
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sergey Kasparov | -- | 2996 | 2023-01-12 17:58:16 | | | |
| 2 | Jessie Wu | -66 word(s) | 2930 | 2023-01-13 02:45:17 | | | | |
| 3 | Jessie Wu | -9 word(s) | 2921 | 2023-01-13 02:50:04 | | | | |
| 4 | Jessie Wu | Meta information modification | 2921 | 2023-01-13 03:01:08 | | |
Video Upload Options
Lactate is a universal metabolite produced and released by all cells in the body. Traditionally it was viewed as energy currency that is generated from pyruvate at the end of the glycolytic pathway and sent into the extracellular space for other cells to take up and consume. In the brain, such a mechanism was postulated to operate between astrocytes and neurons many years ago.
1. Introduction
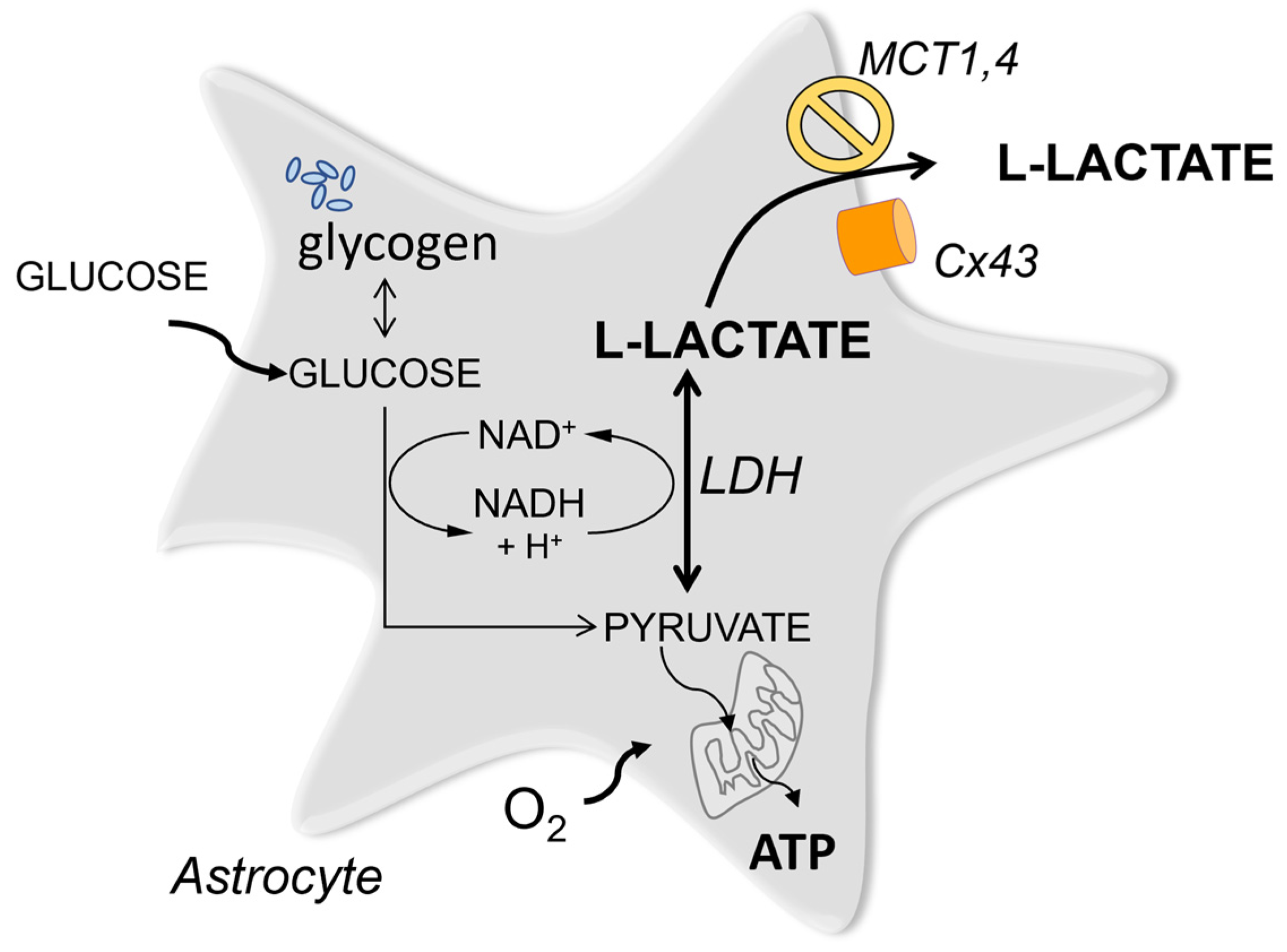
2. Mechanisms Which are Attributed to Lac Entry into Target Neurons
The concept of Lac being passed on between different cell types is well established for peripheral tissues [9]. For the brain, the hypothesis of an analogous Lac shuttle operating between astrocytes and neurons was proposed decades ago [10], originally, as a mechanism to subsidize neurons with energy under conditions of high metabolic demand such as periods of active firing of action potentials [11][12]. Perhaps one of the more contentious aspects of the shuttle hypothesis is the question of why astrocytic Lac, once transferred into neurons, should be used in preference to glucose for ATP generation. The relative importance of astrocytic Lac as a source of neuronal ATP is still controversial.
Many studies argue for the importance of Lac as the source of energy but the actual link between the availability of ATP and the end-point effects is usually assumed, rather than demonstrated directly. Typically the effects of MCT block or LDH block or drugs which interfere with Lac mobilisation in astrocytes are used as arguments to support the caloric role of Lac Many of the approaches used in these studies have limitations.
Overall, the body of studies which support the use of Lac for energy generation in preference to glucose is substantial, for example [13][14], but is it always preferred to glucose and why? A recently published study from the L. Venance group offers an essential clue which may explain the existing controversies [15]. Here, experiments in vitro, in vivo and mathematical modelling are combined to carefully dissect which conditions favor the utilization of glucose vs Lac. The authors used two types of protocols, for example, in vitro, a high frequency (100 Hz) 5 x theta burst stimulation that should require more energy for generation of LTP and, for comparison, spike timing–dependent plasticity (STDP) where frequency of stimulation is relatively low. Both forms of plasticity are dependent on NMDA receptors. It was shown that while the high frequency LTP requires Lac provision, STDP does not. In vivo the authors use a simpler novel object recognition task where the rat needs to detect one new object and compare it with a more complex test (object in place) where several objects have been moved in the arena. Here the simple test is not sensitive to oxamate while the more challenging test is, again pointing to the preferential use of Lac in situations of high energy demand. These experiments are matched by mathematical modelling. This study demonstrates that, while Lac (provided largely by astrocytes) is required to support synaptic activity when the energy consumption is high, the conditions of the experiment are paramount.
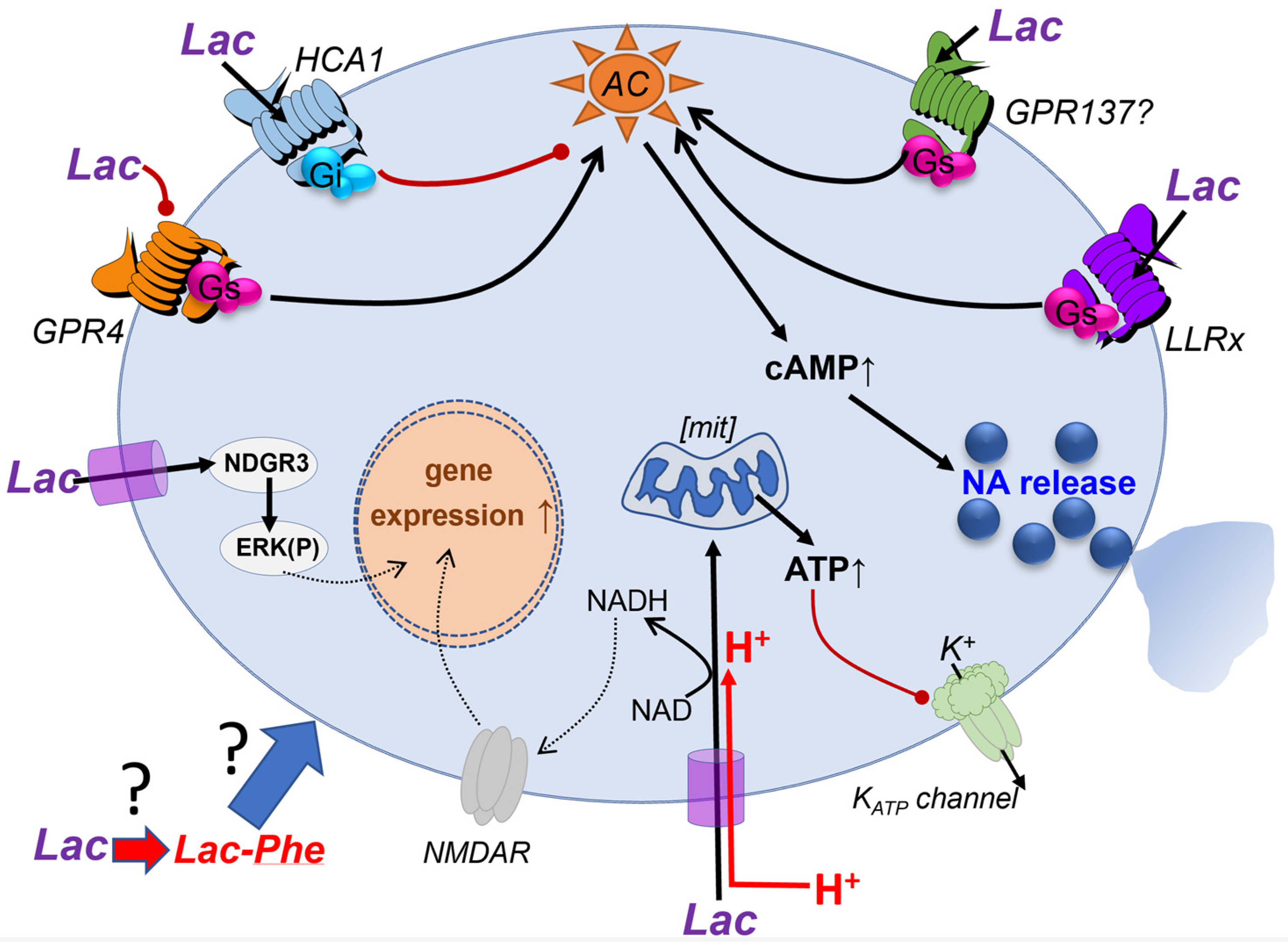
Another line of argument relates effects of Lac to modulation of to NAD+/NADH ratio which could lead to potentiation of NMDA receptors. These effects were noted when high concentrations of Lac (2.5 mM - 20 mM) were used [16][17]. Some studies aimed to trigger release of Lac from astrocytes by optogenetic or chemogenetic activation of astrocytes in hippocampus, assuming that the released Lac will trigger effects via NMDA receptor modulation or energy provision to neurones [16][17] [18][19][20]. These studies are interesting and raise a very important set of questions. How can an activated astrocyte or a group of astrocytes modulate a specific memory trace, given that each astrocyte contacts numerous neurons, potentially many thousands of synapses? Moreover, Lac almost certainly can spread between astrocytes via gap junctions, which would further diffuse the signal within the network. Can astrocytic modulation be targeted to individual synapses which are contacted by distinct end feet of the same astrocyte? Or is the role of astrocytes to provide a wide-scale change in the extracellular concentration of metabolites such as Lac, ATP, glutamate, etc. that results in more general network modulation? Under which physiological conditions could one expect the activation of large pools of astrocytes in the hippocampus and what would be the mechanism? If, as per [21][22], such activation occurs, would that elicit amnesia covering the preceding 24 hours? These are exciting questions and, clearly, a lot of work still needs to be done to explain how the stimulation of astrocytes affects memory formation and retention and whether there may be specific mechanisms for compartmentalization of intra- and inter-cellular Lac signaling in astrocytes [20][23][24][25][26][27][28][29][30].
3. Cell Surface Receptor-Mediated Signaling by Lac in the Brain
References
- Quistorff, B.; Grunnet, N. The isoenzyme pattern of LDH does not play a physiological role; except perhaps during fast transitions in energy metabolism. Aging 2011, 3, 457–460.
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 2005, 94, 1–14.
- Clasadonte, J.; Scemes, E.; Wang, Z.; Boison, D.; Haydon, P.G. Connexin 43-Mediated Astroglial Metabolic Networks Contribute to the Regulation of the Sleep-Wake Cycle. Neuron 2017, 95, 1365–1380.e1365.
- Karagiannis, A.; Sylantyev, S.; Hadjihambi, A.; Hosford, P.S.; Kasparov, S.; Gourine, A.V. Hemichannel-mediated release of lactate. J. Cereb. Blood Flow Metab. 2016, 36, 1202–1211.
- Sotelo-Hitschfeld, T.; Niemeyer, M.I.; Machler, P.; Ruminot, I.; Lerchundi, R.; Wyss, M.T.; Stobart, J.; Fernandez-Moncada, I.; Valdebenito, R.; Garrido-Gerter, P.; et al. Channel-mediated lactate release by K+-stimulated astrocytes. J. Neurosci. 2015, 35, 4168–4178.
- Walz, W.; Mukerji, S. Lactate release from cultured astrocytes and neurons: A comparison. Glia 1988, 1, 366–370.
- Kasparov, S. Are Astrocytes the Pressure-Reservoirs of Lactate in the Brain? Cell Metab 2016, 23, 1–2.
- Machler, P.; Wyss, M.T.; Elsayed, M.; Stobart, J.; Gutierrez, R.; von Faber-Castell, A.; Kaelin, V.; Zuend, M.; San Martin, A.; Romero-Gomez, I.; et al. In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons. Cell Metab. 2016, 23, 94–102.
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785.
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.L.; Stella, N.; Magistretti, P.J. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998, 20, 291–299.
- Beard, E.; Lengacher, S.; Dias, S.; Magistretti, P.J.; Finsterwald, C. Astrocytes as Key Regulators of Brain Energy Metabolism: New Therapeutic Perspectives. Front. Physiol. 2021, 12, 825816.
- Veloz Castillo, M.F.; Magistretti, P.J.; Cali, C. l-Lactate: Food for Thoughts, Memory and Behavior. Metabolites 2021, 11, 548.
- Smith, D.; Pernet, A.; Hallett, W.A.; Bingham, E.; Marsden, P.K.; Amiel, S.A. Lactate: A preferred fuel for human brain metabolism in vivo. J. Cereb. Blood Flow Metab. 2003, 23, 658–664.
- Dembitskaya, Y.; Piette, C.; Perez, S.; Berry, H.; Magistretti, P.J.; Venance, L. Lactate supply overtakes glucose when neural computational and cognitive loads scale up. Proc. Natl. Acad. Sci. USA 2022, 119, e2212004119.
- Jourdain, P.; Allaman, I.; Rothenfusser, K.; Fiumelli, H.; Marquet, P.; Magistretti, P.J. L-Lactate protects neurons against excitotoxicity: Implication of an ATP-mediated signaling cascade. Sci. Rep. 2016, 6, 21250.
- Yang, J.; Ruchti, E.; Petit, J.M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233.
- Jourdain, P.; Rothenfusser, K.; Ben-Adiba, C.; Allaman, I.; Marquet, P.; Magistretti, P.J. Dual action of L-Lactate on the activity of NR2B-containing NMDA receptors: From potentiation to neuroprotection. Sci. Rep. 2018, 8, 13472.
- Stierl, M.; Stumpf, P.; Udwari, D.; Gueta, R.; Hagedorn, R.; Losi, A.; Gartner, W.; Petereit, L.; Efetova, M.; Schwarzel, M.; et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J. Biol. Chem. 2011, 286, 1181–1188.
- Zhou, Z.; Okamoto, K.; Onodera, J.; Hiragi, T.; Andoh, M.; Ikawa, M.; Tanaka, K.F.; Ikegaya, Y.; Koyama, R. Astrocytic cAMP modulates memory via synaptic plasticity. Proc. Natl. Acad. Sci. USA 2021, 118, e2016584118.
- Torres-Torrelo, H.; Ortega-Saenz, P.; Gao, L.; Lopez-Barneo, J. Lactate sensing mechanisms in arterial chemoreceptor cells. Nat. Commun. 2021, 12, 4166.
- Hirase, H.; Akther, S.; Wang, X.; Oe, Y. Glycogen distribution in mouse hippocampus. J. Neurosci. Res. 2019, 97, 923–932.
- Oe, Y.; Baba, O.; Ashida, H.; Nakamura, K.C.; Hirase, H. Glycogen distribution in the microwave-fixed mouse brain reveals heterogeneous astrocytic patterns. Glia 2016, 64, 1532–1545.
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14.
- Halestrap, A.P.; Price, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343 Pt 2, 281–299.
- Chatton, J.Y.; Idle, J.R.; Vagbo, C.B.; Magistretti, P.J. Insights into the mechanisms of ifosfamide encephalopathy: Drug metabolites have agonistic effects on alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptors and induce cellular acidification in mouse cortical neurons. J. Pharmacol. Exp. Ther. 2001, 299, 1161–1168.
- Ovens, M.J.; Manoharan, C.; Wilson, M.C.; Murray, C.M.; Halestrap, A.P. The inhibition of monocarboxylate transporter 2 (MCT2) by AR-C155858 is modulated by the associated ancillary protein. Biochem. J. 2010, 431, 217–225.
- Goncharov, N.V.; Jenkins, R.O.; Radilov, A.S. Toxicology of fluoroacetate: A review, with possible directions for therapy research. J. Appl. Toxicol. 2006, 26, 148–161.
- Fonnum, F.; Johnsen, A.; Hassel, B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia 1997, 21, 106–113.
- Swanson, R.A.; Graham, S.H. Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res. 1994, 664, 94–100.
- Mosienko, V.; Teschemacher, A.G.; Kasparov, S. Is L-lactate a novel signaling molecule in the brain? J. Cereb. Blood Flow Metab. 2015, 35, 1069–1075.
- Ahmed, K.; Tunaru, S.; Tang, C.; Muller, M.; Gille, A.; Sassmann, A.; Hanson, J.; Offermanns, S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010, 11, 311–319.
- Liu, C.; Wu, J.; Zhu, J.; Kuei, C.; Yu, J.; Shelton, J.; Sutton, S.W.; Li, X.; Yun, S.J.; Mirzadegan, T.; et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 2009, 284, 2811–2822.
- Mosienko, V.; Rasooli-Nejad, S.; Kishi, K.; De Both, M.; Jane, D.; Huentelman, M.; Kasparov, S.; Teschemacher, A. Putative Receptors Underpinning l-Lactate Signalling in Locus Coeruleus. Neuroglia 2018, 1, 365–380.
- Bozzo, L.; Puyal, J.; Chatton, J.Y. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS ONE 2013, 8, e71721.
- Briquet, M.; Rocher, A.B.; Alessandri, M.; Rosenberg, N.; de Castro Abrantes, H.; Wellbourne-Wood, J.; Schmuziger, C.; Ginet, V.; Puyal, J.; Pralong, E.; et al. Activation of lactate receptor HCAR1 down-modulates neuronal activity in rodent and human brain tissue. J. Cereb. Blood Flow Metab. 2022, 42, 1650–1665.
- Buscemi, L.; Price, M.; Castillo-Gonzalez, J.; Chatton, J.Y.; Hirt, L. Lactate Neuroprotection against Transient Ischemic Brain Injury in Mice Appears Independent of HCAR1 Activation. Metabolites 2022, 12, 465.
- de Castro Abrantes, H.; Briquet, M.; Schmuziger, C.; Restivo, L.; Puyal, J.; Rosenberg, N.; Rocher, A.B.; Offermanns, S.; Chatton, J.Y. The Lactate Receptor HCAR1 Modulates Neuronal Network Activity through the Activation of G(alpha) and G(betagamma) Subunits. J. Neurosci. 2019, 39, 4422–4433.
- Dienel, G.A. Brain lactate metabolism: The discoveries and the controversies. J. Cereb. Blood Flow Metab. 2012, 32, 1107–1138.
- Zuend, M.; Saab, A.S.; Wyss, M.T.; Ferrari, K.D.; Hosli, L.; Looser, Z.J.; Stobart, J.L.; Duran, J.; Guinovart, J.J.; Barros, L.F.; et al. Arousal-induced cortical activity triggers lactate release from astrocytes. Nat. Metab. 2020, 2, 179–191.
- Ordenes, P.; Villar, P.S.; Tarifeno-Saldivia, E.; Salgado, M.; Elizondo-Vega, R.; Araneda, R.C.; Garcia-Robles, M.A. Lactate activates hypothalamic POMC neurons by intercellular signaling. Sci. Rep. 2021, 11, 21644.
- Durkee, C.A.; Covelo, A.; Lines, J.; Kofuji, P.; Aguilar, J.; Araque, A. G(i/o) protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 2019, 67, 1076–1093.
- Kang, J.; Jiang, L.; Goldman, S.A.; Nedergaard, M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat. Neurosci. 1998, 1, 683–692.
- Nuzzaci, D.; Cansell, C.; Lienard, F.; Nedelec, E.; Ben Fradj, S.; Castel, J.; Foppen, E.; Denis, R.; Grouselle, D.; Laderriere, A.; et al. Postprandial Hyperglycemia Stimulates Neuroglial Plasticity in Hypothalamic POMC Neurons after a Balanced Meal. Cell Rep. 2020, 30, 3067–3078.e3065.
- Tang, F.; Lane, S.; Korsak, A.; Paton, J.F.; Gourine, A.V.; Kasparov, S.; Teschemacher, A.G. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 2014, 5, 3284.
- Aston-Jones, G.; Rajkowski, J.; Cohen, J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog. Brain Res. 2000, 126, 165–182.
- Breton-Provencher, V.; Drummond, G.T.; Feng, J.; Li, Y.; Sur, M. Spatiotemporal dynamics of noradrenaline during learned behaviour. Nature 2022, 606, 732–738.
- Hayat, H.; Regev, N.; Matosevich, N.; Sales, A.; Paredes-Rodriguez, E.; Krom, A.J.; Bergman, L.; Li, Y.; Lavigne, M.; Kremer, E.J.; et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 2020, 6, eaaz4232.
- Marina, N.; Tang, F.; Figueiredo, M.; Mastitskaya, S.; Kasimov, V.; Mohamed-Ali, V.; Roloff, E.; Teschemacher, A.G.; Gourine, A.V.; Kasparov, S. Purinergic signalling in the rostral ventro-lateral medulla controls sympathetic drive and contributes to the progression of heart failure following myocardial infarction in rats. Basic Res. Cardiol. 2013, 108, 317.
- Ludwig, M.G.; Vanek, M.; Guerini, D.; Gasser, J.A.; Jones, C.E.; Junker, U.; Hofstetter, H.; Wolf, R.M.; Seuwen, K. Proton-sensing G-protein-coupled receptors. Nature 2003, 425, 93–98.
- Hosford, P.S.; Mosienko, V.; Kishi, K.; Jurisic, G.; Seuwen, K.; Kinzel, B.; Ludwig, M.G.; Wells, J.A.; Christie, I.N.; Koolen, L.; et al. CNS distribution, signalling properties and central effects of G-protein coupled receptor 4. Neuropharmacology 2018, 138, 381–392.
- Balazova, L.; Balaz, M.; Horvath, C.; Horvath, A.; Moser, C.; Kovanicova, Z.; Ghosh, A.; Ghoshdastider, U.; Efthymiou, V.; Kiehlmann, E.; et al. GPR180 is a component of TGFbeta signalling that promotes thermogenic adipocyte function and mediates the metabolic effects of the adipocyte-secreted factor CTHRC1. Nat. Commun. 2021, 12, 7144.
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.; Lyu, X.; Zushin, P.H.; et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022, 606, 785–790.




