Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mieszko M. Wilk | -- | 2190 | 2023-01-11 11:30:24 | | | |
| 2 | Dean Liu | Meta information modification | 2190 | 2023-01-12 01:50:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Szwejser-Zawislak, E.; Wilk, M.M.; Piszczek, P.; Krawczyk, J.; Wilczyńska, D.; Hozbor, D. Effects of Whole-Cell and Acellular Pertussis Vaccines. Encyclopedia. Available online: https://encyclopedia.pub/entry/40024 (accessed on 07 February 2026).
Szwejser-Zawislak E, Wilk MM, Piszczek P, Krawczyk J, Wilczyńska D, Hozbor D. Effects of Whole-Cell and Acellular Pertussis Vaccines. Encyclopedia. Available at: https://encyclopedia.pub/entry/40024. Accessed February 07, 2026.
Szwejser-Zawislak, Ewa, Mieszko M. Wilk, Piotr Piszczek, Justyna Krawczyk, Daria Wilczyńska, Daniela Hozbor. "Effects of Whole-Cell and Acellular Pertussis Vaccines" Encyclopedia, https://encyclopedia.pub/entry/40024 (accessed February 07, 2026).
Szwejser-Zawislak, E., Wilk, M.M., Piszczek, P., Krawczyk, J., Wilczyńska, D., & Hozbor, D. (2023, January 11). Effects of Whole-Cell and Acellular Pertussis Vaccines. In Encyclopedia. https://encyclopedia.pub/entry/40024
Szwejser-Zawislak, Ewa, et al. "Effects of Whole-Cell and Acellular Pertussis Vaccines." Encyclopedia. Web. 11 January, 2023.
Copy Citation
After the pertussis vaccine had been introduced in the 1940s and was shown to be very successful in reducing the morbidity and mortality associated with the disease, the possibility of improving both vaccine composition and vaccination schedules has become the subject of continuous interest.
pertussis
Bordetella pertussis
acellular pertussis vaccine
whole cell pertussis vaccine
aP
wP
DTP
1. Acellular and Whole-Cell Pertussis Vaccine Induce Different Immune Responses
The natural immunity resulting from previous infection is considered more effective than the immunity resulting from vaccinations, and pertussis is no exception [1]. During the recognition stage, B. pertussis is detected by the resident innate immune cells within the host, providing the first line of immediate defense [2][3]. Upon the recognition of the bacteria, alveolar macrophages present on the mucosal surface of the lungs phagocytose and kill bacteria rapidly upon their recognition [4]. On the other hand, bacterial virulence factors exert potent immunoregulatory activities that can result in modulation of pathogenesis by suppression of antigen delivery into the draining lymph nodes or enhancing transmission of B. pertussis. It was demonstrated that the pertussis toxin (PT) limits the early adaptive immune response by suppressing dendritic cells migration to the draining lymph nodes from the lungs [5]. In addition, Holubova et al. demonstrated that adhesins of B. pertussis, the filamentous hemagglutinin (Fha), and fimbriae (Fim) are critical for the infection of nasal cavity and transmission [6]. The difference between vaccination and natural infection, with regard to the triggering of the immune response, resides mainly in the different routes of entry (mucosa vs. systemic) and presentation of antigens/immunogens to antigen-presenting cells. Several authors have also described differences between the immunity induced by aP immunization and that induced by natural infection and wP vaccines [7][8]. It was demonstrated that natural infection and wP vaccination induce Th1- and Th17-dominated responses [9][10][11][12][13]. In contrast, aP vaccines formulated with alum as an adjuvant induce Th2-dominated responses, as evidenced by a significant increase in the levels of cytokines such as IL-4 and IL-5, and a slight increase in IFN-γ levels [14].
Recent scientific reports described the importance of specific tissue-resident memory T cells (Trm) within the upper and lower respiratory tract in both reducing transmission and inducing long-term protection against pertussis [15][16][17]. IL-17A secreted by CD4+ Trm cells have been shown to be involved in the clearance of B. pertussis from the nasopharynx and to promote the recruitment of neutrophils, particularly Siglec-F+ (a lectin normally expressed on mouse eosinophils) neutrophils, to the nasal mucosa upon reinfection [18]. According to a study by Borkner et al. [17], mice lacking IL-17 are incapable of clearing B. pertussis from nasal mucosa. In the course of B. pertussis infection, CD69+CD4+ Trm cells producing IL-17 accumulate within the upper and lower respiratory tract, including the nasal cavity, and their levels rise significantly following re-exposure to provide protection against colonization and reinfection [15]. The results presented by Wilk et al. [18] show that, in contrast to immunization with an aP-containing vaccine, immunization with a wP-containing vaccine results in the accumulation of CD69+CD4+ Trm cells within the upper respiratory tract following re-exposure to B. pertussis in mice. Additionally, in baboons, the administration of a wP-containing vaccine was shown to significantly reduce the B. pertussis loads within the nasopharynx and prevent the transmission of bacteria to other, non-infected animals [19]. In the same study, immunization with an aP-containing vaccine was shown to protect the animals from the symptoms of disease, while being unable to shorten the B. pertussis dwell times within the nasopharynx and prevent transmission to non-infected baboons [19]. These differences may explain why immunization with aP vaccines is not sufficient for inducing local response to infection and counteracting the persistent burden of B. pertussis within the upper respiratory tract [19][20]. Moreover, in individuals vaccinated with aP, no increase in Th1 and Th17 lymphocyte activity has been detected, even after the subsequent revaccinations with aP vaccines [21][22][23]. In contrast, the expansion of Th1 and Th17 cells was described in individuals immunized with wP vaccine and boosted with wP or aP vaccines [24]. The main differences in the immune responses induced by aP- and wP-containing vaccines are illustrated in Figure 1.
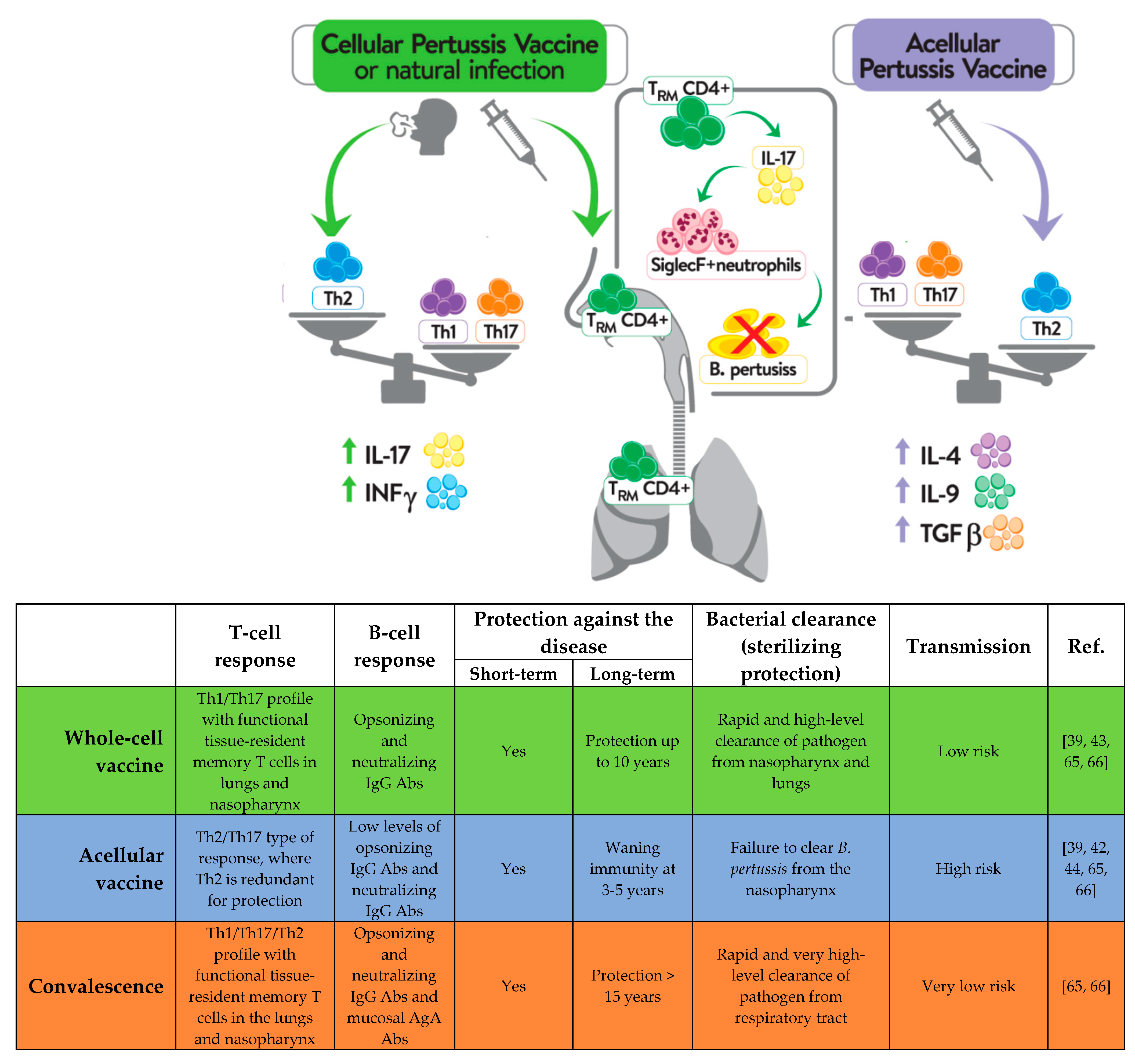
Figure 1. Different immune response to natural B. pertussis infection and immunization with vaccines containing a whole-cell pertussis component or an acellular pertussis component.
Available immunological data indicate that Th1 and Th17-mediated immune responses and formation of Trm cells are required for bacterial clearance and long-lasting protection. However, antibodies are equally important providing protection against disease [25]. While the T cell immunity induced by the two types of vaccines has been well-studied, much less attention has been paid to B cell responses following pertussis vaccination, even though the potentially effective pertussis vaccines were selected on the basis of antigen-specific antibody (Ab) levels. In order to identify better markers of vaccine-induced memory, Weaver et al. [25] analyzed long-term immunity from pertussis vaccines and observed that responses to wP vaccination were characterized by a significant increase in T follicular helper cells in the draining lymph nodes (dLNs) and CXCL13 levels in sera, compared to aP-immunized mice. In addition, the study revealed that only immunization with wP vaccine resulted in the generation of B. pertussis-specific memory B cells [25]. Recent studies also demonstrated that priming mice with wP vaccines led to faster and stronger stimulation of local germinal centers stimulation and memory B cell responses within the dLNs, as well as to plasma cells induced within the bone marrow presenting with a broader antigenic and isotypic diversity, in comparison to prime immunization with aP vaccine [26]. Importantly, the results indicated that, in contrast to T cell responses, where the first dose of aP vaccine determines the immune response to a boost vaccination with wP, the B cell profile and antibody production induced by aP were re-oriented by a wP boost and led to induction of Ab with a broader isotypic diversity [26]. Whether this re-oriented profile of B cell response is long-lasting or wanes soon after revaccination has not been confirmed. Additionally, high-dimensional flow cytometry was used to characterize the kinetics of B cell subsets circulating in the blood of human subjects of different ages and different priming backgrounds upon administration of an aP booster [27]. Diks et al. showed that the total and IgG1+ plasma cell responses were stronger in subjects primed with wP vaccines than in individuals primed with aP vaccine [27]. Consistently with these data, results showing significantly higher IgG4 levels in children who had received an aP vaccine at primary immunization than those in children who had received a wP vaccine were presented at the World Association for Infectious Diseases and Immune Disorders (WAidid) Congress [23]. IgG4 antibodies are not capable of activating the complement system, and consequently initiating antibody-dependent phagocytosis [28], and because of that, it is critical for the efficacy of a pertussis vaccine to induce a broad repertoire of antibodies where, with an induction of IgG1, antibodies being superior to IgG4 subclass [23]. In Figure 2, researchers summarize the differences detected in T and B cells, according to the type of vaccine used in the primary schemes and in the boosters. Altogether, it is important to know whether a B cell bias with broader isotypic profile is seen in humans who received a wP booster after the initial aP dose. This type of study will be valuable for implementation of appropriate life-course vaccination strategy in countries switching from the wP to the aP vaccine.
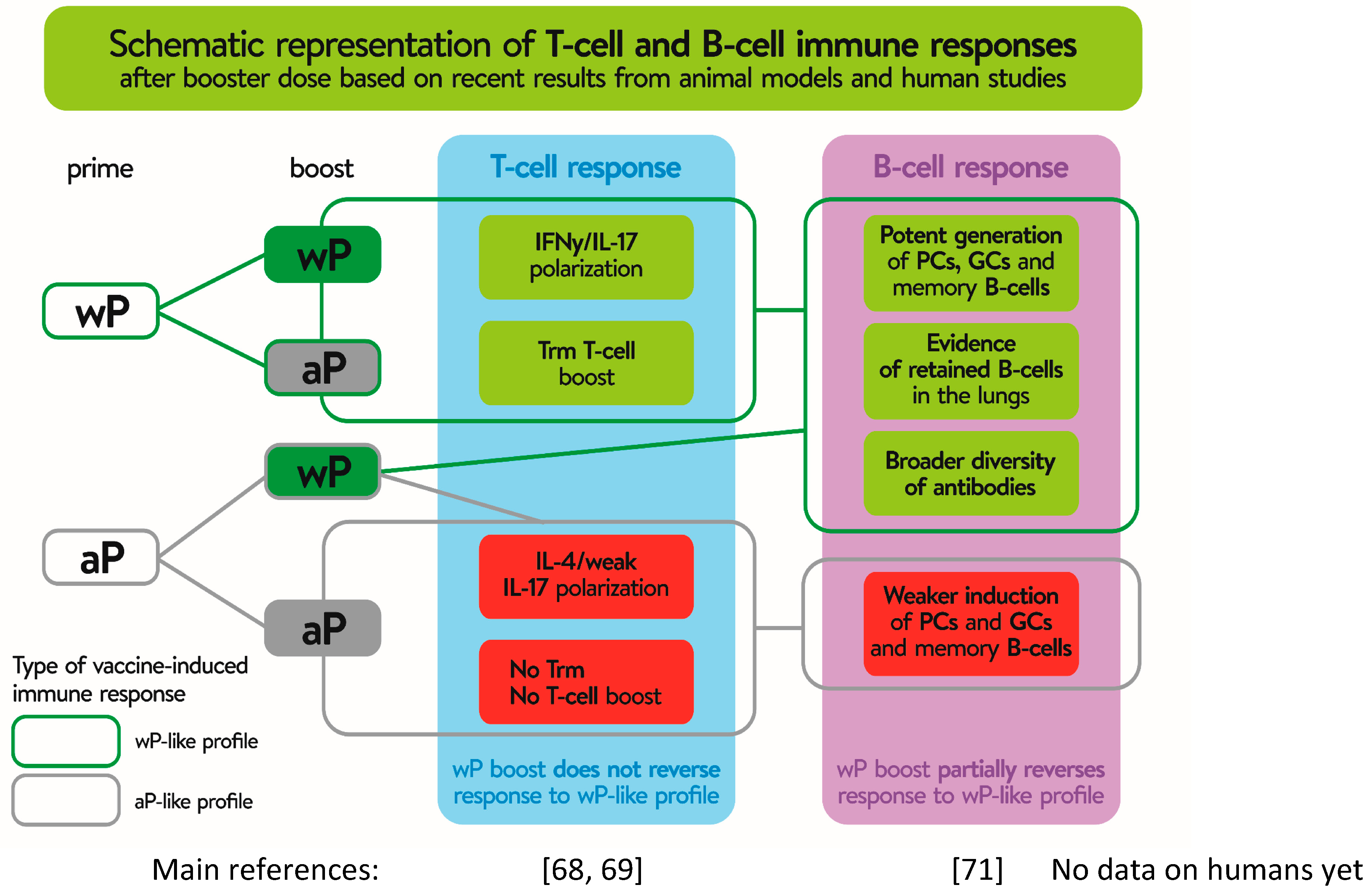
Figure 2. Different T and B cell response, depending on use whole-cell (wP) or acellular (aP) pertussis component during priming and booster immunization. Gray boxes show the effect of priming and booster immunization with aP vaccines. Green boxes show the same effect on T and B cells after prime immunization with wP, irrespective of aP or wP use as a booster, while reversion of the B cell profile after using as a booster wP after priming with aP. Stronger and faster effects of the boost dose on either T or B cell response are shown on a green background. Non-or negative effect on lymphocyte stimulation after boost vaccination is shown in red background. Trm—tissue-resident memory CD4 T cells; PCs—plasma cells; GCs—germinal centers.
2. The Type of Pertussis Vaccine Affects Both the Response in Later Life and the Loss-of-Immunity Rate
Scientific reports indicate that the immunity persists for 10 and 20 years following natural infection and between 10 and 12 years following whole cell vaccination, as compared to about 3 to 5 years following immunization with acellular vaccines [29][30][31][32][33]. The shorter-lasting immunity makes children susceptible to the disease at a younger age (before the administration of subsequent booster doses) and translates to incomplete population immunity. Long-term efficacy studies conducted in Europe and Senegal on children who received 3- or 4-dose series of vaccines suggested that protection waned faster after aP than after wP [34][35][36]. After both three-dose and five-dose primary series of aP vaccination, the protection predictably wanes after the last dose in the series, with the odds of pertussis increasing by a factor of 1.33 (95% CI 1.23–1.43) for every year after administration of diphtheria, tetanus, and acellular pertussis (DTaP) [33]. Epidemiological studies confirm that protection, whereas robustness at the time of vaccination is temporary, with immunity waning as the post-vaccination years pass [31]. The results of the study carried out by Clark et al. [31] show that children who had been fully vaccinated with aP-containing vaccines in infancy were more likely to develop pertussis during the early school years, whereas children who had been vaccinated with wP-containing vaccines were at a higher risk of developing the disease during the adolescence period. Similar differences were also reported by the Vickers’ team [37]. In contrast, in a Swedish 10-year follow-up study, 13% of the population acquired pertussis at a median of 5.5 years after the last dose, irrespective of the type of vaccine (two-component JNIH-6 aP or monovalent Wellcome wP-containing vaccine) [38]. Additionally, in a German 6-year follow-up study of wP and aP vaccines (as manufactured by Wyeth-Lederle), the calculated efficacy for the 6-year follow-up period was 89% (95% CI 79–94) for the aP vaccine and 92% (95% CI 84–96) for the wP vaccine [35]. The limited comparability between studies may result from the use of different vaccines, changes in the manufacturing process or vaccine contents over time, the presence of different immunization schedules (timing and/or number of doses), and the use of different case definitions, surveillance methods and reporting systems.
The decrease in immunity was also reported in those who had completed a full immunization schedule with aP-containing vaccines [39]. In the evaluation of pertussis disease risk in 10- to 17-year-old teenagers, as conducted by Klein et al. [40] after a 2010/2011 pertussis outbreak in the United States, the authors showed that, during the outbreak, DTwP vaccines received in childhood protected the teenagers five times more effectively than DTaP vaccines. Furthermore, the authors estimated the efficacy of DTwP vaccines administered to preschoolers in the United States by investigating the secondary attack rates in household contacts. The results showed that the DTwP vaccine was highly effective in preventing pertussis in preschool children exposed to infection within their households, with protection increasing from 44% for one DTwP vaccine dose to 80% for four or more doses when typical paroxysmal cough was used as a clinical case definition of the disease [41]. Results obtained in the meta-analysis of 11 studies assessing the long-term protection against pertussis after three or five doses of multivalent aP-containing vaccines administered [33] showed that the risk of developing the disease after receiving the last dose of an aP-containing vaccine was estimated to increase by a factor of 1.33 each year (95% CI 1.23–1.43), leading to a conclusion that 8.5 years after the last dose of an aP vaccination schedule, the protection against the disease was maintained only in 10% of children. In another meta-analysis of the efficacy of aP-containing vaccines delivered in accordance with the U.S. immunization schedule [42], the efficacy of the regimen involving administration of six vaccine doses was estimated at 85% (95% CI: 84–86%) in the first year after last dose, with a year-to-year decrease rate of 11.7% (95% CI: 11.1–12.3%), warranting the claim that the immunization program in force might lead to the post-vaccination protection being reduced to 28.2% (95% CI: 27–29%) in patients at the age of 18 [42]. Therefore, it appears that the duration of immune protection following immunization with aP-containing vaccines is insufficient, regardless of the vaccination schedule and the number of doses administered. Increasing the number of aP boosters doses seems to provide short-lasting and rapidly waning protection [22].
In line with what has been described, several studies showed that a combined vaccination schedules that include at least one (at least first) dose of a wP in the vaccination schedule provide a better protection against the disease than those schedules that only consist in the administrations of doses with aP vaccine. Studies showed that the polarization of immune response following the primary immunization in infancy is associated with increased levels of IL-4, IL-9, and TGF-β in children receiving aP-containing vaccines or IFN-γ and IL-17 in children receiving wP-containing vaccines [25][43][44][45][46]. It is considered that the production of IL-9 and IL-17, which varies, particularly depending on the type of anti-pertussis vaccine administered in childhood, may play a significant role in determining this response in later life [24]. Stronger IL-17 polarization from wP vaccination has been reported to be associated with higher protection in baboon models and to be pivotal in the mediation of adaptive immunity by tissue-resident memory T cells after natural infection in mice [9][47].
References
- Chasaide, C.N.; Mills, K.H.G. Next-Generation Pertussis Vaccines Based on the Induction of Protective T Cells in the Respiratory Tract. Vaccines 2020, 8, 621.
- Coutte, L.; Alonso, S.; Reveneau, N.; Willery, E.; Quatannens, B.; Locht, C.; Jacob-Dubuisson, F. Role of adhesin release for mucosal colonization by a bacterial pathogen. J. Exp. Med. 2003, 197, 735–742.
- Ishibashi, Y.; Nishikawa, A. Bordetella pertussis infection of human respiratory epithelial cells up-regulates intercellular adhesion molecule-1 expression: Role of filamentous hemagglutinin and pertussis toxin. Microb. Pathog. 2002, 33, 115–125.
- Jahnsen, F.L.; Strickland, D.H.; Thomas, J.A.; Tobagus, I.T.; Napoli, S.; Zosky, G.R.; Turner, D.J.; Sly, P.D.; Stumbles, P.A.; Holt, P.G. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J. Immunol. 2006, 177, 5861–5867.
- Klimova, N.; Holubova, J.; Streparola, G.; Tomala, J.; Brazdilova, L.; Stanek, O.; Bumba, L.; Sebo, P. Pertussis toxin suppresses dendritic cell-mediated delivery of B. pertussis into lung-draining lymph nodes. PLoS Pathog. 2022, 18, e1010577.
- Holubova, J.; Stanek, O.; Juhasz, A.; Hamidou Soumana, I.; Makovicky, P.; Sebo, P. The Fim and FhaB adhesins play a crucial role in nasal cavity infection and Bordetella pertussis transmission in a novel mouse catarrhal infection model. PLoS Pathog. 2022, 18, e1010402.
- Esposito, S.; Agliardi, T.; Giammanco, A.; Faldella, G.; Cascio, A.; Bosis, S.; Friscia, O.; Clerici, M.; Principi, N. Long-term pertussis-specific immunity after primary vaccination with a combined diphtheria, tetanus, tricomponent acellular pertussis, and hepatitis B vaccine in comparison with that after natural infection. Infect. Immun. 2001, 69, 4516–4520.
- Esposito, S.; Principi, N. Prevention of pertussis: An unresolved problem. Hum. Vaccin Immunother. 2018, 14, 2452–2459.
- Ross, P.J.; Sutton, C.E.; Higgins, S.; Allen, A.C.; Walsh, K.; Misiak, A.; Lavelle, E.C.; McLoughlin, R.M.; Mills, K.H. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella pertussis: Towards the rational design of an improved acellular pertussis vaccine. PLoS Pathog. 2013, 9, e1003264.
- Mahon, B.P.; Sheahan, B.J.; Griffin, F.; Murphy, G.; Mills, K.H. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J. Exp. Med. 1997, 186, 1843–1851.
- Ryan, M.; Murphy, G.; Gothefors, L.; Nilsson, L.; Storsaeter, J.; Mills, K.H. Bordetella pertussis respiratory infection in children is associated with preferential activation of type 1 T helper cells. J. Infect. Dis. 1997, 175, 1246–1250.
- Ryan, M.; Murphy, G.; Ryan, E.; Nilsson, L.; Shackley, F.; Gothefors, L.; Oymar, K.; Miller, E.; Storsaeter, J.; Mills, K.H. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 1998, 93, 1–10.
- Ausiello, C.M.; Urbani, F.; la Sala, A.; Lande, R.; Cassone, A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 1997, 65, 2168–2174.
- Palazzo, R.; Carollo, M.; Bianco, M.; Fedele, G.; Schiavoni, I.; Pandolfi, E.; Villani, A.; Tozzi, A.E.; Mascart, F.; Ausiello, C.M. Persistence of T-cell immune response induced by two acellular pertussis vaccines in children five years after primary vaccination. New Microbiol. 2016, 39, 35–47.
- Wilk, M.M.; Misiak, A.; McManus, R.M.; Allen, A.C.; Lynch, M.A.; Mills, K.H.G. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol. 2017, 199, 233–243.
- Solans, L.; Debrie, A.S.; Borkner, L.; Aguiló, N.; Thiriard, A.; Coutte, L.; Uranga, S.; Trottein, F.; Martín, C.; Mills, K.H.G.; et al. IL-17-dependent SIgA-mediated protection against nasal Bordetella pertussis infection by live attenuated BPZE1 vaccine. Mucosal. Immunol. 2018, 11, 1753–1762.
- Borkner, L.; Curham, L.M.; Wilk, M.M.; Moran, B.; Mills, K.H.G. IL-17 mediates protective immunity against nasal infection with Bordetella pertussis by mobilizing neutrophils, especially Siglec-F+ neutrophils. Mucosal. Immunol. 2021, 14, 1183–1202.
- Wilk, M.M.; Borkner, L.; Misiak, A.; Curham, L.; Allen, A.C.; Mills, K.H.G. Immunization with whole cell but not acellular pertussis vaccines primes CD4 Trm cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg. Microbes Infect. 2019, 8, 169–185.
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792.
- Redhead, K.; Watkins, J.; Barnard, A.; Mills, K.H. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect. Immun. 1993, 61, 3190–3198.
- Allen, A.C.; Wilk, M.M.; Misiak, A.; Borkner, L.; Murphy, D.; Mills, K.H.G. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting Trm cells. Mucosal. Immunol. 2018, 11, 1763–1776.
- Klein, N.P.; Bartlett, J.; Fireman, B.; Baxter, R. Waning Tdap Effectiveness in Adolescents. Pediatrics 2016, 137, e20153326.
- van der Lee, S.; Hendrikx, L.H.; Sanders, E.A.M.; Berbers, G.A.M.; Buisman, A.M. Whole-Cell or Acellular Pertussis Primary Immunizations in Infancy Determines Adolescent Cellular Immune Profiles. Front. Immunol. 2018, 9, 51.
- Da Silva Antunes, R.; Babor, M.; Carpenter, C.; Khalil, N.; Cortese, M.; Mentzer, A.J.; Seumois, G.; Petro, C.D.; Purcell, L.A.; Vijayanand, P.; et al. Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters. J. Clin. Investig. 2018, 128, 3853–3865.
- Weaver, K.L.; Blackwood, C.B.; Horspool, A.M.; Pyles, G.M.; Sen-Kilic, E.; Grayson, E.M.; Huckaby, A.B.; Witt, W.T.; DeJong, M.A.; Wolf, M.A.; et al. Long-Term Analysis of Pertussis Vaccine Immunity to Identify Potential Markers of Vaccine-Induced Memory Associated With Whole Cell But Not Acellular Pertussis Immunization in Mice. Front. Immunol. 2022, 13, 838504.
- Valeri, V.; Sochon, A.; Cousu, C.; Chappert, P.; Lecoeuche, D.; Blanc, P.; Weill, J.C.; Reynaud, C.A. The whole-cell pertussis vaccine imposes a broad effector B-cell response in mouse heterologous prime-boost settings. JCI Insight 2022, 8, e157034.
- Diks, A.M.; Versteegen, P.; Teodosio, C.; Groenland, R.J.; de Mooij, B.; Buisman, A.M.; Torres-Valle, A.; Pérez-Andrés, M.; Orfao, A.; Berbers, G.A.M.; et al. Age and Primary Vaccination Background Influence the Plasma Cell Response to Pertussis Booster Vaccination. Vaccines 2022, 10, 136.
- Aalberse, R.C.; Stapel, S.O.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477.
- Sallusto, F. Heterogeneity of Human CD4(+) T Cells Against Microbes. Annu. Rev. Immunol. 2016, 34, 317–334.
- Jenkinson, D. Duration of effectiveness of pertussis vaccine: Evidence from a 10 year community study. Br. Med. J. 1988, 296, 612–614.
- Clark, T.A.; Messonnier, N.E.; Hadler, S.C. Pertussis control: Time for something new? Trends Microbiol. 2012, 20, 211–213.
- Burdin, N.; Handy, L.K.; Plotkin, S.A. What Is Wrong with Pertussis Vaccine Immunity? The Problem of Waning Effectiveness of Pertussis Vaccines. Cold Spring Harb Perspect Biol. 2017, 9, a029454.
- McGirr, A.; Fisman, D.N. Duration of pertussis immunity after DTaP immunization: A meta-analysis. Pediatrics 2015, 135, 331–343.
- Salmaso, S.; Mastrantonio, P.; Tozzi, A.E.; Stefanelli, P.; Anemona, A.; Ciofi degli Atti, M.L.; Giammanco, A.; Group, S.I.W. Sustained efficacy during the first 6 years of life of 3-component acellular pertussis vaccines administered in infancy: The Italian experience. Pediatrics 2001, 108, E81.
- Lugauer, S.; Heininger, U.; Cherry, J.D.; Stehr, K. Long-term clinical effectiveness of an acellular pertussis component vaccine and a whole cell pertussis component vaccine. Eur. J. Pediatr. 2002, 161, 142–146.
- Lacombe, K.; Yam, A.; Simondon, K.; Pinchinat, S.; Simondon, F. Risk factors for acellular and whole-cell pertussis vaccine failure in Senegalese children. Vaccine 2004, 23, 623–628.
- Vickers, D.; Ross, A.G.; Mainar-Jaime, R.C.; Neudorf, C.; Shah, S. Whole-cell and acellular pertussis vaccination programs and rates of pertussis among infants and young children. CMAJ 2006, 175, 1213–1217.
- Tindberg, Y.; Blennow, M.; Granström, M. A ten year follow-up after immunization with a two component acellular pertussis vaccine. Pediatr. Infect. Dis. J. 1999, 18, 361–365.
- Witt, M.A.; Arias, L.; Katz, P.H.; Truong, E.T.; Witt, D.J. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin. Infect. Dis. 2013, 56, 1248–1254.
- Klein, N.P.; Bartlett, J.; Fireman, B.; Rowhani-Rahbar, A.; Baxter, R. Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics 2013, 131, e1716–e1722.
- Onorato, I.M.; Wassilak, S.G.; Meade, B. Efficacy of whole-cell pertussis vaccine in preschool children in the United States. JAMA 1992, 267, 2745–2749.
- Chit, A.; Zivaripiran, H.; Shin, T.; Lee, J.K.H.; Tomovici, A.; Macina, D.; Johnson, D.R.; Decker, M.D.; Wu, J. Acellular pertussis vaccines effectiveness over time: A systematic review, meta-analysis and modeling study. PLoS ONE 2018, 13, e0197970.
- DeAngelis, H.; Scarpino, S.V.; Fitzpatrick, M.C.; Galvani, A.P.; Althouse, B.M. Epidemiological and Economic Effects of Priming With the Whole-Cell Bordetella pertussis Vaccine. JAMA Pediatr. 2016, 170, 459–465.
- Le, T.; Cherry, J.D.; Chang, S.J.; Knoll, M.D.; Lee, M.L.; Barenkamp, S.; Bernstein, D.; Edelman, R.; Edwards, K.M.; Greenberg, D.; et al. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: The APERT Study. J. Infect. Dis. 2004, 190, 535–544.
- van Twillert, I.; Bonačić Marinović, A.A.; Kuipers, B.; van Gaans-van den Brink, J.A.; Sanders, E.A.; van Els, C.A. Impact of age and vaccination history on long-term serological responses after symptomatic B. pertussis infection, a high dimensional data analysis. Sci. Rep. 2017, 7, 40328.
- Mooi, F.R.; van Loo, I.H.; van Gent, M.; He, Q.; Bart, M.J.; Heuvelman, K.J.; de Greeff, S.C.; Diavatopoulos, D.; Teunis, P.; Nagelkerke, N.; et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg. Infect. Dis. 2009, 15, 1206–1213.
- Warfel, J.M.; Edwards, K.M. Pertussis vaccines and the challenge of inducing durable immunity. Curr. Opin. Immunol. 2015, 35, 48–54.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
13 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




