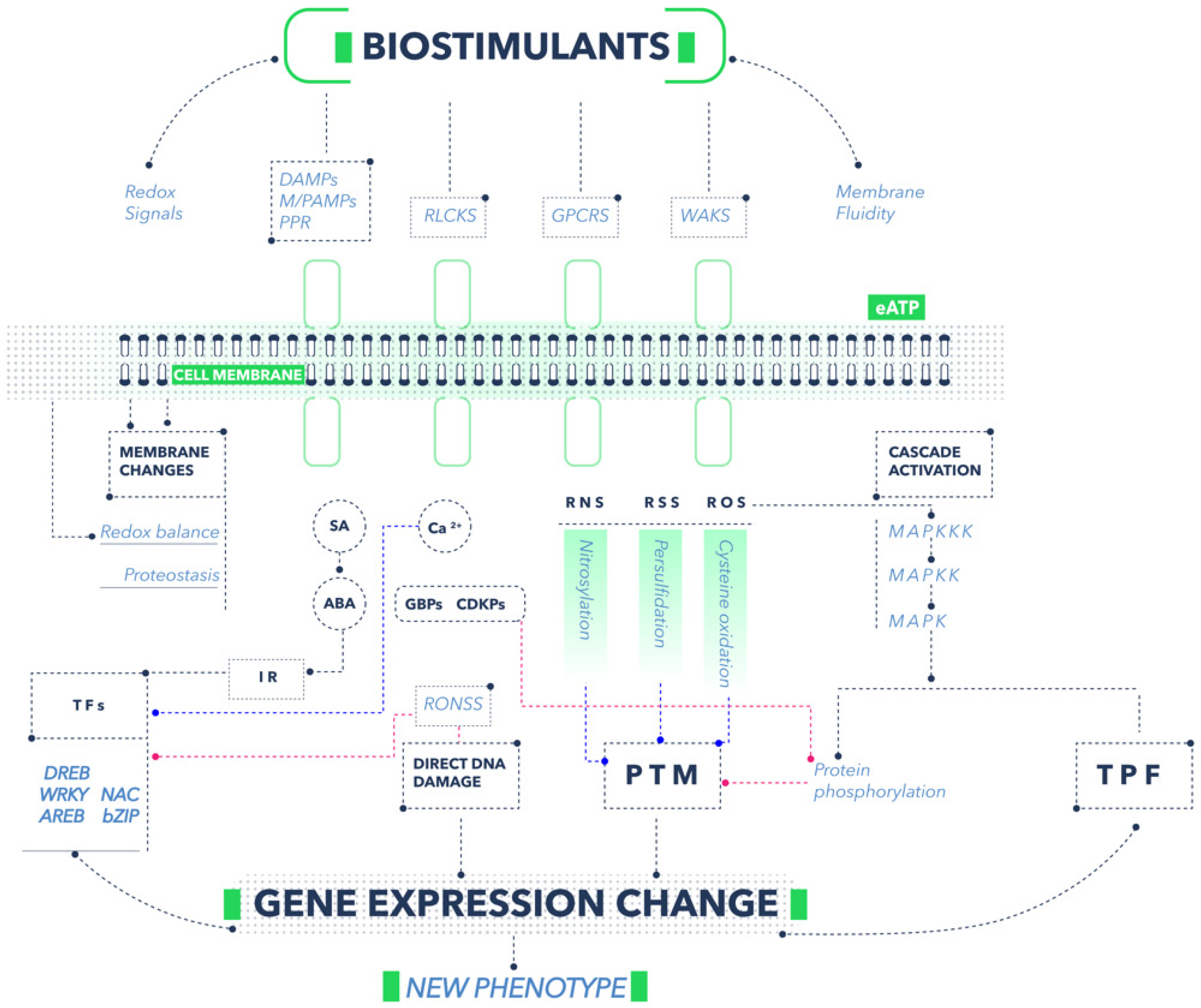
Figure 2. Mechanisms of action of biostimulants. The abbreviations used are defined in the text of this section. Figure prepared by the researchers.
Additionally, there is evidence that some ROS are necessary to activate MAPK signaling cascades [6]. Likewise, NO fulfills various roles of PTM through mechanisms such as metal nitrosylation, tyrosine nitration, and S-nitrosylation [21]. On the other hand, H2S causes the persulfidation of proteins and residues such as cysteine, causing changes in the proteome and gene expression [22]. Thanks to their physical characteristics, gasotransmitters such as NO and H2S can move quickly between organelles and through other cells, which increases their ability to induce plant transcriptional changes. In addition, all signalers are detected by other types of intracellular receptors and transcription factors, such as DREB, WRKY, AREB, NAC, and bZIP, thus modifying gene expression [23]. Signals can also travel directly to the nucleus of cells, causing changes in DNA and resulting in overexpression or repression of genes [24]. A final way in which plants respond to the stimuli of the environment is through changes in the fluidity and structure of membranes, which is like the observed effect when plants are subjected to stress due to salinity or drought [25]. Such membrane changes are perceived by putative sensors that subsequently modify gene expression [26]. Furthermore, some biostimulants have a large active surface per unit volume; examples are nanomaterials, zeolites, and biochar. The above materials can induce changes in plant behavior; this could be due to a physical interaction mechanism in the interphases of the material and cell walls or related to the considerable ion exchange capacity of the materials. The specific direct or indirect mechanisms by which the different categories of biostimulants positively affect the growth and development of plants, depending on their chemical, biochemical, biological, or physical nature, are described in the subsequent sections. As a result of all the previously mentioned mechanisms, a new phenotype better adapted to the environment is obtained, with greater tolerance to biotic and abiotic stress and better growth, development, and quality of harvestable products.
3. Use of Chemical and Biochemical Biostimulants in Strawberry Cropping
This group includes humic substances, protein hydrolysates, seaweed extracts, botanical extracts, chitosan, other biopolymers, beneficial elements, nanomaterials, compost, biochar, and cell signalers (H2O2, H2S, NO).
3.1. Humic Substances (HS)
Humic substances (HS) are organic compounds formed from plant or animal residues present in soils, which are degraded in a process known as humification resulting from the activity of microorganisms such as fungi and bacteria [27]. These substances represent approximately 25% of the total organic carbon present on the planet [28]. Depending on their characteristics, HS can be classified as humic acids (HA) and fulvic acids (FA), which differ mainly by their solubility, depending on the pH of the medium in which they are found [29]. The beneficial effects of HS on plants have been widely documented [30]. Part of the mechanisms of action is the ability to induce changes in the structure of the root system, promoting its growth and improving the assimilation of nutrients. On the other hand, HS can act as an antioxidant compound, favoring some oxidation-reduction reactions in soils, substrates, or plant cells [31]. It is also likely that plants recognize the disordered molecular structure of HS, being detected as DAMPs and triggering a cascade of signals, as explained in previous paragraphs. Likewise, HS can improve soil structure, increase cation-exchange capacity, promote P solubility, and improve nitrate assimilation [32]. Therefore, in recent years, HS has been considered a plant biostimulant [33], with positive effects on plant growth and development. Different impacts of HS have been reported in the case of strawberry cultivation, which varies depending on the nature of the HS, dose, and forms of application of the products. The main positive effects reported include variables related to vegetative growth and yield, such as fruit quality, mineral concentration, and antioxidant compounds. However, there is very little, or no information related to metabolic aspects such as photosynthesis, and few studies related to the postharvest life of the fruit and tolerance to pathogens.
3.2. Protein Hydrolysates (PHs)
Protein hydrolysates (PHs) are products that can be derived from animal origin (blood meal, leather byproducts, fish byproducts, and bird feathers) or vegetable origin (alfalfa hay, legume seeds, and other vegetables) [34]. Methods for producing PHs range from chemistry to thermal and enzymatic hydrolysis, depending on the source material [35]. The final content of free amino acids and other compounds will depend on the hydrolysis method, as some compounds are degraded during the process [36]. One of the main mechanisms of action of PHs depends on the high concentration of free amino acids and peptides, which function as signaling molecules, N sources, and metal-complexation or antioxidant metabolites [37]. The different peptides containing PHs can be recognized by plants through specific receptors, such as putative leucine-rich repeats (LRRs), triggering a cascade of signaling and transcriptional responses [35]. In addition to the above, some PHs also contains fatty acids, carbohydrates, phytohormones, and macro- and micronutrients, which fulfill their respective roles in plants [38]. On the other hand, PHs increase the activity of enzymes such as nitrate reductase (NR), nitrite reductase (NiR), and glutamine synthetase (GS); all of these are related to the assimilation of N in addition to promoting carbon metabolism, increasing the production of auxins and gibberellins, antioxidant enzymes, and photosynthetic pigments and secondary metabolites [34]. Furthermore, PH applications have been shown to stimulate flavonoid biosynthesis and the phenylpropanoid pathway [36]. Using PHs from various sources with various application forms has shown positive effects on strawberry cultivation. In most cases, PHs are reported to increase variables related to vegetative growth and, to a lesser extent, to antioxidant compounds, chlorophylls, and minerals in tissues. However, information on aspects of primary metabolism and postharvest life of fruits is very scarce.
3.3. Seaweed and Algal and Microalgal Extracts
Extracts of marine algae have gained importance in recent years due to the beneficial effects reported in various crops [39]. The main species producing these extracts are Ascophyllum nodosum, Sargassum spp., and Laminaria spp., among others [7]. The production of seaweed extracts is based on different methodologies but mainly involves subjecting the biomass to high temperatures and pressures and using alkaline solutions to ensure the extraction of the active compounds [40]. An abundance of phenolic compounds, as well as the presence of phytohormones such as gibberellins, could be found within the specific mechanisms of action of seaweed extracts [41]. One of the main compounds found in these extracts is alginic acid, which can be perceived by plants and trigger a positive response; in addition, this substance favors the chelation of minerals in the soil, increasing the assimilation and accumulation of nutrients in plants [38]. In general, the positive effects of extracts on crop growth and quality are partially explained by the regulation of the genes RD29A, RD22, SOS, CBF3, COR15A, and the increase in osmolytes, greater efficiency in water use, and increase in photosynthetic pigments and mineral concentration [39]. Furthermore, these extracts improve plants' enzymatic and nonenzymatic systems, providing greater tolerance to abiotic stress [42]. Seaweed extracts of several species with various forms of application have been reported in strawberry cultivation, highlighting some aspects of vegetative plant growth and fruit quality, mineral concentration, and enzymatic-nonenzymatic antioxidant systems. However, it is essential to have information related to transcriptomics and proteomics, the resistance of plants to pathogens, and the postharvest life of fruits.
3.4. Botanical Extracts
Botanical extracts are products generally derived from fresh plant tissues, especially from plants recognized for their high concentrations of bioactive compounds, minerals, phytohormones, and amino acids [43][44]. Several species have been used to produce extracts; an example is the plant Moringa oleifera, of which there are several reports on its positive effects on plants [45][46]. However, despite all the above, the group of botanical extracts has not yet been sufficiently studied as a biostimulant because such products are mainly used as pesticides [4]. The methods for elaborating botanical extracts use solvents such as water or different alcohols, which are mixed with the biomass to be later stirred, blended, and even applied with ultrasound techniques [46]. The specific mechanism of action of botanical extracts is not yet well known. However, it is related to the high availability of minerals, amino acids, bioactive compounds, and phytohormones, which fulfill specific functions such as promoting growth and vegetative development, improving the antioxidant system, and greater tolerance to biotic and abiotic stress, among others [47]. Several works have been reported using botanical extracts as biostimulants in strawberry cultivation. An experiment in the open field with soil conditions and foliar applications of M. oleifera extract at concentrations of 2, 4, and 6% increased the fresh and dry weight of plants, the number of leaves, plant height, SPAD, carbohydrates, and the concentration of N, P, K, Ca, Mg Fe, Mn, and Cu, as well as some characteristics of fruits, such as weight, firmness, TSS, Vit. C, anthocyanins, and total yield [48]. On the other hand, foliar applications of a mixture of three grass species, Lolium perenne L. (60%), Festuca spp. (20%), and Poa pratensis L. (20%) promotes root and shoot dry weight and chlorophyll concentration in strawberry plants grown under greenhouse conditions [49]. A similar experiment was carried out using the same botanical extract in the strawberry plant cv. Diamond, and foliar applications increased shoot and root dry weight, chlorophyll, and concentrations of succinic, malic, and citric acid in root tips, as well as concentrations of P, K, Mg, and Ca in different organs of the plant [50]. On the other hand, drench applications of a Pelargonium hortorum extract increased some parameters of the radicular system, such as root diameter and root volume, as well as the photosynthetic rate in strawberry plants cv. Duch [51].
3.5. Chitosan and Other Biopolymers
Biopolymers are compounds widely used in the pharmaceutical, cosmetic, textile, and food industries. The main ones are cellulose, collagen, alginate, chitin, and chitosan, which have the most significant applications in agriculture [52]. Chitosan is a biopolymer obtained through the chemical or enzymatic deacetylation of chitin, mainly from crustaceans or insects, where the result can be D-glucosamine and N-acetyl-D-glucosamine [53]. Deacetylation consists of replacing acetyl groups (CH3CO) with amino groups (NH2), where the degree of this process (reaction time and temperature) defines the final form of chitosan (D-glucosamine or N-acetyl-D-glucosamine) [53]. The multiple applications of chitosan are due to its biocompatibility, biodegradability, high absorption capacity, and nontoxicity [54]. In plants, chitosan is mainly used to improve the response against pathogens and resistance to abiotic factors and promote vegetative growth [52]. The primary mechanism of action of chitosan applications could be related to the octadecanoid pathway, which begins in the cell's chloroplast and ends in the production of response genes related to enzymes such as PAL and CAT, as well as other response mechanisms such as stomatal opening/closing [55]. Signals ranging from chitosan perception to transduction factors include NO, Ca2+, and phytohormones such as JA, SA, and ABA [56]. Currently, no specific receptors have been identified for chitosan. However, the first perception could be related to the difference in charges between the amino groups of chitosan (positive charge) and the cell membrane (negative charge) [55]. The forms of chitosan application in plants range from seed priming, drench, and leaf sprays, while beneficial effects range from increased biomass gain, more photosynthetic pigments, and antioxidant compounds [57].
3.6. Beneficial Elements
Beneficial elements are not considered essential for plants, but their presence or application positively affects growth and development parameters [58]. The most studied elements in this group are silicon (Si), selenium (Se), iodine (I), vanadium (V), cobalt (Co), and titanium (Ti) [59]. These elements can be considered biostimulants because they can promote plant growth and provide tolerance to stress through mechanisms such as strengthening cell walls, osmoregulation, synthesis of phytohormones, greater assimilation of essential elements, and reduction of transpiration, among others [4]. Si is the most beneficial element studied; several authors have considered it a biostimulant for plants [60]. Among the main functions of Si in plants is its ability to accumulate in cell walls, providing greater rigidity to tissues and reducing damage by organisms such as insects or microorganisms [61]. In addition, Si can reduce the absorption of ions such as Na+ and Cl− when plants are under saline stress conditions [62] and increase the production of antioxidant compounds in the face of various biotic and abiotic stress [60]. On the other hand, Se promotes the quenching of ROS, regulates enzymatic and nonenzymatic antioxidants, and improves the photosynthesis and homeostasis of elements in plants [63]. Likewise, iodine has been an element of interest in recent years, where its functions are mostly related to the increase in antioxidant compounds when this element is at low concentrations; however, high concentrations produce phytotoxicity in cells [64]. Finally, V, Co, and Ti are the elements less studied. However, it has been reported that these elements promote the assimilation of other nutrients, are involved in redox reactions, and stimulate enzymatic activity and photosynthesis [58][59][65].. These elements have been applied in strawberry cultivation, obtaining favorable responses in various groups of variables, such as agronomic (growth and development), fruit quality (size, weight, firmness, TSS, anthocyanins), the antioxidant system of the plant, aspects related to photosynthesis (photosynthetic rate, stomatal conductance), and the concentration of minerals in the tissues. However, further studies related to the tolerance against pathogens and postharvest quality of the fruits are needed.
3.7. Metal, Carbon, Zeolite, and Chitosan Nanomaterials
Nanotechnology has gained importance in recent years due to its applications in industry, medicine, and agriculture, with uses such as pesticides or fertilizers found in the latter [66]. Nanomaterials (NMs) are considered products of a size between 1–100 nm, ranging from metals (ZnO, FeO3, SiO), carbon (carbon and graphene nanotubes), zeolite, and nanochitosan [67]. Recently, nanomaterials (NMs) have been proposed as plant biostimulants [5]. The positive effects of NMs in plants can be explained by the specific mechanisms by which NMs induce biostimulation in plants, which can be encompassed in two main phases: The first phase is due to the initial contact of the material with the cell walls or membranes, where interactions occur due to the difference in corona composition, surface charges, size, shape, and hydrophobicity of the NMs. NMs cause damage or modifications in the structures of integral proteins, cell walls, or membranes. These, in turn, can produce cascades of signalers (signaling metabolites, redox balance alterations, membrane potential, and transcriptional and posttranslational modifications) inside or between cells and trigger a biostimulation response [5][68]. Once NMs cross the cell membrane through existing pores, inducing new pores or mechanisms such as diffusion or endocytosis, a series of similar reactions usually occur between NMs and organelles, such as the nucleus, mitochondria, or chloroplasts [69]. In the second phase, once the NMs are internalized and transported through plant cells, the biotransformation of the NM core into specific ions (e.g., Zn, Fe, Cu, Si) occurs. The ions will be available in the cytoplasm of the cells and can fulfill specific roles in the metabolism of plants [70].
3.8. Compost
The decomposition of organic matter forms composts with the help of soil microorganisms. The primary sources of organic matter come from plant wastes or manure of animal species used in livestock, such as birds, cows, pigs, and horses [71]. In addition to the conventional form of composting, it is possible to use worms to obtain a product known as vermicompost [72]. Although some authors do not consider compost as a biostimulant, the applications of these products to soil or any other culture medium have shown some beneficial effects of other biostimulants [73]. Due to the limited study of this category as a biostimulant, the mechanisms of action are also unknown. However, most of them are related to indirect mechanisms, such as the increase in the populations of beneficial microorganisms, buffer for electrons and protons in the soil volume, increased moisture retention, and increased fertility, among others [73][74]. The composts contain a high concentration of humic substances that fulfill the roles previously explained, in addition to having high amounts of beneficial fungi and bacteria with biostimulant potential. Although the primary way of applying compost is direct as a mixture with the soil or substrates, it is also possible to elaborate extracts known as “compost tea”, which can be applied in a drench or foliar [75]. Composts from various sources have been used at different levels and forms in strawberry cultivation. Most studies report beneficial effects on vegetative growth, yield, quality of fruits, and the concentration of minerals in leaves and fruits. However, there is a lack of information on variables such as photosynthesis, antioxidant compounds, postharvest quality of fruits, and resistance of plants to pathogens.
3.9. Biochar
Biochar, also called biocarbon or vegetable carbon, is a product obtained from transforming organic matter with high temperatures and the absence of oxygen, a process known as pyrolysis [76]. The composition and physicochemical characteristics vary depending on the origin of organic matter and pyrolysis temperature. Biochar is a compound with a porosity of up to 124 m2 g−1 [77], rich in N, and with high concentrations of humic substances [78]. Like compost, biochar is not commonly studied as a biostimulant; however, some of its effects on soil characteristics promote plant growth, development, and quality [79]. Among the indirect mechanisms by which biochar could be considered a biostimulant are its abilities to improve soil structure by increasing porosity that facilitates the movement of air, water, and nutrients in the soil [80]. In addition to the aforementioned effects, biochar can increase soil pH, promote cation-exchange capacity, and increase efficiency in using N, among others [74]. The application of biochar to the soil favors root colonization and the activity of plant growth-promoting rhizobacteria (PGPR) [81]. One of the main effects of biochar applications in strawberry cultivation is the capacity to reduce the incidence of diseases in leaves and fruits. A study reported that wood-biochar and greenhouse-waste biochar (mixed with soil at 1–3%) mediate the systemic response of strawberry plants against Botrytis cinerea, Colletotrichum acutatum, and Podosphaera apahanis, promoting the overexpression of defense genes such as FaPR1, Faolp2, Falox, and FaWRKY1 [82]. On the other hand, a recent investigation reported that biochar application mixed with peat substrate had a positive effect on the resistance of strawberry fruits against Botrytis cinerea, which was attributed to changes in the microbial community of the substrate [83]. Biochar application (1% in peat substrate) promotes fresh and dry weight and a lower susceptibility to the fungal pathogen Botrytis cinerea on both leaves and fruits of strawberry plants [84]. On the other hand, animal-bone biochar (130 kg ha−1) and plant-based biochar (1 ton ha−1) improve the number of fruits and the total yield of strawberries grown in soil under open field conditions [85].
3.10. H2O2, NO, H2S, H2, CH4, and CO
Cell signalers play a key role in the biostimulant response of plants. In recent years, the exogenous application of these compounds has been studied due to the positive effects observed in various plant species [86]. In some cases, it is possible to directly apply the molecule of interest (such as H2O2); however, in the case of gasotransmitters, precursor compounds must be used, such as sodium nitroprusside (SNP; source of NO) and NaHS (source of H2S) [87]. All these compounds are applied in very low doses since high concentrations could cause damage to plants. The primary responses are related to the increase in the activity of antioxidant enzymes and the production of nonenzymatic antioxidant compounds to maintain redox balance [88]. Some exogenous applications of signalers have been reported in strawberry plants, with greater emphasis given to H2O2, NO, and H2S and the response of enzymatic and nonenzymatic antioxidant compounds, vegetative growth, and fruit quality.
4. Use of Biological Biostimulants in Strawberry Cropping
Biological biostimulants, also known as biopreparations or bioformulations, are products characterized as containing some living organisms, usually microorganisms such as bacteria and fungi, as the main active ingredient.
4.1. Beneficial Bacteria
4.1.1. PGPR
The plant growth-promoting rhizobacteria (PGPR) group includes multiple species, including the genera Bacillus, Pseudomonas, Azospirillum, Rhizobium, and Streptomyces stand out [89]. In the market, it is possible to find commercial formulations with one or several species of bacteria combined, where applications have shown positive effects on crop growth and development [90]. The mechanisms of action of PGPR in plants can be direct or indirect. Among the direct mechanisms are the production of phytohormones such as auxins, indole acetic acid, gibberellins, and cytokinins, which regulate the growth and development of plants [7]. Additionally, some species of PGPR can produce volatile compounds that promote plant growth [91] and increase tolerance to various types of stress through the induction of the production of antioxidant enzymes in plants, modulation of membrane integrity, and accumulation of osmolytes [81]. In contrast, indirect mechanisms are the biological fixation of N, solubilization of P and other elements in soils, and production of metabolites, among others [92]. For products containing soil-colonizing bacteria, the application forms must be carried out directly to the root zone, either in drench, direct mixing with the soil or substrate, or root dipping, before transplanting to the final place [91].
4.1.2. Endophytic Bacteria
Endophytic bacteria are characterized by colonizing the internal tissues of plants and crossing the root epidermis to reach the vascular bundles, through which they can reach the stems, leaves, flowers, and fruits [90]. Most endophytic species include Bacillus, Pseudomonas, Azospirillum, Rhizobium, and Streptomyces [89]. The mechanisms of action of this group of microorganisms are like those mentioned in the section PGPR, to which are added: the increase of cellulose, providing greater resistance to the attack of herbivores; reduction of toxicity by heavy metals through extracellular precipitation, sequestration or biotransformation; and modifications in gene expression to increase defense by pathogens [93]. On the other hand, one of the main characteristics of endophytic bacteria is the production of siderophores, which function as chelating agents of Fe, promoting the assimilation of this element by the roots [94]..
4.2. Beneficial Fungi
4.2.1. Arbuscular Mycorrhizal Fungi (AMF)
Arbuscular mycorrhizal fungi (AMF) are different species of fungi characterized by a symbiotic association with plant roots [95]. The main species of AMF are Rhizophagus intraradices (formerly known as Glomus intraradices), Funneliformis mosseae (formerly known as Glomus mosseae), and some species of the genus Gigaspora [96]. One of the main characteristics that identify AMF is the ability to form an extension of up to 40 times the root system of plants, exploring a greater volume of soil [95]. This functional root surface expansion explains the main mechanisms of action by which AMF are considered biostimulants since they allow an increase in the absorption of water and nutrients, produce P solubilizing compounds in the soil, alter the architecture of the root, produce antioxidant compounds and induce signaling phytohormones such as ABA [38]. In addition, AMF provides plants with greater resistance to abiotic stress—such as drought, salinity, nutritional deficiencies, heavy metals, and changes in pH—due to the production of ascorbic acid, phenolic compounds, flavonoids, and carotenoids when the roots perceive the stimulus caused by AMF [96].
4.2.2. Trichoderma
Trichoderma is a genus of beneficial fungi for plants that comprise more than 200 species; Trichoderma harzianum is the most studied [97]. These fungi are characterized by their usual endophytic growth habit, penetrating through the roots of plants [98]. Therefore, plants perceive the stimulus by the spores or mycelia of the fungus, obtaining a response similar to the microorganisms. Among Trichoderma's primary mechanisms of action is the modulation of hormonal signaling by ABA, ET, JA, and IAA, in addition to favoring the activity of MAPK cascades [97]. On the other hand, inoculation with Trichoderma increases the assimilation of elements such as P, Mg, Zn, Fe, and B [34]. There are also reports where the absorption and efficiency in using N were increased [98]. On the other hand, Trichoderma can produce antioxidant compounds such as glucosinolates and phytoalexins, which allow for counteracting the attack of other phytopathogenic microorganisms [99]. Additionally, some reports indicate that Trichoderma increases the populations of some beneficial bacteria in soils [100]. Colonization with Trichoderma also induces changes in the plant proteome, modifying the synthesis of proteins involved in essential processes such as carbohydrate metabolism and photosynthesis [97].
5. Use of Physical Biostimulants in Strawberry Cropping
This group includes supplementary applications of light (mainly through LEDs), priming with extreme temperatures (high or low), and treatments with magnetism.
5.1. Biostimulation and Priming Using UV and Visible Light
Supplementation with artificial light, either visible or UV light, has been shown to have positive effects on plant growth and development [101]. In the first instance, visible light supplementation, mainly within the photosynthetically active radiation range (PAR: 400–700 nm), increases the photosynthetic activity of plants [102], resulting in more significant dry matter gain and crop yields. However, another mechanism is the ability to stimulate plants, induce morphological and anatomical changes, and regulate some developmental processes, such as flowering [103]. Plants have specific receptors for different wavelengths, including phytochromes (red/far red light, 600–750 nm), cryptochromes (blue, 350–500 nm), phototropins, F-box-containing flavin-binding proteins (blue/UV-A, 320–500 nm), and UVR8 (UV-B, 280–320 nm) [104]. Once these receptors perceive a light stimulus, signal transduction is carried out mainly through ROS [105] and hormonal signalers such as IAA, brassinosteroids, and ethylene [106][107]. Once TFs detect the signals, the changes in gene expression are like those reported for other groups of biostimulants. Some studies have shown the positive effects of different types of supplementary light on strawberry cultivation. Due to the nature of this biostimulant method, most research has focused on studying some photosynthetic parameters (e.g., stomatal conductance, CO2 assimilation, photosynthetic rate) and vegetative growth and fruit quality. Information on antioxidant compounds, pathogen resistance, and the postharvest life of fruits is still scarce.
5.2. Biostimulation and Priming Using Heat Shock and Chill Priming
Plants have various mechanisms to respond to temperature changes in the air or rhizosphere. This category of biostimulation consists of subjecting plants for a certain time to high or low temperatures, without them becoming lethal, which triggers a response to achieve acclimatization. Some of the thermo-sensors identified in plants are glutamate receptor-like (GKR) and cyclic nucleotide-gated channels (CNGCs) [108]; however, plants also use some of their photoreceptors, such as phytochromes and phototropins, to perceive stimuli by temperature [109] and begin the transduction of signals, mainly through signaling by Ca2+cyt, H2O2, and NO [110]. These signalers reach the heat shock transcription factors (HSFs), which have been identified as at least 20 members, from which the overexpression of the HSP90 and HSP70 genes occurs [111]. These genes produce heat shock proteins (HSPs), which are proteins that reduce molecular damage caused by temperature extremes [112]. In an experiment carried out in strawberry fruits subjected to a temperature of 45 °C for 3.5 h, an increment was found in the activity of the enzymes chitinase (CHI), β-1,3-glucanase, PAL, SOD, CAT, and APX, providing resistance against the fungus B. cinerea [113]. In addition, Widiastuti et al. [114] performed root dipping of strawberry seedlings in water at different temperatures (40, 45, and 50 °C) for 20 s, as well as immersion of the basal leaf in water at 50 °C for 20 s. In both cases, they found overexpression of the CHI2-1 gene, the precursor of the CHI enzyme. They also reported an increase in the concentration of salicylic acid (SA) in leaves. All the above resulted in decreased incidence of the fungus Colletotrichum gloeosporioides, which causes strawberry crown rots. In another work carried out by Brown et al. [115], strawberry roots were placed in a water bath at 37 °C for 1 h, resulting in the overexpression of genes related to the synthesis of heat shock proteins (HSP), such as HSP90 and HSP70, which would mean a greater tolerance to heat shock stress in strawberry plants. Kesici et al. [116] placed strawberry plants in growth chambers under different high-temperature treatments (35, 40, 45, and 50 °C) for 24 h. Also, they found overexpression of the HSP90, HSP70, and small heat shock protein (sHSPS) genes, seen as an increase in plant soluble protein.
5.3. Magnetopriming
Magnetopriming consists of subjecting seeds or other plant organs to a magnetic field for a specific time to produce changes in metabolism [117]. The mechanisms by which magnetic fields act in plants are not yet well known. However, it is most likely that they are related to changes in the electrical charges of cellular components, producing reorganizations of the various structures [118]. Likewise, magnetopriming increases the production of ROS such as H2O2 and O2− [119], favoring signaling cascades in plants. On the other hand, it has been reported that magnetism induces the production of enzymatic and nonenzymatic antioxidant compounds, providing greater tolerance to different abiotic stresses, such as saline stress [120]. Therefore, magnetopriming can be considered a form of biostimulation since numerous works have reported positive effects on plants, such as more significant vegetative growth, increased photosynthesis, and favored germination [121]. Currently, there are no reports on the use of magnetism for the biostimulation of strawberry plants.
As a general summary, Figure 3 presents the main ways of applying biostimulants in strawberry plants and the increased parameters of interest in this crop.
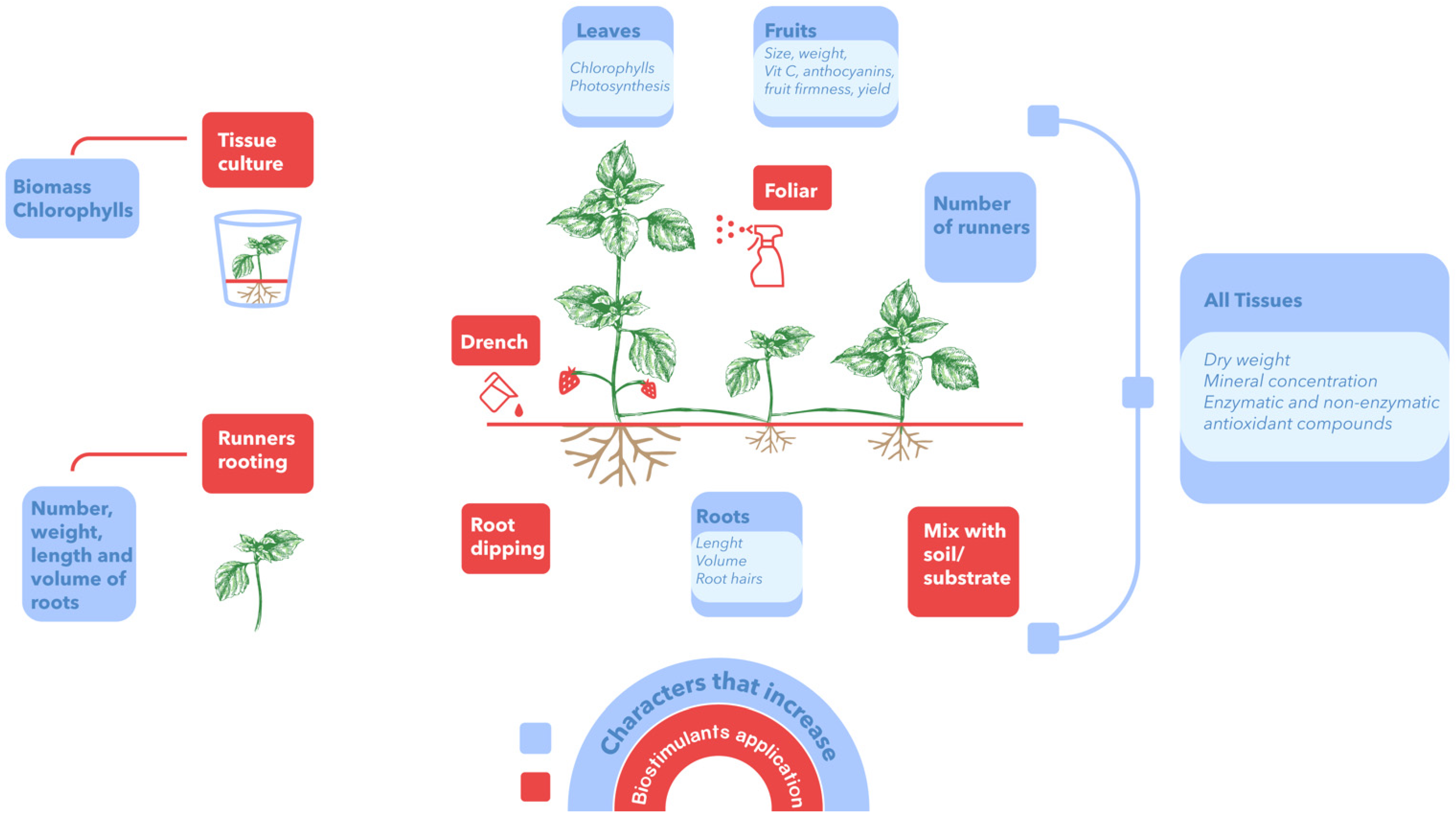
Figure 3. Forms of application of biostimulants and main effects on strawberry plants.
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501.
- Clapp, J.; Newell, P.; Brent, Z.W. The global political economy of climate change, agriculture and food systems. J. Peasant Stud. 2018, 45, 80–88.
- FAO Food and Agriculture Data. Available online: https://www.fao.org/faostat/en/#data (accessed on 20 October 2022).
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14.
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162.
- Liu, Y.; He, C. A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol. 2017, 11, 192–204.
- González-Morales, S.; Solís-Gaona, S.; Valdés-Caballero, M.V.; Juárez-Maldonado, A.; Loredo-Treviño, A.; Benavides-Mendoza, A. Transcriptomics of Biostimulation of Plants Under Abiotic Stress. Front. Genet. 2021, 12, 583888.
- Zipfel, C. Plant pattern-recognition receptors. Trends Immunol. 2014, 35, 345–351.
- Liang, X.; Zhou, J.M. Receptor-Like Cytoplasmic Kinases: Central Players in Plant Receptor Kinase-Mediated Signaling. Annu. Rev. Plant Biol. 2018, 69, 267–299.
- Kanneganti, V.; Gupta, A.K. Wall associated kinases from plants—An overview. Physiol. Mol. Biol. Plants 2008, 14, 109–118.
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85.
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for plant growth and mitigation of abiotic stresses: A metabolomics perspective. Metabolites 2020, 10, 505.
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK machinery in plants. Plant Signal. Behav. 2010, 5, 1370–1378.
- Heinemann, B.; Künzler, P.; Eubel, H.; Braun, H.P.; Hildebrandt, T.M. Estimating the number of protein molecules in a plant cell: Protein and amino acid homeostasis during drought. Plant Physiol. 2021, 185, 385–404.
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440.
- Cao, Y.; Tanaka, K.; Nguyen, C.T.; Stacey, G. Extracellular ATP is a central signaling molecule in plant stress responses. Curr. Opin. Plant Biol. 2014, 20, 82–87.
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571.
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regul. 2015, 76, 25–40.
- Kapoor, D.; Sharma, R.; Handa, N.; Kaur, H.; Rattan, A.; Yadav, P.; Gautam, V.; Kaur, R.; Bhardwaj, R. Redox homeostasis in plants under abiotic stress: Role of electron carriers, energy metabolism mediators and proteinaceous thiols. Front. Environ. Sci. 2015, 3, 13.
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236.
- Astier, J.; Lindermayr, C. Nitric oxide-dependent posttranslational modification in plants: An update. Int. J. Mol. Sci. 2012, 13, 15193–15208.
- González-Morales, S.; López-Sánchez, R.C.; Juárez-Maldonado, A.; Robledo-Olivo, A.; Benavides-Mendoza, A. A Transcriptomic and Proteomic View of Hydrogen Sulfide Signaling in Plant Abiotic Stress. In Plant in Challenging Environments; Gupta, D., Palma, J.M., Corpas, F.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 161–186. ISBN 9783030736774.
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription factors and plants response to drought stress: Current understanding and future directions. Front. Plant Sci. 2016, 7, 1029.
- Li, X.; Gu, Y. Structural and functional insight into the nuclear pore complex and nuclear transport receptors in plant stress signaling. Curr. Opin. Plant Biol. 2020, 58, 60–68.
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021, 171, 653–676.
- Hayes, S.; Schachtschabel, J.; Mishkind, M.; Munnik, T.; Arisz, S.A. Hot topic: Thermosensing in plants. Plant Cell Environ. 2021, 44, 2018–2033.
- Muscolo, A.; Sidari, M.; Nardi, S. Humic substance: Relationship between structure and activity. deeper information suggests univocal findings. J. Geochem. Explor. 2013, 129, 57–63.
- Weber, J.; Chen, Y.; Jamroz, E.; Miano, T. Preface: Humic substances in the environment. J. Soils Sediments 2018, 18, 2665–2667.
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface: From environmental aspects to molecular factors. Plant Signal. Behav. 2010, 5, 635–643.
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Timothy, R. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89.
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.; Sander, M. Antioxidant Properties of Humic Substances. Environ. Sci. Technol. 2012, 46, 4916–4925.
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 871, 1655.
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27.
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715.
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for production, characterization and application of protein-based biostimulants in agriculture: A review. Chil. J. Agric. Res. 2020, 80, 274–289.
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202.
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23.
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41.
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48.
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed: An overview. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2020; pp. 31–55. ISBN 9781119357193.
- Al-Juthery, H.W.A.; Abbas Drebee, H.; Al-Khafaji, B.M.K.; Hadi, R.F. Plant Biostimulants, Seaweeds Extract as a Model (Article Review). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Al-Qadisiyah, Iraq, 31 May–1 June 2020; Volume 553.
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and beneficial effect on soil-plant systems. Plants 2020, 9, 359.
- Kocira, S.; Szparaga, A.; Krawczuk, A.; Bartoš, P.; Zaguła, G.; Plawgo, M.; Černý, P. Plant material as a novel tool in designing and formulating modern biostimulants—Analysis of botanical extract from Linum usitatissimum L. Materials 2021, 14, 6661.
- Szparaga, A.; Kocira, S.; Kapusta, I. Identification of a biostimulating potential of an organic biomaterial based on the botanical extract from arctium lappa l. Roots. Materials 2021, 14, 4920.
- Arif, Y.; Bajguz, A.; Hayat, S. Moringa oleifera Extract as a Natural Plant Biostimulant. J. Plant Growth Regul. 2022, 1–16.
- Godlewska, K.; Ronga, D.; Michalak, I. Plant extracts-importance in sustainable agriculture. Ital. J. Agron. 2021, 16, 1851.
- Hayat, S.; Ahmad, H.; Ali, M.; Hayat, K.; Khan, M.A.; Cheng, Z. Aqueous garlic extract as a plant biostimulant enhances physiology, improves crop quality and metabolite abundance, and primes the defense responses of receiver plants. Appl. Sci. 2018, 8, 1505.
- Ismail, S.A.A.; Ganzour, S.K. Efficiency of foliar spraying with moringa leaves extract and potassium nitrate on yield and quality of strawberry in sandy soil. Int. J. Agric. Stat. Sci. 2021, 17, 383–398.
- Pestana, M.; Domingos, I.; Gama, F.; Dandlen, S.; Miguel, M.; Castro Pinto, J.; de Varennes, A.; Correia, P.J. Strawberry recovers from iron chlorosis after foliar application of a grass-clipping extract. J. Plant Nutr. Soil Sci. 2011, 174, 473–479.
- Saavedra, T.; Gama, F.; Correia, P.J.; Da Silva, J.P.; Miguel, M.G.; de Varennes, A.; Pestana, M. A novel plant extract as a biostimulant to recover strawberry plants from iron chlorosis. J. Plant Nutr. 2020, 43, 2054–2066.
- Dong, C.; Wang, G.; Du, M.; Niu, C.; Zhang, P.; Zhang, X.; Ma, D.; Ma, F.; Bao, Z. Biostimulants promote plant vigor of tomato and strawberry after transplanting. Sci. Hortic. 2020, 267, 9355.
- Palacio-Márquez, A.; Ramírez-Estrada, C.A.; Sánchez, E.; Ojeda-Barrios, D.L.; Chávez-Mendoza, C.; Sida-Arreola, J.P.; Preciado-Rangel, P. Use of biostimulant compounds in agriculture: Chitosan as a sustainable option for plant development. Not. Sci. Biol. 2022, 14, 11124.
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819.
- Stasinska, M.; Hawrylak-Nowak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecule 2022, 27, 2801.
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65.
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211.
- González-García, Y.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Impact of chitosan and chitosan-based nanoparticles on plants growth and development. In Role of Chitosan and Chitosan-Bases Nanomaterials in Plant Sciences; Kumar, S., Madihally, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 255–272. ISBN 9780323853910.
- Pilon-Smits, E.A.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274.
- Vatansever, R.; Ozyigit, I.I.; Filiz, E. Essential and Beneficial Trace Elements in Plants, and Their Transport in Roots: A Review. Appl. Biochem. Biotechnol. 2017, 181, 464–482.
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81.
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411.
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Lee, I.J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017, 8, 510.
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.A.H.; Dhankher, O.P.; Tripathi, R.D. Understanding selenium metabolism in plants and its role as a beneficial element. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1937–1958.
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26.
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a beneficial element for crop production. Front. Plant Sci. 2017, 8, 597.
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778.
- Hossain, A.; Skalicky, M.; Brestic, M.; Mahari, S.; Kerry, R.G.; Maitra, S.; Sarkar, S.; Saha, S.; Bhadra, P.; Popov, M.; et al. Application of Nanomaterials to Ensure Quality and Nutritional Safety of Food. J. Nanomater. 2021, 2021, 9336082.
- Benavides-Mendoza, A.; Gonzalez-Moscoso, M.; Ojeda-Barrios, D.L.; Fuentes-Lara, L.O. Biostimulation and Toxicity: Two Levels of Action of Nanomaterials in Plants. In Nanotechnology in Plant Growth Promotion and Protection: Recent Advances and Impacts; Ingle, A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2021; pp. 283–303. ISBN 9781119745853.
- González-Morales, S.; Cárdenas-Atayde, P.A.; Garza-Alonso, C.A.; Robledo-Olivo, A.; Benavides-Mendoza, A. Plant Biostimulation with Nanomaterials: A Physiological and Molecular Standpoint Susana. In Inorganic Nanopesticides and Nanofertilizers; Fraceto, L.F., Pereira de Carvalho, H.W., De Lima, R., Ghoshal, S., Santaella, C., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2022; pp. 153–185. ISBN 9783030941543.
- Juárez-Maldonado, A.; Tortella, G.; Rubilar, O.; Fincheira, P.; Benavides-Mendoza, A. Biostimulation and toxicity: The magnitude of the impact of nanomaterials in microorganisms and plants. J. Adv. Res. 2021, 31, 113–126.
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting parameters and compost quality: A literature review. Org. Agric. 2018, 8, 141–158.
- Ali, U.; Sajid, N.; Khalid, A.; Riaz, L.; Rabbani, M.; Syed, J.; Malik, R. A Review on Vermicomposting of Organic Wastes Usman. Environ. Prog. Sustain. Energy 2015, 34, 1050–1062.
- Martínez-Blanco, J.; Lazcano, C.; Christensen, T.H.; Muñoz, P.; Rieradevall, J.; Møller, J.; Antón, A.; Boldrin, A. Compost benefits for agriculture evaluated by life cycle assessment. A review. Agron. Sustain. Dev. 2013, 33, 721–732.
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170.
- Islam, M.K.; Yaseen, T.; Traversa, A.; Ben Kheder, M.; Brunetti, G.; Cocozza, C. Effects of the main extraction parameters on chemical and microbial characteristics of compost tea. Waste Manag. 2016, 52, 62–68.
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490.
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215.
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18.
- Palansooriya, K.N.; Ok, Y.S.; Awad, Y.M.; Lee, S.S.; Sung, J.K.; Koutsospyros, A.; Moon, D.H. Impacts of biochar application on upland agriculture: A review. J. Environ. Manag. 2019, 234, 52–64.
- Wang, D.; Jiang, P.; Zhang, H.; Yuan, W. Biochar production and applications in agro and forestry systems: A review. Sci. Total Environ. 2020, 723, 137775.
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 871, 1473.
- Harel, Y.M.; Kolton, M.; Elad, Y.; Dalia, R.-D.; Cytryn, E.; Ezra, D.; Borenstein, M.; Shulchani, R.; Graber, E.R. Induced systemic resistance in strawberry (Fragaria × ananassa) to powdery mildew using various control agents. IOBC/wprs Bull 2011, 71, 47–51.
- De Tender, C.; Vandecasteele, B.; Verstraeten, B.; Ommeslag, S.; Kyndt, T.; Debode, J. Biochar-Enhanced Resistance to Botrytis cinerea in Strawberry Fruits (But Not Leaves) Is Associated with Changes in the Rhizosphere Microbiome. Front. Plant Sci. 2021, 12, 479.
- De Tender, C.A.; Debode, J.; Vandecasteele, B.; D’Hose, T.; Cremelie, P.; Haegeman, A.; Ruttink, T.; Dawyndt, P.; Maes, M. Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar. Appl. Soil Ecol. 2016, 107, 1–12.
- Koron, D.; Lavrič, L.; Someus, E. Comparison of animal bone biochar and plant-based biochar in strawberry production. Acta Hortic. 2018, 1217, 313–315.
- Fang, L.; Ju, W.; Yang, C.; Jin, X.; Liu, D.; Li, M.; Yu, J.; Zhao, W.; Zhang, C. Exogenous application of signaling molecules to enhance the resistance of legume-rhizobium symbiosis in Pb/Cd-contaminated soils. Environ. Pollut. 2020, 265, 114744.
- Beavers, A.; Koether, M.; McElroy, T.; Greipsson, S. Effects of exogenous application of plant growth regulators (SNP and GA3) on phytoextraction by switchgrass (Panicum virgatum L.) grown in lead (Pb) contaminated soil. Sustainability 2021, 13, 10866.
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270.
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285.
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856.
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134.
- Kumari, B.; Mallick, M.A.; Solanki, M.K.; Solanki, A.C. Plant Growth-Promoting Rhizobacteria (PGPR): Modern Prospects for Sustainable Agriculture. In Plant Health Under Biotic Stress; Ansario, R., Mahmood, I., Eds.; Springer: Singapore, 2019; pp. 107–127. ISBN 9789811360398.
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732.
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49.
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inocula. Agronomy 2020, 10, 108.
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108.
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123.
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-based biostimulants optimize N use efficiency and stimulate growth of leafy vegetables in greenhouse intensive cropping systems? Agronomy 2020, 10, 121.
- Formisano, L.; Miras-Moreno, B.; Ciriello, M.; El-Nakhel, C.; Corrado, G.; Lucini, L.; Colla, G.; Rouphael, Y. Trichoderma and phosphite elicited distinctive secondary metabolite signatures in zucchini squash plants. Agronomy 2021, 11, 1205.
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743.
- Smith, B.J.; Rezazadeh, A.; Stafne, E.T.; Sakhanokho, H.F. Effect of Light-emitting Diodes, Ultraviolet-B, and Fluorescent Supplemental Greenhouse Lights on Strawberry Plant Growth and Response to Infection by the Anthracnose Pathogen Colletotrichum gloeosporioides. HortScience 2022, 57, 856–863.
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130243.
- Zakurin, A.O.; Shchennikova, A.V.; Kamionskaya, A.M. Artificial-Light Culture in Protected Ground Plant Growing: Photosynthesis, Photomorphogenesis, and Prospects of LED Application. Russ. J. Plant Physiol. 2020, 67, 413–424.
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121.
- Devireddy, A.R.; Liscum, E.; Mittler, R. Phytochrome B is required for systemic stomatal responses and reactive oxygen species signaling during light stress. Plant Physiol. 2020, 184, 1563–1572.
- Küpers, J.J.; Oskam, L.; Pierik, R. Photoreceptors regulate plant developmental plasticity through auxin. Plants 2020, 9, 940.
- Luo, Y.; Shi, H. Direct Regulation of Phytohormone Actions by Photoreceptors. Trends Plant Sci. 2019, 24, 105–108.
- Song, J.; Wu, W.; Hu, B. Light and temperature receptors and their convergence in plants. Biol. Plant. 2020, 64, 159–166.
- Casal, J.J.; Qüesta, J.I. Light and temperature cues: Multitasking receptors and transcriptional integrators. New Phytol. 2018, 217, 1029–1034.
- Saidi, Y.; Finka, A.; Goloubinoff, P. Heat perception and signalling in plants: A tortuous path to thermotolerance. New Phytol. 2011, 190, 556–565.
- von Koskull-Döring, P.; Scharf, K.D.; Nover, L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007, 12, 452–457.
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252.
- Jin, P.; Zheng, C.; Huang, Y.P.; Wang, X.L.; Luo, Z.S.; Zheng, Y. Hua Hot air treatment activates defense responses and induces resistance against Botrytis cinerea in strawberry fruit. J. Integr. Agric. 2016, 15, 2658–2665.
- Widiastuti, A.; Yoshino, M.; Saito, H.; Maejima, K.; Zhou, S.; Odani, H.; Narisawa, K.; Hasegawa, M.; Nitta, Y.; Sato, T. Heat shock-induced resistance in strawberry against crown rot fungus Colletotrichum gloeosporioides. Physiol. Mol. Plant Pathol. 2013, 84, 86–91.
- Brown, R.; Wang, H.; Dennis, M.; Slovin, J.; Turechek, W.W. The Effects of Heat Treatment on the Gene Expression of Several Heat Shock Protein Genes in Two Cultivars of Strawberry. Int. J. Fruit Sci. 2016, 16, 239–248.
- Kesici, M.; Ipek, A.; Ersoy, F.; Ergin, S.; Gülen, H. Genotype-Dependent Gene Expression in Strawberry (Fragaria × ananassa) Plants Under High Temperature Stress. Biochem. Genet. 2020, 58, 848–866.
- Araújo, S.d.S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advantages and challenges in seed technology. Front. Plant Sci. 2016, 7, 646.
- Bukhari, S.A.; Farah, N.; Mustafa, G.; Mahmood, S.; Naqvi, S.A.R. Magneto-Priming Improved Nutraceutical Potential and Antimicrobial Activity of Momordica charantia L. without Affecting Nutritive Value. Appl. Biochem. Biotechnol. 2019, 188, 878–892.
- Gupta, M.K.; Anand, A.; Paul, V.; Dahuja, A.; Singh, A.K. Reactive oxygen species mediated improvement in vigour of static and pulsed magneto-primed cherry tomato seeds. Indian J. Plant Physiol. 2015, 20, 197–204.
- Rathod, G.R.; Anand, A. Effect of seed magneto-priming on growth, yield and Na/K ratio in wheat (Triticum aestivum L.) under salt stress. Indian J. Plant Physiol. 2016, 21, 15–22.
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445.
More
Information
Subjects:
Agronomy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
13 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




