Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohamed Hamouda | -- | 1848 | 2023-01-12 10:29:30 | | | |
| 2 | Jessie Wu | Meta information modification | 1848 | 2023-01-13 04:47:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Arimbrathodi, S.P.; Javed, M.A.; Hamouda, M.A.; Hassan, A.A.; Ahmed, M.E. Production of BioH2 Using Microalgae. Encyclopedia. Available online: https://encyclopedia.pub/entry/40109 (accessed on 03 March 2026).
Arimbrathodi SP, Javed MA, Hamouda MA, Hassan AA, Ahmed ME. Production of BioH2 Using Microalgae. Encyclopedia. Available at: https://encyclopedia.pub/entry/40109. Accessed March 03, 2026.
Arimbrathodi, Shirin P., Muhammad Asad Javed, Mohamed A. Hamouda, Ashraf Aly Hassan, Mahmoud E. Ahmed. "Production of BioH2 Using Microalgae" Encyclopedia, https://encyclopedia.pub/entry/40109 (accessed March 03, 2026).
Arimbrathodi, S.P., Javed, M.A., Hamouda, M.A., Hassan, A.A., & Ahmed, M.E. (2023, January 12). Production of BioH2 Using Microalgae. In Encyclopedia. https://encyclopedia.pub/entry/40109
Arimbrathodi, Shirin P., et al. "Production of BioH2 Using Microalgae." Encyclopedia. Web. 12 January, 2023.
Copy Citation
Demand for clean energy has increased due to the proliferation of climate change impact from excessive emission of greenhouse gases (GHG) from the combustion of fossil fuels. H2 is a clean energy source since water vapor is the only byproduct after its combustion. Growing microalgae offers a promising low-energy and low-cost approach for bioH2 production. Diverse microalgae can generate bioH2, including Chlorella sp., Scenedesmus sp., Monoraphidium sp., Platymonas sp., Tetraspora sp., Closterium sp., and Chlamydomonas sp. Even though green microalgae have high potential as a renewable energy source, only about 70 species from more than 30 genera have been researched so far.
bioH2
microalgae
H2
1. The Techniques to Produce BioH2 Using Microalgae
Microalgae contain pigment molecules capable of absorbing solar energy and converting it into chemical energy by simultaneously splitting water into oxygen (O2) and protons (H+). The photosynthetic electron transfer constitutes light and dark reactions. The light reaction helps to obtain electrons by splitting water in photosystem II (PSII) and transfer the electrons through an electron transport chain from PSII to Photosystem I (PSI). This results in the generation of Adenosine triphosphate (ATP) and strong reductants (NAD(P)H). Photobiological bioH2 production is associated with photosynthesis, where the final electron acceptor, ferredoxin (Fd), donates electrons to enzymes involved in H2 metabolism [1]. Biophotolysis is the initial step of microalgal bioH2 production. In direct biophotolysis, microalgae convert solar energy into chemical energy, and H2 is derived from the electrons and protons generated by the water splitting at PSII. Nevertheless, some of the restrictions of biophotolysis include O2 generation by the activity of PSII, the requirement for a customized photobioreactor, the sensitivity of H2ase to O2, and low yield [2][3]. For indirect biophotolysis, electrons and protons are mainly supplied by the degradation of intracellular carbon compounds. Indirect photolysis has two stages: first, the carbohydrate biomass is generated from photosynthesis, and in the next stage, H2 and CO2 are produced due to the fermentation of carbohydrate-rich biomass. In these two steps, oxygen and H2 will be separated. This prevents enzyme deactivation and removes CO2 from the H2 and CO2 mixture, making H2 purification easier. Some drawbacks include high H2 selectivity, the restricted effect of O2 on the H2ase, and low yield [4]. Figure 1 shows a schematic diagram of hydrogen production by direct and indirect biophotolysis.
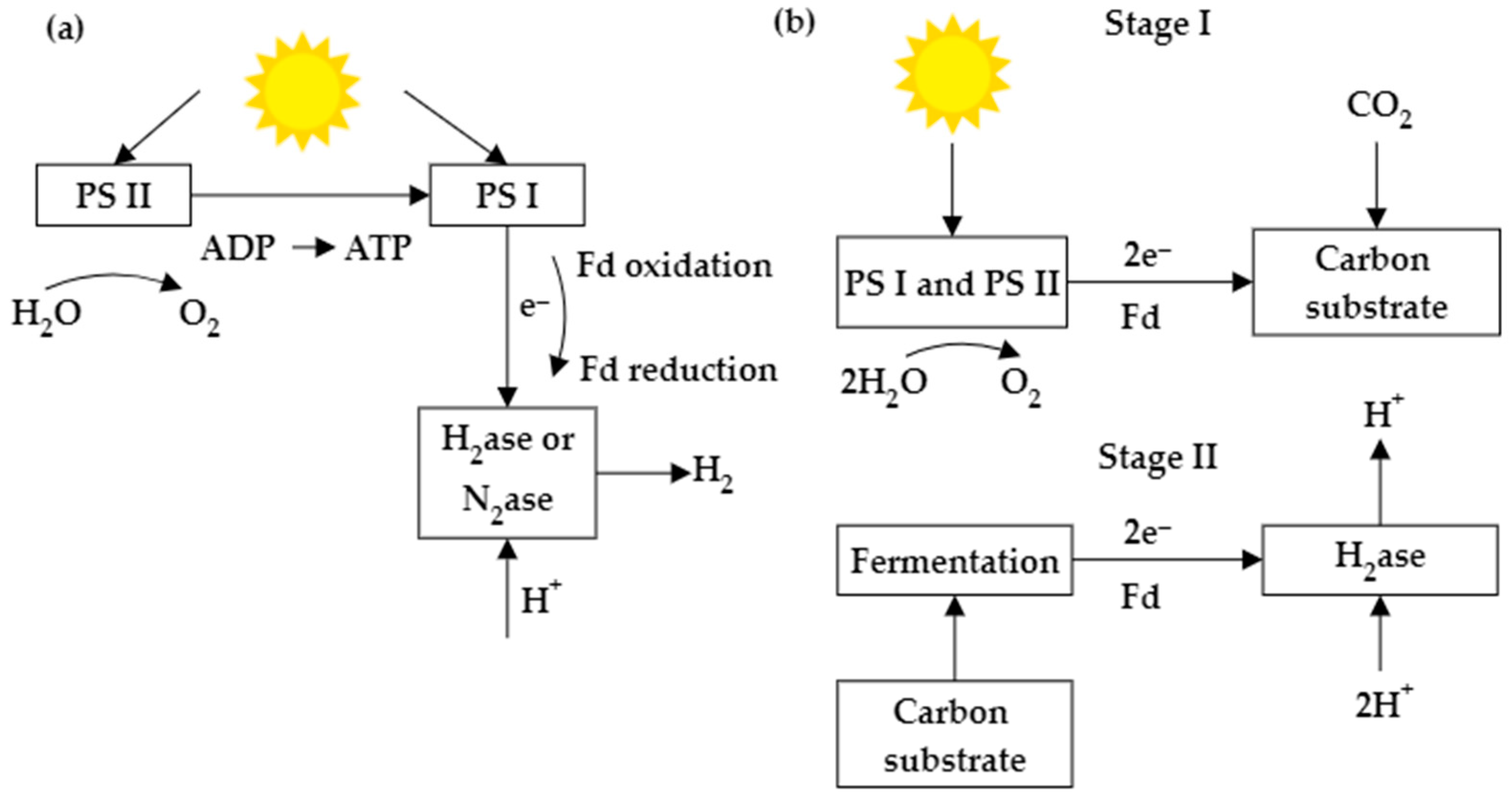
Figure 1. Schematic diagram of bioH2 production through (a) direct biophotolysis and (b) indirect biophotolysis.
Dark fermentation has gained attention because of its relatively high bioH2 production rates. The complex organic substances (lipids, carbohydrates, and proteins) are subjected to four stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. Firstly, lipids, carbohydrates, and proteins are hydrolyzed into sugars, fatty acids, and amino acids. In the acidogenesis stage, the hydrolyzed products are acidified to form H2, CO2, fatty acids, and other intermediates. In the next step, acetogenesis, the fatty acids produced are again fermented to generate H2 and acetate. The final products, methane (CH4) and CO2, are formed from the decarboxylation of acetate by the acetoclastic methanogens. H2-utilizing methanogens consume H2 gas as an electron donor while reducing the carbon dioxide to methane. In dark fermentation, H2 is produced as an intermediate metabolite in the acidogenesis and acetogenesis transformations. Two mechanisms which involve specific coenzymes are responsible for the evolution of H2 gas, either by formic acid catabolic transformation or by re-oxidation of nicotinamide adenine dinucleotide (NADH) catalyzed by H2ase pathway; H2ase is the major enzyme in the process [5][6][7]. Dark fermentation has many advantages: it is capable of continually producing H2 without depending on sunlight, has high energy efficiency, is an eco-friendly and economical process, is easy to commercialize, has a less complicated bioreactor design, and has a wide range of organic acid as byproducts. The major disadvantages are low bioH2 production due to the accumulation of O2, methanogenic bacteria utilizing H2 as an electron donor, more tedious and expensive H2 recovery due to the generation of CO2 and other gaseous products, and low substrate conversion efficiency [5][8][9].
In photo fermentation, H2 is produced by the photosynthetic bacteria by breaking down the organic compounds with the help of nitrogenase (N2ase) enzymes under nitrogen-deprived conditions. Atmospheric nitrogen is converted into ammonium ions used by microorganisms as a nitrogen source through nitrogen (N2) fixation by nitrogenase. They are only found in cyanobacteria and non-sulfur purple and green sulfur bacteria. These bacteria consume acetic acid and use ATP as an energy source. This leads to the transfer of the electrons by ferredoxin to the enzyme nitrogenase and results in N2 fixation. Moreover, nitrogenase protons are converted to H2 in the absence of nitrogen [6][9]. The major advantages are that photosynthetic bacteria use various spectral energy and substrates, can treat effluents from dark fermentation, have higher substrate transformation efficiency, and hence have a high H2 yield. One disadvantage is the requirement of a light source; the photosynthesis efficiency directly depends on the availability of light. Moreover, it requires a large area and an anaerobic bioreactor, which increases costs [5][8].
Thermochemical processes include liquefaction, gasification, and pyrolysis. The wet microalgae biomass is converted into gaseous bioH2 during hydrothermal gasification. The process includes heating biomass at higher temperatures in a compressed water medium. The reaction is quick due to the higher temperatures; the main products obtained are H2, CO2, and methane (CH4). The compressed water medium usually has a low percentage of effluents and can be reused [10][11]. The microalgae should be dried up to low moisture content in conventional thermal gasification for higher efficiency. Nevertheless, the high moisture content of microalgae often results in high energy consumption during thermal gasification. Thus, the main advantage of applying supercritical water gasification (SCWG) is that it can be conducted without the drying process, but rather in an aqueous state. Lastly, pyrolysis occurs in the absence of oxygen at higher temperatures. Dry microalgae are needed to be fed to the reactor, which demands a large amount of energy. The conversion efficiency of microalgae depends on parameters such as reaction temperature, retention time, and the composition of feedstock [12][13].
Bioelectrochemical systems are an alternate method for bioH2 production. Microalgae catalyze the oxidation–reduction at the anode and cathode, respectively, and act as electrochemical catalysts. Microbial fuel cells and microbial electrolysis cells are the two categories of bioelectrochemical systems. Microbial Fuel Cells (MFCs) are eco-friendly bioelectrochemical devices that produce electrical energy from chemical energy obtained from biomass. The H2 forms at the cathodes as a result of the reduction reaction and is collected by an external system. The primary restriction of MFC is membrane fouling, which occurs due to the long-term growth of biofilm in separators. It can also occur due to the accumulation of microbes, which leads to the formation of thick biofilm on the surface of the membrane. This prevents the transfer of H2 ions from the anode to the cathode. Another restriction is the removal of heavy metals, which causes lower efficiency performance of microbes and pH imbalance. The higher cost of the membrane prevents the large-scale expansion of MFC. Microbial Electrolysis Cells (MECs) are a different form of MFC; the bioH2 is produced by the oxidation of organic matter, which is catalyzed by electroactive biofilms. It is yet to be developed in an efficient and scalable design. One major advantage of MEC is the higher efficiency in metal ions removal. The drawbacks are the higher cost as well as the H2 loss and contamination [9][14][15].
2. Factors Affecting BioH2 Production Using Microalgae
Efficient production of bioH2 yield from microalgae biomass depends on factors such as nutrients, pH, temperature, light intensity, photoreactor configuration, substrate concentration, and cell density. Compared to a near-neutral pH, higher H2 productivity was observed when the pH was around 6. At a highly acidic pH, H2 yield declines because of the inactivation of the acetate-producing bacteria. Temperature is another parameter that influences the metabolic pathways of H2ase. A temperature range of 15–35 °C is good for microalgal growth. Moreover, the proper configuration of the photoreactor is important as it is critical for the effective use of light and the provision of sufficient surface area for the growth of microalgae. Additive subtracts such as biotin, cyanocobalamin, and thiamine are required to add to the culture to support the maximum cell growth and bioH2 production. For optimal bioH2 production, the culture needs a balance of carbohydrate-based substrate. Moreover, a carbon source is also required for the microalgae to flourish in all conditions except photoautotrophic conditions [16][17].
Furthermore, high bioH2 production and good microalgal growth can be achieved by introducing the proper fraction of nutrients such as nitrogen, phosphorus, and trace elements. The nitrogen element mainly regulates the protein synthesis and growth metabolites of microalgae, while the phosphorus element regulates most of the cell’s activities and metabolism. The trace elements like magnesium, sodium, and zinc are important supplements that play a role in improving microalgal cultivation. The problem associated with the inhibition of H2ase is caused by the presence of oxygen and can be resolved by sulfur deprivation. BioH2 production is relatively low at lower light intensities. Exposure to high light intensity can increase bioH2 production rates by inhibiting photosynthetic O2 [9][18]. In addition, optimal light conditions can reduce the lag period of microalgae and increase H2 yield [9][18]. Cell density controls the amount of light that passes through the microalgal cell, and it depends on the nature of the cultivation process. The low cell concentration will not allow the uptake of the dissolved O2 into the microalgae culture. In contrast, a high cell density may cause the cumulation of starch and hinder the productivity rate. Therefore, to have significant bioH2 production, an active growth phase and cell density for the culture should be maintained [16]. The application of bioH2 production using microalgae is still limited due to the lack of proper distribution, capture, storage, and transformation technologies.
3. Strategies to Improve the BioH2 Production from Microalgae
Various strategies can be adopted to improve bioH2 production using microalgae, such as immobilization of microalgae, pretreatment techniques, nanoparticles, and genetic engineering. The pretreatment immediately disrupts the microalgal cell walls and enhances the accessibility of carbohydrates present in the cells. Different pretreatment methods include chemical, thermal, mechanical, enzyme, and combined methods. The best pretreatment method and its optimal conditions are yet to be determined [9][16].
Microalgal immobilization is the mechanism of the entrapment of microalgal cells on or into solid support. It has many advantages, such as high cell density, alleviating manipulation of cultures, and easy microalgae cell harvesting. Further, this approach protects the cells from unwanted contaminations and sudden changes in other culture parameters. This also results in high bioH2 production due to the enhanced permeability of cell walls. In addition, the microalgal cells wash out, get reduced, and cause an overall increase in H2 yields. The major drawbacks are the slow infusion of nutrients from the medium into microalgae and the high sunlight gradient within the cells because of high cell density [19][20]. Nanotechnology is capable of bioH2 production due to its role in intracellular electron transfer, microalgal growths, and enzymes involved in bioH2 generation. Genetic engineering and metabolic engineering can be used to modify specific pathways to increase bioH2 production. The photosynthetic barriers and inhibition factors can be suppressed [8].
References
- Maneeruttanarungroj, C.; Lindblad, P.; Incharoensakdi, A. A Newly Isolated Green Alga, Tetraspora sp., CU2551, from Thailand with Efficient Hydrogen Production. Int. J. Hydrog. Energy 2010, 35, 13193–13199.
- Mona, S.; Kumar, S.S.; Kumar, V.; Parveen, K.; Saini, N.; Deepak, B.; Pugazhendhi, A. Green Technology for Sustainable Biohydrogen Production (Waste to Energy): A Review. Sci. Total Environ. 2020, 728, 138481.
- Chen, J.; Li, J.; Li, Q.; Wang, S.; Wang, L.; Liu, H.; Fan, C. Engineering a Chemoenzymatic Cascade for Sustainable Photobiological Hydrogen Production with Green Algae. Energy Environ. Sci. 2020, 13, 2064–2068.
- Dalena, F.; Senatore, A.; Tursi, A.; Basile, A. Bioenergy Production from Second- and Third-Generation Feedstocks. In Bioenergy Systems for the Future; Elsevier: Amsterdam, The Netherlands, 2017; pp. 559–599. ISBN 978-0-08-101031-0.
- Goswami, R.K.; Mehariya, S.; Obulisamy, P.K.; Verma, P. Advanced Microalgae-Based Renewable Biohydrogen Production Systems: A Review. Bioresour. Technol. 2021, 320, 124301.
- Javed, M.A.; Zafar, A.M.; Aly Hassan, A.; Zaidi, A.A.; Farooq, M.; El Badawy, A.; Lundquist, T.; Mohamed, M.M.A.; Al-Zuhair, S. The Role of Oxygen Regulation and Algal Growth Parameters in Hydrogen Production via Biophotolysis. J. Environ. Chem. Eng. 2022, 10, 107003.
- Show, K.-Y.; Yan, Y.; Zong, C.; Guo, N.; Chang, J.-S.; Lee, D.-J. State of the Art and Challenges of Biohydrogen from Microalgae. Bioresour. Technol. 2019, 289, 121747.
- Li, S.; Li, F.; Zhu, X.; Liao, Q.; Chang, J.-S.; Ho, S.-H. Biohydrogen Production from Microalgae for Environmental Sustainability. Chemosphere 2022, 291, 132717.
- Musa Ardo, F.; Wei Lim, J.; Ramli, A.; Kee Lam, M.; Kiatkittipong, W.; Alaaeldin Abdelfattah, E.; Kashif Shahid, M.; Usman, A.; Wongsakulphasatch, S.; Tasnim Sahrin, N. A Review in Redressing Challenges to Produce Sustainable Hydrogen from Microalgae for Aviation Industry. Fuel 2022, 330, 125646.
- Jiao, J.-L.; Wang, F.; Duan, P.-G.; Xu, Y.-P.; Yan, W.-H. Catalytic Hydrothermal Gasification of Microalgae for Producing Hydrogen and Methane-Rich Gas. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 851–860.
- Kumar, M.; Oyedun, A.O.; Kumar, A. A Parametric Study through the Modelling of Hydrothermal Gasification for Hydrogen Production from Algal Biomass. Can. J. Chem. Eng. 2021, 99, S39–S54.
- Aziz, M. Integrated Hydrogen Production and Power Generation from Microalgae. Int. J. Hydrog. Energy 2016, 41, 104–112.
- Demirbas, A. Hydrogen from Mosses and Algae via Pyrolysis and Steam Gasification. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 32, 172–179.
- Chader, S.; Mahmah, B.; Chetehouna, K.; Amrouche, F.; Abdeladim, K. Biohydrogen Production Using Green Microalgae as an Approach to Operate a Small Proton Exchange Membrane Fuel Cell. Int. J. Hydrog. Energy 2011, 36, 4089–4093.
- Chatzitakis, A.; Nikolakaki, E.; Sotiropoulos, S.; Poulios, I. Hydrogen Production Using an Algae Photoelectrochemical Cell. Appl. Catal. B Environ. 2013, 142–143, 161–168.
- Ahmed, S.F.; Mofijur, M.; Nahrin, M.; Chowdhury, S.N.; Nuzhat, S.; Alherek, M.; Rafa, N.; Ong, H.C.; Nghiem, L.D.; Mahlia, T.M.I. Biohydrogen Production from Wastewater-Based Microalgae: Progresses and Challenges. Int. J. Hydrog. Energy 2021, 47, 37321–37342.
- Saifuddin, N.; Ong, M.Y.; Priatharsini, P. Optimization of Photosynthetic Hydrogen Gas Production by Green Alga in Sulfur Deprived Condition. Indian J. Sci. Technol. 2016, 9, 93390.
- Mujalin Pholchan, K.K. Effect of Light Intensities and Atmospheric Gas Conditions on Biohydrogen Production of Microalgae Isolated from Fisheries Wastewater. Environ. Nat. Resour. J. 2017, 15, 21–29.
- Maswanna, T.; Lindblad, P.; Maneeruttanarungroj, C. Improved Biohydrogen Production by Immobilized Cells of the Green Alga Tetraspora sp., CU2551 Incubated under Aerobic Conditions. J. Appl. Phycol. 2020, 32, 2937–2945.
- Maswanna, T.; Phunpruch, S.; Lindblad, P.; Maneeruttanarungroj, C. Enhanced Hydrogen Production by Optimization of Immobilized Cells of the Green Alga Tetraspora sp., CU2551 Grown under Anaerobic Condition. Biomass Bioenergy 2018, 111, 88–95.
More
Information
Subjects:
Engineering, Environmental; Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
13 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





