
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Livia Marques Casanova | -- | 3349 | 2023-01-11 15:55:55 | | | |
| 2 | Lindsay Dong | + 2 word(s) | 3351 | 2023-01-13 02:35:25 | | |
Video Upload Options
Microalgae are a diverse group of prokaryotic and eukaryotic photosynthetic unicellular organisms. More than 50,000 microalgal species live in various environmental conditions, including water domains such as streams, rivers, lakes, oceans, and terrestrial ecosystems. Microalgae are regarded as a promising source of biodiesel. In contrast with conventional crops currently used to produce commercial biodiesel, microalgae can be cultivated on non-arable land, besides having a higher growth rate and productivity. However, microalgal biodiesel is not yet regarded as economically competitive, compared to fossil fuels and crop-based biodiesel; therefore, it is not commercially produced.
1. Introduction
The world’s energy expenditure is expected to increase by approximately 50% between 2018 and 2050 [1]. Fossil fuels, a non-renewable energy source, provide around 80% of all energy consumed worldwide [1][2]. Their use, however, leads to large emissions of greenhouse gases (GHGs), mainly CO2, which is a major contributor to global warming [3][4]. Consequently, the industrial sectors are looking for ecological solutions and green technologies to reduce these emissions, resulting in alternative and innovative solutions [5][6].
Biofuels are one of the main alternatives to fossil fuel exploitation [7][8]. These fuels, produced from biomass or waste feedstocks, have the advantages of renewability and a significantly reduced contribution to global warming. The main biofuels available are biodiesel and bioethanol [2][9][10]. Biodiesel is produced from lipids mainly by transesterification reactions having oils as the starting material [11][12]. Commercial biodiesel is currently obtained from different oil crops, such as soybean, corn, sunflower, and oil palm [9]. One of the main concerns related to these biodiesel sources is the use of arable lands resulting in competition with other segments, such as bioethanol and agriculture/livestock feed/food production [13].
High lipid contents make microalgae a promising alternative for biodiesel production. Besides this, microalgae are the major source of oxygen on the planet, and their CO2 biosequestration by photosynthesis point to the biodiesel from microalgae as a promising carbon-neutral fuel [14]. These microorganisms show higher growth rates and productivity and can be cultivated using wastewater, thus avoiding competition for freshwater and increasing sustainability [8][9][15]. Several countries, including Brazil, are investing in the development of algal biotechnology [16]. However, there are bottlenecks to be overcome, such as expensive and energy-intensive cultivation, microbial contamination, and the biodiesel conversion processes. All these factors lead to a higher production cost, the major challenge for biodiesel production after scaling-up [17].
2. Microalgae for Biodiesel Production
Microalgae are a diverse group of prokaryotic and eukaryotic photosynthetic unicellular organisms. More than 50,000 microalgal species live in various environmental conditions, including water domains such as streams, rivers, lakes, oceans, and terrestrial ecosystems [18][19][20].
Cyanobacteria are prokaryotic microalgae (Cyanophyta), while eukaryotic microalgae include Bacillariophyta (diatoms), Cryptophyta (golden algae), Rhodophyta (red algae), Xanthophyta (yellow/green algae), and Chlorophyta (green algae), amongst others [19][21]. The latter is the most promising group for biodiesel production. The selection of species for this purpose comprises criteria such as growth rate, tolerance to different environmental conditions, harvesting facility, and, most importantly, the lipid content, which ranges from 2 to 85% of dry biomass, depending on the species/strain and cultivation conditions [22][23].
Microalgae, similar to other organisms, use neutral lipids for energy storage, while polar lipids are membrane constituents (Figure 1). They store acylglycerols, mostly triacylglycerols (TAG), during the day when photosynthesis occurs, and consume them at night to keep metabolic activities. TAG accumulation is induced by stress conditions, such as nutritional restriction, high temperature, and high salinity [22][24].
Microalgae can accumulate TAG to around 20–50% of their dry weight [9][22][25]. The fatty acids that constitute the acylglycerols in microalgae vary from C12:0 to C22:6. Qualitative and quantitative composition is diverse among different species. It is also highly dependent on nutritional and environmental conditions. However, most of their fatty acids have saturated and unsaturated C16 and C18 (Figure 1) carbon chains, such as palmitic (16:0), palmitoleic (16:1), oleic (18:1), linoleic (18:2), and linolenic (18:3) acids [19][22][26][27]. The fatty acid composition is relevant to the quality of the resulting biodiesel, influencing its outflow property, ignition quality, and oxidative stability [19][22][26].
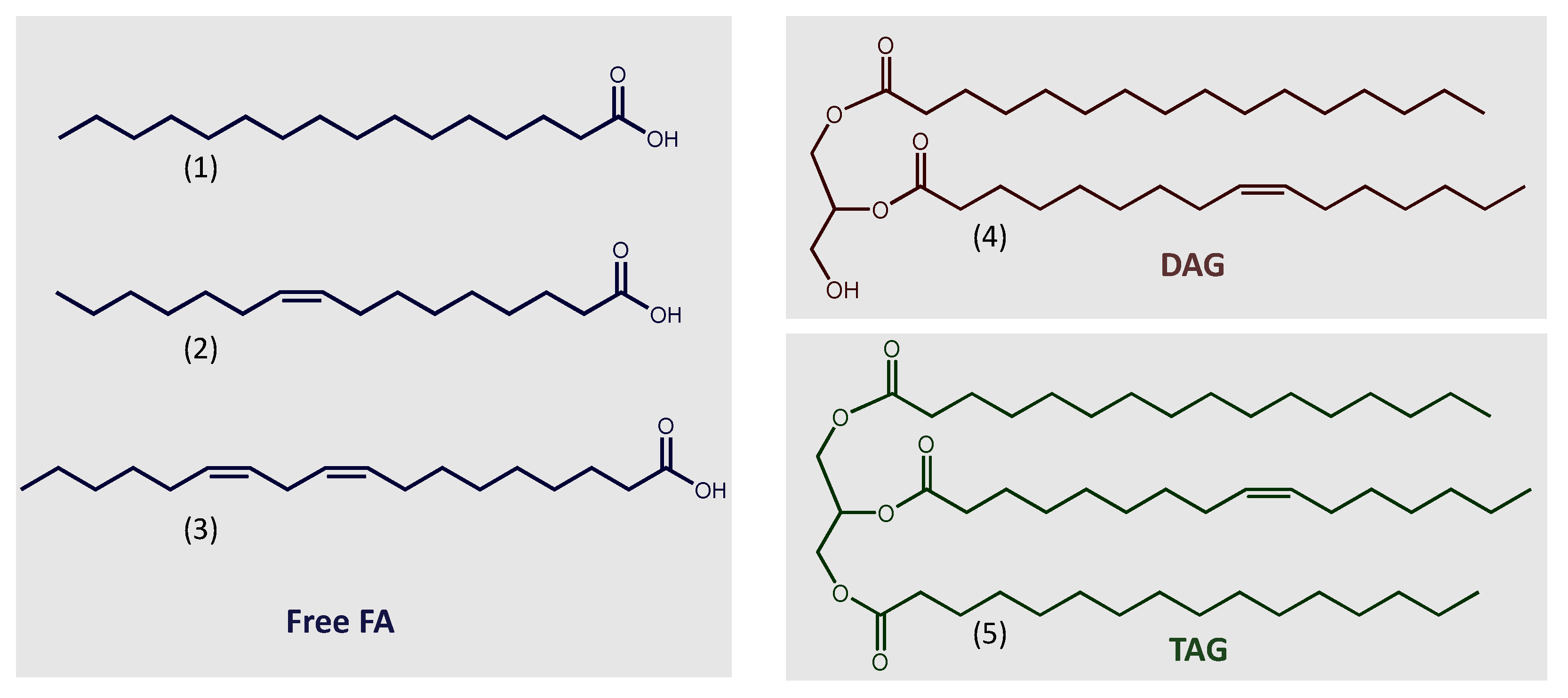
Figure 1. Chemical structures of commonly representative lipids. (1) Palmitic acid (16:0); (2) Palmitoleic acid (16:1); (3) Linoleic acid (18:2); (4) DAG (16:0/16:1); (5) TAG (16:0/16:1/16:0). Abbreviations: DAG, diacylglycerol; FA, fatty acid; TAG, triacylglycerol.
3. Microalgal Production: Open X Closed Systems
The cultivation of microalgae can be carried out in open systems, closed photobioreactors, and, to a lesser extent, fermenters in the case of heterotrophic and mixotrophic conditions [28][29].
In open cultivation systems, microalgae can be grown in lakes, lagoons, or ponds. Raceway shallow ponds are the most used for industrial purposes (food and cosmetic production). Open raceway ponds (OPR) are considered cost-effective, as they are cheaper to build, more straightforward to scale, and easier to operate when compared to photobioreactors. Another advantage is free sunlight energy [28][29][30][31].
On the other hand, open systems have many limitations, such as low biomass productivity, high harvesting costs, high water evaporation rates, and a high risk of contamination with other algae, bacteria, fungi, viruses, and predator protozoa species [28][29][30][31]. Additionally, open ponds suffer from poor CO2 mass transfer. Atmospheric CO2 usually does not meet the productivity requirements; thus, aeration and bubbling are necessary [28][31].
The lack of control over environmental factors affects productivity, which depends on weather conditions, photoperiod, and seasonal variations [28][31]. Therefore, choosing a location for microalgae cultivation in open systems is crucial, and must consider parameters such as solar radiation, pluviometric index, and local temperatures, besides land costs [31]. The most suitable locations for this cultivation are dry coastal areas in tropical and subtropical regions, with high solar irradiance throughout the year [32].
Culturing in photobioreactors (PBRs) can overcome the limitations of open cultures. They enable a higher degree of process control and, consequently, higher productivity. PBRs have great versatility: several designs are available (e.g., tubular, flat plate, airlift, bubbling column), and various combinations of source light can be used, sunlight included [28][29][33]. In addition, they provide better CO2 utilization and maximum light exposure [28][34].
Despite the advantages, there is a significant limitation to using PBRs for large-scale production: the capital and operational costs [28][35][36]. The energy consumption by these systems is a major concern [28]. Economic assessments of biodiesel production costs in OPRs and PBRs have evidenced that the predicted costs of the latter are generally higher [37].
As both open and closed culture systems have advantages and drawbacks, researchers proposed the combination of both for more cost-effective production. In a hybrid system, microalgae are grown in a PBR during the first stage, which favors high biomass productivity and minimum contamination. Subsequently, the produced biomass is transferred to OPR, aiming to achieve high lipid accumulation by applying stress conditions. Thus, the cultivation period in open ponds is shorter, and contaminants do not remain long enough to harm the culture [28][36][38][39]. Hybrid systems have shown higher lipid and biomass productivity when compared to open systems [38][39][40][41], and can therefore be considered promising for large-scale cultivation.
4. Biomass X Lipid Content: A Challenge
Despite the higher lipid yield of microalgae when compared to terrestrial crops, the overall costs to produce microalgal biodiesel are still high. Economic studies have pointed out lipid productivity as being a critical factor in enabling microalgal biodiesel to achieve favorable costs, compared with petroleum diesel [42][43][44].
Stress conditions generally induce lipid accumulation in microalgae. One of the most common strategies to enhance microalgal lipid content is limiting nutrients, mainly nitrogen, followed by phosphorus, sulfur, iron, and trace metals. Other stress conditions that affect lipid metabolism include high salinity and variations in the medium’s temperature, light, and pH [19][22][45][46][47]. Nevertheless, stress conditions often negatively affect microalgal growth, resulting in lower biomass yield [48][49][50]. Due to these opposing traits, two situations are typical: high biomass production with low lipid content, and low biomass production with high lipid content. Both cases result in low lipid productivity [49][50][51]. Thus, finding conditions to induce high lipid accumulation, without interfering with growth and biomass production, is an important challenge for commercial biodiesel production [42][49][51].
Multiple methods are proposed to increase lipid productivity such as two-stage cultivation, the addition of phytohormones and other chemicals, and co-cultivation. Approaches based on metabolic engineering and synthetic biology are also promising [45].
5. Strategies to Increase Microalgal Lipid Productivity
5.1. Two Stage-Cultivation
Two stage-cultivation strategies aim to decouple biomass growth from lipid accumulation. In the first stage, microalgal growth is carried out under optimal conditions, while in the second stage, lipid accumulation is induced by applying stress conditions [39][48][50][51].
Nutrient starvation is the most frequent strategy to induce lipid accumulation [52]. Using a two-stage cultivation strategy for the species Nannochloropsis oculata, grown in nitrogen-sufficient conditions until the stationary phase and then transferred to a nitrogen-deficient medium, enabled lipid productivity almost 3-fold higher than with one-stage cultivation [53]. Nayak et al. [54] reported a higher than 1.5-fold improvement in the lipid productivity of Chlorella sp. by employing this strategy.
Applying the two-stage cultivation strategy with nutrient restriction requires concentration of the biomass obtained in the first stage by harvesting, followed by transfer to a new culture medium [50][52]. The requirement of an intermediate harvesting step and transfer often demands more cost and energy [52]. An alternative is the use of salt stress in a two-stage cultivation strategy. As salt can be directly added to the medium after the culture reaches the stationary phase, thus the extra harvesting and transfer steps can be eliminated, which contributes to the economic feasibility of the approach [50]. This strategy was successfully applied to Scenedesmus obtusus. Adding NaCl into the medium, when cultures reached the late exponential phase, resulted in lipid productivity 1.2 times higher [55].
5.2. Phytohormones Addition
Phytohormones are small organic chemical messengers that play a broad spectrum of physiological roles in higher plants [56][57]. They are classified into five groups: auxins, abscisic acid, gibberellins, cytokinins, and ethylene. Other substances also act as phytohormones, including brassinosteroids, jasmonates, polyamines, salicylic acid, and signal peptides [57][58]. Some representatives of these groups are shown in Figure 2. The hormone systems of higher plants are likely to have evolved from a similar pre-existing system in microalgae. In fact, several phytohormones are known to be produced by microalgae. However, knowledge of their biosynthesis and physiological roles are still scarce [56][57][58][59].
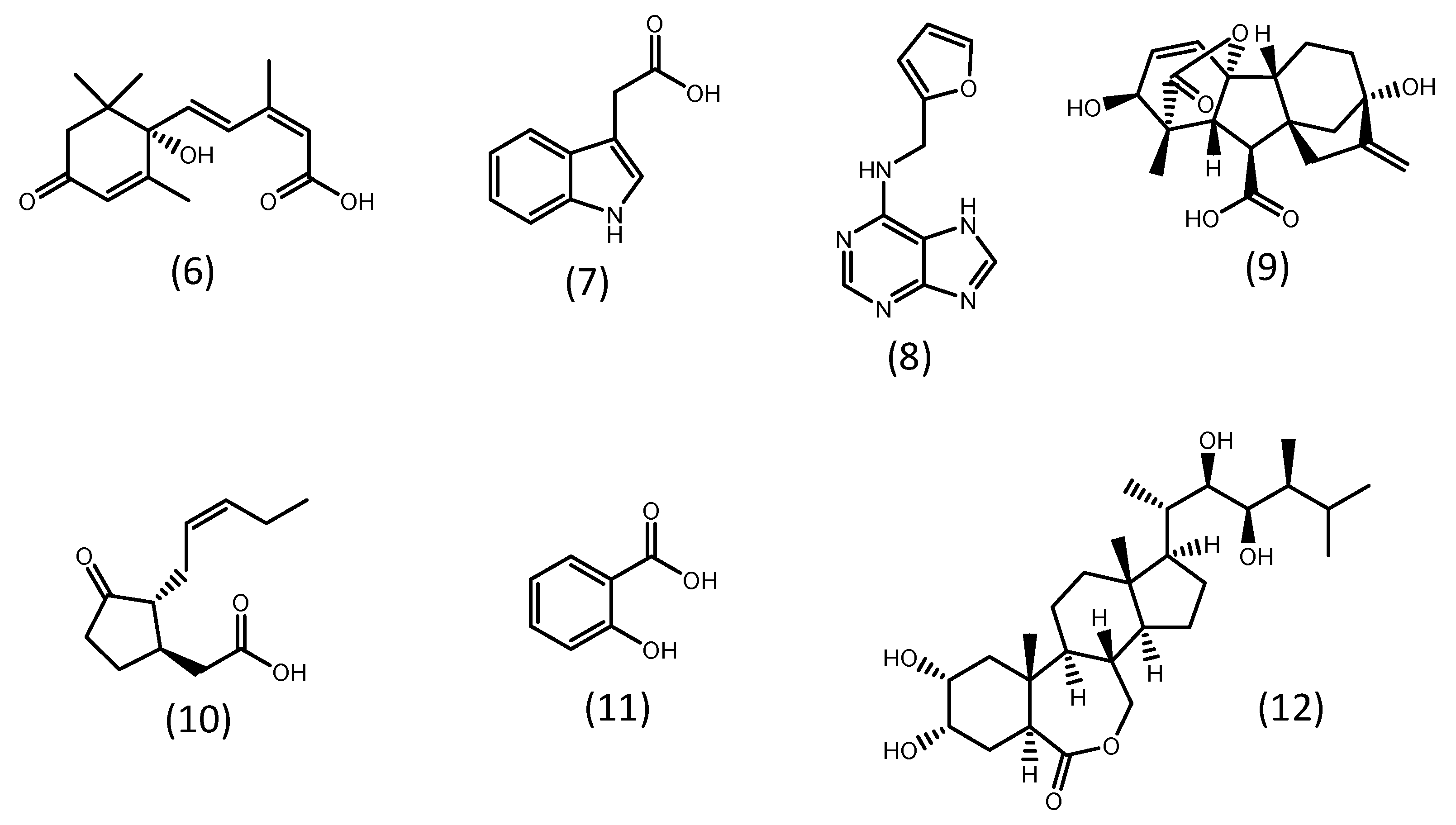
The improvement in microalgal biomass production by phytohormones is often correlated with the increase in photosynthetic activity due to an increment in the expression of photosynthetic enzymes and chlorophyll content [57][59][60][61][62][63]. Concerning the accumulation of lipids, in several studies, the upregulation of enzymes related to the biosynthesis of fatty acids, such as acetyl-CoA carboxylase (ACCase), an acyl carrier protein (ACP), malonyl-CoA: ACP-transacylase (MCTK), and fatty acid desaturase (FAD) was reported [61][62][64][65][66].
It is generally observed that phytohormones enhance the adaptability of microalgae to biotic and abiotic stress conditions. Thus, phytohormones can aid microalgae in overcoming constrained biomass production under stress conditions, resulting in higher lipid productivity [57][58][59][60]. The combined effect of stress and phytohormones has been evaluated in some studies with positive results. It is hypothesized that stress conditions inhibit cell growth due to the accumulation of reactive oxygen species (ROS). Phytohormones can aid the cells in keeping the ROS balance and reducing oxidative stress under these conditions by increasing the level of detoxifying enzymes and antioxidants [59][61][67][68].
The combined use of phytohormones on microalgae has also been assessed in some studies. Kozlova et al. [69] have evidenced the synergistic effects of 2,4-epibrassinolide (brassinosteroid) and indol-3-acetic acid (auxin) on cell growth and fatty acid accumulation of Scenedesmus quadricauda. A range of nano-concentrations of the combined phytohormones produced a 1.7-fold increase in cell density, and greater biomass and fatty acid production. These two phytohormones act synergistically in higher plants, and the cross-talk between their molecular pathways has been demonstrated [69].
5.3. Addition of Antioxidants and Other Bioactive Substances
Some studies have evidenced that adding antioxidant substances (Figure 3) to culture media can enhance microalgal lipid productivity. This is the case for propyl gallate and butylated hydroxytoluene (BHT), two well-known antioxidants used in the food and pharmaceutical industries, which enhanced biomass and lipid productivities in Schizochytrium sp. [70].
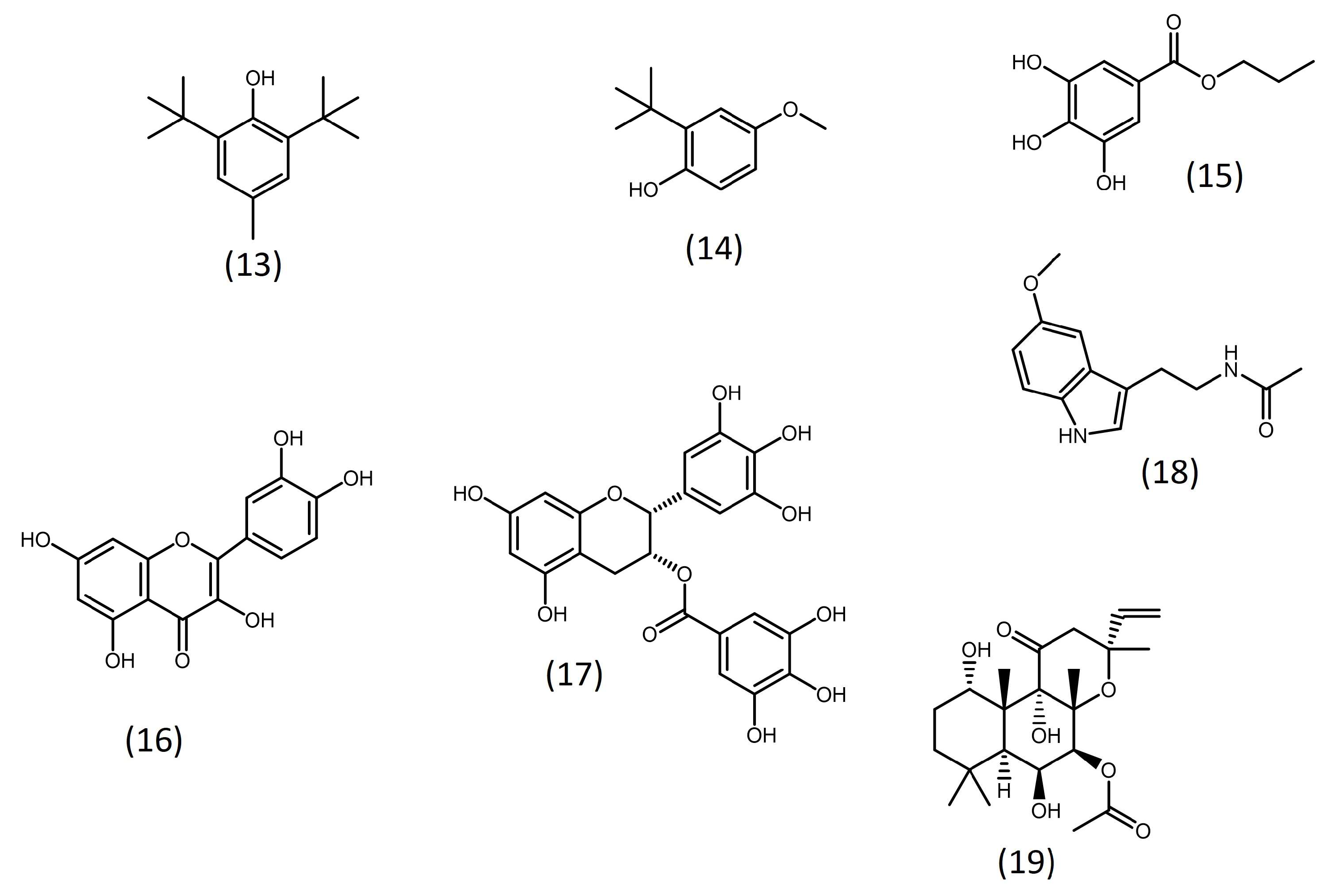
Moreover, butylated hydroxyanisole (BHA) and propyl gallate, as well as the natural polyphenolic antioxidant (−)-epigallocatechin gallate, were shown to increase lipid accumulation in Nannochloropsis salina by up to 60% without negatively affecting growth [71]. Similarly, the plant polyphenolic quercetin increased the biomass productivity and lipid content of Chlorella vulgaris by 2.5-fold and 1.8-fold, respectively [72].
Melatonin, another natural antioxidant, has also shown a positive effect on the lipid productivity of microalgae. It increased lipid accumulation of Monoraphidium sp. under normal and nitrogen-stress conditions by 1.2 to 1.4-fold, respectively [73][74]. The substance also increased the lipid productivity of Monoraphidium sp. under saline-induced stress [75].
The effect of antioxidants on microalgal biomass and lipid content is not entirely understood. The over-production of ROS negatively affects photosynthesis, and can damage macromolecules, including lipids. Thus, antioxidants could reduce ROS levels and improve growth performance and lipid biosynthesis, especially under stress conditions, in which ROS levels are known to rise [59][71][76].
Besides antioxidants, some studies evaluated other compounds as potential modulators of lipid accumulation. Franz et al. [71] conducted a phenotypic screening with 54 commercially available substances to identify small molecules that are able to increase the growth and lipid accumulation of four microalgal strains (Nannochloropsis salina, Nannochloropsis oculata, Nannochloris sp., and Phaeodactylum tricornutum). Bioactive molecules such as forskolin (Figure 3), cyclic adenosine monophosphate (cAMP), orlistat, quinacrine, as well as previously mentioned common antioxidants, were among the compounds considered most promising [71].
5.4. Co-Cultivation
In nature, microorganisms are found in complex and dynamic communities. Microalgae are not different; they live symbiotically with associated bacteria and fungi [77][78]. Thus, not surprisingly, co-cultures of microalgae with other microorganisms can potentially increase biomass and lipid productivity [78][79].
Among the possible strategies of this kind, the co-culturing of microalgae and oleaginous yeasts is one of the most studied. In this cultivation system, the microalgae provide O2 to the yeast, while the former provides CO2 to the microalgae. Moreover, organic acids produced by the yeast, which can inhibit its growth, can be taken up by the microalgae. The yeast can also metabolize complex sugars into simpler ones to be taken up by the microalgae. Another advantage of the association is the pH balance. While microalgae in monoculture tend to make the medium more alkaline, yeasts grown alone make the medium more acidic, which is detrimental to their growth. Together, they can keep the pH in an optimal range for both [78][80]. For instance, the co-cultivation of the microalgae Chlorella pyrenoidosa and the red yeast Rhodotorula glutinis in cassava bagasse hydrolysate reached a significantly higher biomass and lipid productivity when compared to monocultures [81].
Mutualistic interactions between microalgae and bacteria are also documented. Bacteria produce important substances for microalgal development, such as macronutrients, vitamins, siderophores, and growth stimulants [77][82][83]. Some studies reported the benefits of microalgae and bacteria co-cultivation for improving algal lipid productivity. For instance, Toyama et al. [84] observed that the co-cultivation of Euglena gracilis with the bacterium Emticicia sp. EG3 in wastewater enhanced the microalgae’s biomass and lipid content by 3.2 and 2.9-fold, respectively. Co-cultivation systems with bacteria can also improve and reduce harvesting costs by promoting cell bioflocculation [85][86].
6. Large-Scale Production: Current Scenario and Perspectives
Microalgal biodiesel production is in its infancy and has not yet achieved the commercial stage. An important challenge for the viability of commercial microalgal biodiesel production is the lower biomass and lipid productivity of large-scale outdoor microalgae cultivation [87]. For instance, Lu et al. [88] observed that the biomass productivity of Chlorella sp. in an outdoor PBR was more than 50% lower compared to bench-scale indoor conditions, while the lipid productivity was three times lower.
Even though most of the research on the subject has been carried out on a laboratory scale, some recent reports on pilot-scale studies and scale-up experiments are available [87][89][90][91][92][93][94][95][96][97][98][99]. According to Chisti [100], to have competitive prices relative to petroleum, algal biomass must be produced at around US$0.25/kg (dry weight). The biomass production of Synechocystis sp. in a large-scale cultivation OPR system was estimated to be approximately US$2–3/kg [90]. Similarly, the biomass cost of Scenedesmus acuminatus in OPR was predicted to be US$1.76/kg. On the other hand, the biomass production of the same species in PRB was calculated to be US$6.91/kg [101]. In another report, the biomass cost of Chlorella vulgaris in a pilot-scale outdoor PBR was estimated at US$14.3/kg [98]. These results are close to theoretical estimates, which projected the costs of biomass production in OPR to be around US$0.4–2.1/kg (€0.3–1.8) and in PRB around US$4.5–11.8/kg (€3.8–10) [102], confirming that production costs in OPRs are lower than in PBRs. However, the current costs are still far from the desirable condition, which evidences the need for optimization and technological improvement in the process.
A promising possibility to enhance the economic competitiveness of microalgal biodiesel is the production within a biorefinery concept. In a biorefinery, the biodiesel production from lipids would be integrated with the simultaneous production of other high value biomass components, such as carbohydrates, proteins, vitamins, terpenes, and carotenoids. These components can be extracted and transformed into a variety of by-products, such as bioethanol, biogas, chemicals, food supplements, animal feed, fertilizers, cosmetics, and nutraceuticals [103][104][105].
The aggregation of other valuable goods to the biodiesel production chain allows better material and energy utilization, enables greater product flexibility, reduces the generation of residues, and thus improves the feasibility of the whole process [103][104][105]. Some techno-economic evaluations have shown that biodiesel production in integrated biorefineries is more economically and environmentally favorable [103][106].
Another way to improve the economic feasibility of microalgal biodiesel is by reducing nutrient supply costs. Currently, large-scale microalgal cultivation uses commercial CO2 and agricultural fertilizers. It is, however, possible to project cultivation systems in which the CO2 is obtained from flue gases emitted by industries and/or in which nitrogen, phosphorus, and other nutrients are obtained from wastewater. These approaches can potentially reduce costs and the environmental impact of the process [100][103][107]. Nayak et al. [107] demonstrated the suitability of cultivating Scenedesmus sp. with domestic wastewater and CO2 from flue gas in both PBR and OPR.
7. Biodiesel from Microalgae in Brazil
The Brazilian government started to invest in biodiesel in 2004 with the creation of the National Program for the Production and Use of Biodiesel (PNPB), whose objective was to stimulate biodiesel production in the country. New technology routes and research development were established, and Brazil, together with the USA and Indonesia, are the major world producers and consumers of biodiesel. The biodiesel currently produced in Brazil is mainly obtained from animal fats and vegetable oils (1G biodiesel). Microalgae biomass has received attention for third-generation biofuel production due to its carbohydrate (for bioethanol) and TAG content for biodiesel production. However, their use has yet to achieve full industrial scale [108]. Several microalgae were isolated from Brazilian biomes, such as the Amazon Forest, the Cerrado, and the Pantanal flooded grasslands. In addition, wastewater deposits generated by industrial and agricultural activities are a source of microalgae [109].
Microalgal Brazilian biodiversity is very significant. For instance, Botryococcus found in Brazil, is a microalgae genus rich in monounsaturated fatty acids and is an excellent candidate for biodiesel production [110]. Brazilian companies, such as Petrobras (Petróleo Brasileiro S.A, Rio de Janeiro, Brazil) and Embrapa (Brasília, Brazil) have invested in microalgae research in recent years. A Program created by Embrapa isolated, identified and evaluated biotechnologically important microalgae species in Brazil, integrating the biorefinery concept into the biofuels [111].
Petrobras has invested in partnerships with public research universities to develop microalgae research since 2009. With this integrated approach, the company has overcome many scale-up challenges. Solutions for other challenges are in development and are being successfully applied in a project developed by Petrobras, with the State University of Campinas (UNICAMP), Federal University of Viçosa (UFV), the Federal University of Rio de Janeiro (UFRJ), the Federal University of Rio Grande (FURG), and the Federal University of Rio Grande do Norte (UFRN). With the last institution, an open ponds pilot plant to test microalgae for biodiesel production was developed (Figure 4) [112].
In conclusion, the future of microalgae biodiesel is bright and increasingly within a circular economic framework where waste residues from one industry serve as inputs to its production and where algae not only produce biodiesel but other bioproducts too. The improved economics of the scale-up process is in progress, and commercial production will be possible within a low-carbon economy shortly.

Figure 4. Petrobras pilot-scale open pond microalgae production facility in Rio Grande do Norte (Brazil).
References
- Halkos, G.E.; Gkampoura, E.-C. Reviewing Usage, Potentials, and Limitations of Renewable Energy Sources. Energies 2020, 13, 2906.
- International Energy Agency World Energy Outlook 2019. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 2 September 2022).
- Liu, Z.; Wang, K.; Chen, Y.; Tan, T.; Nielsen, J. Third-Generation Biorefineries as the Means to Produce Fuels and Chemicals from CO2. Nat. Catal. 2020, 3, 274–288.
- Jackson, R.B.; Friedlingstein, P.; Andrew, R.M.; Canadell, J.G.; Le Quéré, C.; Peters, G.P. Persistent Fossil Fuel Growth Threatens the Paris Agreement and Planetary Health. Environ. Res. Lett. 2019, 14, 121001.
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adelekan, I.; Adler, C.; Adrian, R.; Aldunce, P.; Ali, E.; Ara Begum, R.; BednarFriedl, B. Climate Change 2022: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022.
- ÓhAiseadha, C.; Quinn, G.; Connolly, R.; Connolly, M.; Soon, W. Energy and Climate Policy—An Evaluation of Global Climate Change Expenditure 2011–2018. Energies 2020, 13, 4839.
- Chen, Y.; Xu, C.; Vaidyanathan, S. Microalgae: A Robust “Green Bio-Bridge” between Energy and Environment. Crit. Rev. Biotechnol. 2018, 38, 351–368.
- Arenas, E.G.; Rodriguez Palacio, M.C.; Juantorena, A.U.; Fernando, S.E.L.; Sebastian, P.J. Microalgae as a Potential Source for Biodiesel Production: Techniques, Methods, and Other Challenges. Int. J. Energy Res. 2017, 41, 761–789.
- González-González, L.M.; Correa, D.F.; Ryan, S.; Jensen, P.D.; Pratt, S.; Schenk, P.M. Integrated Biodiesel and Biogas Production from Microalgae: Towards a Sustainable Closed Loop through Nutrient Recycling. Renew. Sustain. Energy Rev. 2018, 82, 1137–1148.
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36.
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Yuvaraj, D.; Jayamuthunagai, J.; Kumar, R.P.; Palani, S. Biodiesel Production Using Chemical and Biological Methods—A Review of Process, Catalyst, Acyl Acceptor, Source and Process Variables. Renew. Sustain. Energy Rev. 2014, 38, 368–382.
- Daroch, M.; Geng, S.; Wang, G. Recent Advances in Liquid Biofuel Production from Algal Feedstocks. Appl. Energy 2013, 102, 1371–1381.
- Bharti, R.K.; Singh, A.; Dhar, D.W.; Kaushik, A. Biological Carbon Dioxide Sequestration by Microalgae for Biofuel and Biomaterials Production. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 137–153.
- Karlsson, H.; Ahlgren, S.; Sandgren, M.; Passoth, V.; Wallberg, O.; Hansson, P.-A. Greenhouse Gas Performance of Biochemical Biodiesel Production from Straw: Soil Organic Carbon Changes and Time-Dependent Climate Impact. Biotechnol. Biofuels 2017, 10, 217.
- Georgianna, D.R.; Mayfield, S.P. Exploiting Diversity and Synthetic Biology for the Production of Algal Biofuels. Nature 2012, 488, 329–335.
- Matos, Â.P. Advances in Microalgal Research in Brazil. Braz. Arch. Biol. Technol. 2021, 64, e21200531.
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An Overview to Process Design, Simulation and Sustainability Evaluation of Biodiesel Production. Biotechnol. Biofuels 2021, 14, 129.
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Microalgae Taxonomy and Breeding. In Biofuel Crops: Production, Physiology and Genetics; CABI: Wallingford, UK, 2013; pp. 44–53.
- Sajjadi, B.; Chen, W.-Y.Y.; Raman, A.A.A.; Ibrahim, S. Microalgae Lipid and Biomass for Biofuel Production: A Comprehensive Review on Lipid Enhancement Strategies and Their Effects on Fatty Acid Composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232.
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232.
- Evert, R.F.; Eichhorn, S.E. Raven Biology of Plants, 8th ed.; Freeman and Co.: New York, NY, USA, 2013.
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal Lipids: A Review of Lipids Potential and Quantification for 95 Phytoplankton Species. Biomass Bioenergy 2021, 150, 106108.
- Bhujade, R.; Chidambaram, M.; Kumar, A.; Sapre, A. Algae to Economically Viable Low-Carbon-Footprint Oil. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 335–357.
- Heredia, V.; Gonçalves, O.; Marchal, L.; Pruvost, J. Producing Energy-Rich Microalgae Biomass for Liquid Biofuels: Influence of Strain Selection and Culture Conditions. Energies 2021, 14, 1246.
- Chen, H.-H.H.; Jiang, J.-G.G. Lipid Accumulation Mechanisms in Auto- and Heterotrophic Microalgae. J. Agric. Food Chem. 2017, 65, 8099–8110.
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and Studies on Lipid and Pigments of Microalgae: A Review. Renew. Sustain. Energy Rev. 2016, 58, 832–841.
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The Lipid Biochemistry of Eukaryotic Algae. Prog. Lipid Res. 2019, 74, 31–68.
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from Microalgae: A Critical Evaluation from Laboratory to Large Scale Production. Appl. Energy 2013, 103, 444–467.
- Veillette, M.; Giroir-Fendler, A.; Faucheux, N.; Heitz, M. Biodiesel from Microalgae Lipids: From Inorganic Carbon to Energy Production. Biofuels 2018, 9, 175–202.
- Hossain, N.; Mahlia, T.M.I. Progress in Physicochemical Parameters of Microalgae Cultivation for Biofuel Production. Crit. Rev. Biotechnol. 2019, 39, 835–859.
- Kumar, K.; Mishra, S.K.; Shrivastav, A.; Park, M.S.; Yang, J.-W. Recent Trends in the Mass Cultivation of Algae in Raceway Ponds. Renew. Sustain. Energy Rev. 2015, 51, 875–885.
- Correa, D.F.; Beyer, H.L.; Possingham, H.P.; Thomas-Hall, S.R.; Schenk, P.M. Global Mapping of Cost-effective Microalgal Biofuel Production Areas with Minimal Environmental Impact. GCB Bioenergy 2019, 11, gcbb.12619.
- Wang, B.; Lan, C.Q.; Horsman, M. Closed Photobioreactors for Production of Microalgal Biomasses. Biotechnol. Adv. 2012, 30, 904–912.
- Behera, B.; Acharya, A.; Gargey, I.A.; Aly, N.P.B. Bioprocess Engineering Principles of Microalgal Cultivation for Sustainable Biofuel Production. Bioresour. Technol. Rep. 2019, 5, 297–316.
- Pawar, S. Effectiveness Mapping of Open Raceway Pond and Tubular Photobioreactors for Sustainable Production of Microalgae Biofuel. Renew. Sustain. Energy Rev. 2016, 62, 640–653.
- Singh, K.; Kaloni, D.; Gaur, S.; Kushwaha, S.; Mathur, G. Current Research and Perspectives on Microalgae-Derived Biodiesel. Biofuels 2017, 11, 1–18.
- Banerjee, S.; Ramaswamy, S. Comparison of Productivity and Economic Analysis of Microalgae Cultivation in Open Raceways and Flat Panel Photobioreactor. Bioresour. Technol. Rep. 2019, 8, 100328.
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of Microalgae Cultivation in Photobioreactor, Open Raceway Pond, and a Two-Stage Hybrid System. Front. Energy Res. 2016, 4, 1–10.
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Two-Stage Cultivation of Microalgae for Production of High-Value Compounds and Biofuels: A Review. Algal Res. 2021, 57, 102353.
- Liu, W.; Chen, Y.; Wang, J.; Liu, T. Biomass Productivity of Scenedesmus dimorphus (Chlorophyceae) Was Improved by Using an Open Pond–Photobioreactor Hybrid System. Eur. J. Phycol. 2019, 54, 127–134.
- Yun, J.-H.; Cho, D.-H.; Lee, S.; Heo, J.; Tran, Q.-G.; Chang, Y.K.; Kim, H.-S. Hybrid Operation of Photobioreactor and Wastewater-Fed Open Raceway Ponds Enhances the Dominance of Target Algal Species and Algal Biomass Production. Algal Res. 2018, 29, 319–329.
- Passell, H.; Dhaliwal, H.; Reno, M.; Wu, B.; Ben Amotz, A.; Ivry, E.; Gay, M.; Czartoski, T.; Laurin, L.; Ayer, N. Algae Biodiesel Life Cycle Assessment Using Current Commercial Data. J. Environ. Manage. 2013, 129, 103–111.
- Delrue, F.; Setier, P.-A.; Sahut, C.; Cournac, L.; Roubaud, A.; Peltier, G.; Froment, A.-K. An Economic, Sustainability, and Energetic Model of Biodiesel Production from Microalgae. Bioresour. Technol. 2012, 111, 191–200.
- Remmers, I.M.; Wijffels, R.H.; Barbosa, M.J.; Lamers, P.P. Can We Approach Theoretical Lipid Yields in Microalgae? Trends Biotechnol. 2018, 36, 265–276.
- Aratboni, H.A.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and Lipid Induction Strategies in Microalgae for Biofuel Production and Other Applications. Microb. Cell Fact. 2019, 18, 178.
- Solovchenko, A.; Khozin-Goldberg, I.; Selyakh, I.; Semenova, L.; Ismagulova, T.; Lukyanov, A.; Mamedov, I.; Vinogradova, E.; Karpova, O.; Konyukhov, I.; et al. Phosphorus Starvation and Luxury Uptake in Green Microalgae Revisited. Algal Res. 2019, 43, 101651.
- da Silva Ferreira, V.; Sant’Anna, C. The Effect of Physicochemical Conditions and Nutrient Sources on Maximizing the Growth and Lipid Productivity of Green Microalgae. Phycol. Res. 2017, 65, 3–13.
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and Novel Strategies for Enhancing Lipid Accumulation and Quality in Microalgae. Renew. Sustain. Energy Rev. 2016, 55, 1–16.
- Tan, K.W.M.; Lee, Y.K. The Dilemma for Lipid Productivity in Green Microalgae: Importance of Substrate Provision in Improving Oil Yield without Sacrificing Growth. Biotechnol. Biofuels 2016, 9, 255.
- Aziz, M.M.A.; Kassim, K.A.; Shokravi, Z.; Jakarni, F.M.; Liu, H.Y.; Zaini, N.; Tan, L.S.; Islam, A.B.M.S.; Shokravi, H. Two-Stage Cultivation Strategy for Simultaneous Increases in Growth Rate and Lipid Content of Microalgae: A Review. Renew. Sustain. Energy Rev. 2020, 119, 109621.
- Nagappan, S.; Devendran, S.; Tsai, P.-C.; Dahms, H.-U.; Ponnusamy, V.K. Potential of Two-Stage Cultivation in Microalgae Biofuel Production. Fuel 2019, 252, 339–349.
- Aléman-Nava, G.S.; Muylaert, K.; Cuellar Bermudez, S.P.; Depraetere, O.; Rittmann, B.; Parra-Saldívar, R.; Vandamme, D. Two-Stage Cultivation of Nannochloropsis oculata for Lipid Production Using Reversible Alkaline Flocculation. Bioresour. Technol. 2017, 226, 18–23.
- Su, C.H.; Chien, L.J.; Gomes, J.; Lin, Y.S.; Yu, Y.K.; Liou, J.S.; Syu, R.J. Factors Affecting Lipid Accumulation by Nannochloropsis oculata in a Two-Stage Cultivation Process. J. Appl. Phycol. 2011, 23, 903–908.
- Nayak, M.; Suh, W.I.; Chang, Y.K.; Lee, B. Exploration of Two-Stage Cultivation Strategies Using Nitrogen Starvation to Maximize the Lipid Productivity in Chlorella Sp. HS2. Bioresour. Technol. 2019, 276, 110–118.
- Xia, L.; Ge, H.; Zhou, X.; Zhang, D.; Hu, C. Photoautotrophic Outdoor Two-Stage Cultivation for Oleaginous Microalgae Scenedesmus obtusus XJ-15. Bioresour. Technol. 2013, 144, 261–267.
- Lu, Y.; Xu, J. Phytohormones in Microalgae: A New Opportunity for Microalgal Biotechnology? Trends Plant Sci. 2015, 20, 273–282.
- Han, X.; Zeng, H.; Bartocci, P.; Fantozzi, F.; Yan, Y. Phytohormones and Effects on Growth and Metabolites of Microalgae: A Review. Fermentation 2018, 4, 25.
- Wang, C.; Qi, M.; Guo, J.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. The Active Phytohormone in Microalgae: The Characteristics, Efficient Detection, and Their Adversity Resistance Applications. Molecules 2021, 27, 46.
- Zhao, Y.; Wang, H.; Han, B.; Yu, X. Coupling of Abiotic Stresses and Phytohormones for the Production of Lipids and High-Value by-Products by Microalgae: A Review. Bioresour. Technol. 2019, 274, 549–556.
- Renuka, N.; Guldhe, A.; Singh, P.; Ansari, F.A.; Rawat, I.; Bux, F. Evaluating the Potential of Cytokinins for Biomass and Lipid Enhancement in Microalga Acutodesmus obliquus under Nitrogen Stress. Energy Convers. Manag. 2017, 140, 14–23.
- Babu, A.G.; Wu, X.; Kabra, A.N.; Kim, D. Cultivation of an Indigenous Chlorella sorokiniana with Phytohormones for Biomass and Lipid Production under N-Limitation. Algal Res. 2017, 23, 178–185.
- Guldhe, A.; Renuka, N.; Singh, P.; Bux, F. Effect of Phytohormones from Different Classes on Gene Expression of Chlorella Sorokiniana under Nitrogen Limitation for Enhanced Biomass and Lipid Production. Algal Res. 2019, 40, 101518.
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone Addition Coupled with Nitrogen Depletion Almost Tripled the Lipid Productivities in Two Algae. Bioresour. Technol. 2018, 247, 904–914.
- Du, H.; Ren, J.; Li, Z.; Zhang, H.; Wang, K.; Lin, B.; Zheng, S.; Zhao, C.; Meng, C.; Gao, Z. Plant Growth Regulators Affect Biomass, Protein, Carotenoid, and Lipid Production in Botryococcus braunii. Aquac. Int. 2020, 28, 1319–1340.
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Aziz, A.; Cha, T.S. Indole-3-Acetic Acid (IAA) Induced Changes in Oil Content, Fatty Acid Profiles and Expression of Four Fatty Acid Biosynthetic Genes in Chlorella vulgaris at Early Stationary Growth Phase. Phytochemistry 2015, 111, 65–71.
- Song, X.; Zhao, Y.; Li, T.; Han, B.; Zhao, P.; Xu, J.; Yu, X. Enhancement of Lipid Accumulation in Monoraphidium Sp. QLY-1 by Induction of Strigolactone. Bioresour. Technol. 2019, 288, 121607.
- Udayan, A.; Sabapathy, H.; Arumugam, M. Stress Hormones Mediated Lipid Accumulation and Modulation of Specific Fatty Acids in Nannochloropsis oceanica CASA CC201. Bioresour. Technol. 2020, 310, 123437.
- González-Garcinuño, Á.; Sánchez-Álvarez, J.M.; Galán, M.A.; Martin del Valle, E.M. Understanding and Optimizing the Addition of Phytohormones in the Culture of Microalgae for Lipid Production. Biotechnol. Prog. 2016, 32, 1203–1211.
- Kozlova, T.A.; Hardy, B.P.; Levin, D.B. The Combined Influence of 24-epibrassinolide and 3-indoleacetic Acid on Growth and Accumulation of Pigments and Fatty Acids in the Microalgae Scenedesmus quadricauda (CPCC-158). Algal Res. 2018, 35, 22–32.
- Singh, D.; Mathur, A.S.; Tuli, D.K.; Puri, M.; Barrow, C.J. Propyl Gallate and Butylated Hydroxytoluene Influence the Accumulation of Saturated Fatty Acids, Omega-3 Fatty Acid and Carotenoids in Thraustochytrids. J. Funct. Foods 2015, 15, 186–192.
- Franz, A.K.; Danielewicz, M.A.; Wong, D.M.; Anderson, L.A.; Boothe, J.R. Phenotypic Screening with Oleaginous Microalgae Reveals Modulators of Lipid Productivity. ACS Chem. Biol. 2013, 8, 1053–1062.
- Ma, Y.; Balamurugan, S.; Yuan, W.; Yang, F.; Tang, C.; Hu, H.; Zhang, H.; Shu, X.; Li, M.; Huang, S.; et al. Quercetin Potentiates the Concurrent Hyper-Accumulation of Cellular Biomass and Lipids in Chlorella vulgaris. Bioresour. Technol. 2018, 269, 434–442.
- Zhao, Y.; Li, D.; Xu, J.-W.; Zhao, P.; Li, T.; Ma, H.; Yu, X. Melatonin Enhances Lipid Production in Monoraphidium sp. QLY-1 under Nitrogen Deficiency Conditions via a Multi-Level Mechanism. Bioresour. Technol. 2018, 259, 46–53.
- Li, D.; Zhao, Y.; Ding, W.; Zhao, P.; Xu, J.-W.; Li, T.; Ma, H.; Yu, X. A Strategy for Promoting Lipid Production in Green Microalgae Monoraphidium Sp. QLY-1 by Combined Melatonin and Photoinduction. Bioresour. Technol. 2017, 235, 104–112.
- Zhao, Y.; Song, X.; Zhao, P.; Li, T.; Xu, J.-W.; Yu, X. Role of Melatonin in Regulation of Lipid Accumulation, Autophagy and Salinity-Induced Oxidative Stress in Microalga Monoraphidium Sp. QLY-1. Algal Res. 2021, 54, 102196.
- Meng, Y.; Chen, H.; Liu, J.; Zhang, C.-Y. Melatonin Facilitates the Coordination of Cell Growth and Lipid Accumulation in Nitrogen-Stressed Chlamydomonas reinhardtii for Biodiesel Production. Algal Res. 2020, 46, 101786.
- Lian, J.; Wijffels, R.H.; Smidt, H.; Sipkema, D. The Effect of the Algal Microbiome on Industrial Production of Microalgae. Microb. Biotechnol. 2018, 11, 806–818.
- Arora, N.; Patel, A.; Mehtani, J.; Pruthi, P.A.; Pruthi, V.; Poluri, K.M. Co-Culturing of Oleaginous Microalgae and Yeast: Paradigm Shift towards Enhanced Lipid Productivity. Environ. Sci. Pollut. Res. 2019, 26, 16952–16973.
- Ray, A.; Nayak, M.; Ghosh, A. A Review on Co-Culturing of Microalgae: A Greener Strategy towards Sustainable Biofuels Production. Sci. Total Environ. 2022, 802, 149765.
- Dias, C.; Santos, J.; Reis, A.; Lopes da Silva, T. Yeast and Microalgal Symbiotic Cultures Using Low-Cost Substrates for Lipid Production. Bioresour. Technol. Rep. 2019, 7, 100261.
- Liu, L.; Chen, J.; Lim, P.-E.; Wei, D. Enhanced Single Cell Oil Production by Mixed Culture of Chlorella pyrenoidosa and Rhodotorula glutinis Using Cassava Bagasse Hydrolysate as Carbon Source. Bioresour. Technol. 2018, 255, 140–148.
- Palacios, O.A.; López, B.R.; De-Bashan, L.E. Microalga Growth-Promoting Bacteria (MGPB): A Formal Term Proposed for Beneficial Bacteria Involved in Microalgal–Bacterial Interactions. Algal Res. 2022, 61, 102585.
- Tandon, P.; Jin, Q.; Huang, L. A Promising Approach to Enhance Microalgae Productivity by Exogenous Supply of Vitamins. Microb. Cell Fact. 2017, 16, 219.
- Toyama, T.; Hanaoka, T.; Yamada, K.; Suzuki, K.; Tanaka, Y.; Morikawa, M.; Mori, K. Enhanced Production of Biomass and Lipids by Euglena gracilis via Co-Culturing with a Microalga Growth-Promoting Bacterium, Emticicia Sp. EG3. Biotechnol. Biofuels 2019, 12, 205.
- Lakshmikandan, M.; Wang, S.; Murugesan, A.G.; Saravanakumar, M.; Selvakumar, G. Co-Cultivation of Streptomyces and Microalgal Cells as an Efficient System for Biodiesel Production and Bioflocculation Formation. Bioresour. Technol. 2021, 332, 125118.
- Feng, Y.; Xiao, J.; Cui, N.; Zhao, Y.; Zhao, P. Enhancement of Lipid Productivity and Self-Flocculation by Cocultivating Monoraphidium Sp. FXY-10 and Heveochlorella Sp. Yu under Mixotrophic Mode. Appl. Biochem. Biotechnol. 2021, 193, 3173–3186.
- Mendes, L.B.B.; Viegas, C.V.; Joao, R.R.; da Silva, R.B. Microalgae Production: A Sustainable Alternative for a Low-Carbon Economy Transition. Open Microalgae Biotechnol. 2021, 1, 1–7.
- Lu, W.; Wang, Z.; Wang, X.; Yuan, Z. Cultivation of Chlorella sp. Using Raw Dairy Wastewater for Nutrient Removal and Biodiesel Production: Characteristics Comparison of Indoor Bench-Scale and Outdoor Pilot-Scale Cultures. Bioresour. Technol. 2015, 192, 382–388.
- Corrêa, D.d.O.; Duarte, M.E.R.; Noseda, M.D. Biomass Production and Harvesting of Desmodesmus subspicatus Cultivated in Flat Plate Photobioreactor Using Chitosan as Flocculant Agent. J. Appl. Phycol. 2019, 31, 857–866.
- Ashokkumar, V.; Chen, W.-H.; Ngamcharussrivichai, C.; Agila, E.; Ani, F.N. Potential of Sustainable Bioenergy Production from Synechocystis Sp. Cultivated in Wastewater at Large Scale—A Low Cost Biorefinery Approach. Energy Convers. Manag. 2019, 186, 188–199.
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Santos, M.; Freitas, M.; Mata, T.; Martins, A.; Caetano, N. Potential of Phaeodactylum tricornutum for Biodiesel Production under Natural Conditions in Chile. Energies 2017, 11, 54.
- He, Q.; Yang, H.; Hu, C. Culture Modes and Financial Evaluation of Two Oleaginous Microalgae for Biodiesel Production in Desert Area with Open Raceway Pond. Bioresour. Technol. 2016, 218, 571–579.
- Kumar, A.K.; Sharma, S.; Shah, E.; Parikh, B.S.; Patel, A.; Dixit, G.; Gupta, S.; Divecha, J.M. Cultivation of Ascochloris Sp. ADW007-Enriched Microalga in Raw Dairy Wastewater for Enhanced Biomass and Lipid Productivity. Int. J. Environ. Sci. Technol. 2019, 16, 943–954.
- Koley, S.; Mathimani, T.; Bagchi, S.K.; Sonkar, S.; Mallick, N. Microalgal Biodiesel Production at Outdoor Open and Polyhouse Raceway Pond Cultivations: A Case Study with Scenedesmus accuminatus Using Low-Cost Farm Fertilizer Medium. Biomass Bioenergy 2019, 120, 156–165.
- Tamil Selvan, S.; Velramar, B.; Ramamurthy, D.; Balasundaram, S.; Sivamani, K. Pilot Scale Wastewater Treatment, CO2 Sequestration and Lipid Production Using Microalga, Neochloris aquatica RDS02. Int. J. Phytoremediation 2020, 0, 1–18.
- Wen, X.; Du, K.; Wang, Z.; Peng, X.; Luo, L.; Tao, H.; Xu, Y.; Zhang, D.; Geng, Y.; Li, Y. Effective Cultivation of Microalgae for Biofuel Production: A Pilot-Scale Evaluation of a Novel Oleaginous Microalga Graesiella Sp. WBG-1. Biotechnol. Biofuels 2016, 9, 123.
- Lam, M.K.; Lee, K.T. Cultivation of Chlorella vulgaris in a Pilot-Scale Sequential-Baffled Column Photobioreactor for Biomass and Biodiesel Production. Energy Convers. Manag. 2014, 88, 399–410.
- Huntley, M.E.; Johnson, Z.I.; Brown, S.L.; Sills, D.L.; Gerber, L.; Archibald, I.; Machesky, S.C.; Granados, J.; Beal, C.; Greene, C.H. Demonstrated Large-Scale Production of Marine Microalgae for Fuels and Feed. Algal Res. 2015, 10, 249–265.
- Abu Jayyab, M.; Al-Zuhair, S. Use of Microalgae for Simultaneous Industrial Wastewater Treatment and Biodiesel Production. Int. J. Environ. Res. 2020, 14, 311–322.
- Chisti, Y. Constraints to Commercialization of Algal Fuels. J. Biotechnol. 2013, 167, 201–214.
- Jin, H.; Zhang, H.; Zhou, Z.; Li, K.; Hou, G.; Xu, Q.; Chuai, W.; Zhang, C.; Han, D.; Hu, Q. Ultrahigh-cell-density Heterotrophic Cultivation of the Unicellular Green Microalga Scenedesmus acuminatus and Application of the Cells to Photoautotrophic Culture Enhance Biomass and Lipid Production. Biotechnol. Bioeng. 2020, 117, 96–108.
- Slade, R.; Bauen, A. Micro-Algae Cultivation for Biofuels: Cost, Energy Balance, Environmental Impacts and Future Prospects. Biomass Bioenergy 2013, 53, 29–38.
- Kumar, D.; Singh, B. Algal Biorefinery: An Integrated Approach for Sustainable Biodiesel Production. Biomass Bioenergy 2019, 131, 105398.
- Zhu, L. Biorefinery as a Promising Approach to Promote Microalgae Industry: An Innovative Framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384.
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Freitas, M.A.V.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Biotechnological Potential of Phaeodactylum tricornutum for Biorefinery Processes. Fuel 2020, 268, 117357.
- Giwa, A.; Adeyemi, I.; Dindi, A.; Lopez, C.G.-B.; Lopresto, C.G.; Curcio, S.; Chakraborty, S. Techno-Economic Assessment of the Sustainability of an Integrated Biorefinery from Microalgae and Jatropha: A Review and Case Study. Renew. Sustain. Energy Rev. 2018, 88, 239–257.
- Nayak, M.; Karemore, A.; Sen, R. Sustainable Valorization of Flue Gas CO2 and Wastewater for the Production of Microalgal Biomass as a Biofuel Feedstock in Closed and Open Reactor Systems. RSC Adv. 2016, 6, 91111–91120.
- Ramos, M.D.N.; Milessi, T.S.; Candido, R.G.; Mendes, A.A.; Aguiar, A. Enzymatic Catalysis as a Tool in Biofuels Production in Brazil: Current Status and Perspectives. Energy Sustain. Dev. 2022, 68, 103–119.
- Andrade, D.S.; Telles, T.S.; Leite Castro, G.H. The Brazilian Microalgae Production Chain and Alternatives for Its Consolidation. J. Clean. Prod. 2020, 250, 119526.
- Cabanelas, I.T.D.; Marques, S.S.I.; de Souza, C.O.; Druzian, J.I.; Nascimento, I.A. Botryococcus, What to Do with It? Effect of Nutrient Concentration on Biorefinery Potential. Algal Res. 2015, 11, 43–49.
- Brasil, B.S.A.; Silva, F.C.P.; Siqueira, F.G. Microalgae Biorefineries: The Brazilian Scenario in Perspective. New Biotechnol. 2017, 39, 90–98.
- Brantes, L.; Mendes, B.; Vermelho, A.B. Allelopathy as a Potential Strategy to Improve Microalgae Cultivation. Biotechnol. Biofuels 2013, 6, 1.





