Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bárbara Costa | -- | 2074 | 2023-01-10 11:52:45 | | | |
| 2 | Rita Xu | Meta information modification | 2074 | 2023-01-11 03:18:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Costa, B.; Vale, N. Efavirenz. Encyclopedia. Available online: https://encyclopedia.pub/entry/39961 (accessed on 08 February 2026).
Costa B, Vale N. Efavirenz. Encyclopedia. Available at: https://encyclopedia.pub/entry/39961. Accessed February 08, 2026.
Costa, Bárbara, Nuno Vale. "Efavirenz" Encyclopedia, https://encyclopedia.pub/entry/39961 (accessed February 08, 2026).
Costa, B., & Vale, N. (2023, January 10). Efavirenz. In Encyclopedia. https://encyclopedia.pub/entry/39961
Costa, Bárbara and Nuno Vale. "Efavirenz." Encyclopedia. Web. 10 January, 2023.
Copy Citation
Efavirenz (Sustiva®) is a first-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) used to treat human immunodeficiency virus (HIV) type 1 infection or to prevent the spread of HIV. In 1998, the FDA authorized efavirenz for the treatment of HIV-1 infection. Patients formerly required three 200 mg efavirenz capsules daily, which was rapidly updated to a 600 mg tablet that only required one tablet per day.
efavirenz
NNRTI
HIV
1. History
Efavirenz is the first-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) used to treat immunodeficiency virus (HIV) type 1 infection or prevent the spread of HIV. It is sold under the brand names Sustiva® and Stocrin® and is the lead compound of a series of benzoxazinones developed initially by DuPont Pharmaceuticals (Figure 1). In 1997, clinical studies started to assess the efficacy of the triple combination of efavirenz with nelfinavir, indinavir, ritonavir, or other retroviral for the treatment of opportunistic and pediatric viral infections. Then, the effectiveness of efavirenz was studied both alone and in combination with zidovudine and lamivudine. Later, a study involving eight HIV-positive patients was presented at the 12th World AIDS Conference in July 1998, demonstrating that the administration of efavirenz in dual and triple combinations reduced the level of detectable HIV-RNA in plasma [1].
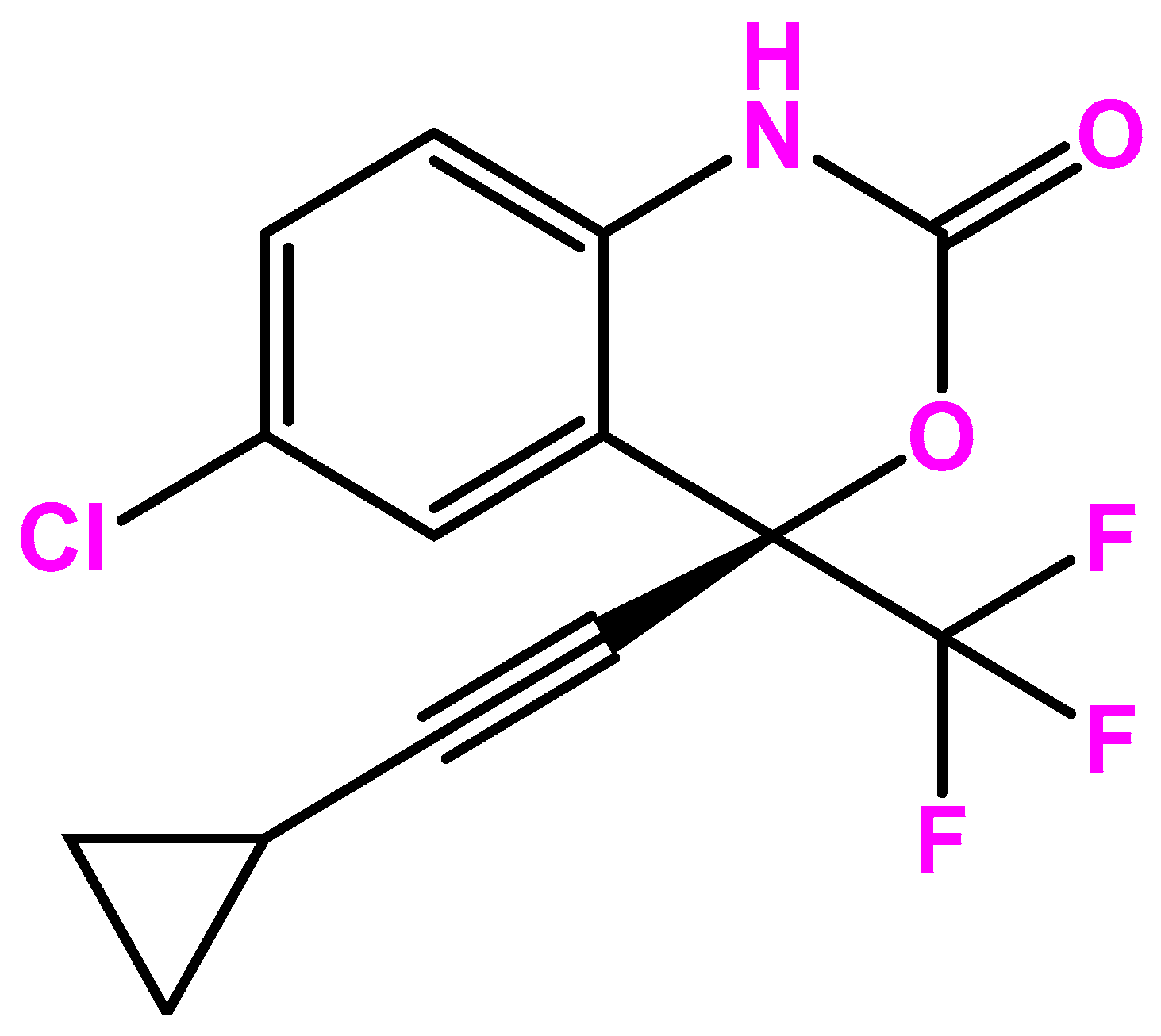
Figure 1. Efavirenz chemical structure.
The Food and Drug Administration (FDA) authorized efavirenz on 7 June 1998, with dose of 600 mg orally once-daily (200 mg × 3 capsules, once-daily) for the treatment of HIV infection, and in the European Union in 1999. On 17 February 2016, FDA gave its approval for the generic tablet formulation to Mylan Pharmaceuticals. After getting WHO clearance, Thailand’s Government Pharmaceutical Organization (GPO) declared that it would begin manufacturing efavirenz in late 2018. Today, this drug is registered on the World Health Organization’s (WHO) list of essential medicines for priority diseases [2]. Efavirenz was one of the first acquired immunodeficiency syndrome (AIDS) medications to be authorized for once-daily use. Patients formerly required three 200 mg efavirenz capsules daily, which was later updated to a 600 mg tablet that only required one tablet per day [3][4].
These findings have given rise to discussion on the ideal efavirenz dosage. In specific circumstances, such as when cytochrome P450 polymorphism is present (which has been linked to greater plasma efavirenz concentrations), some studies have recommended lowering the dose of efavirenz [5]. This, however, is not practical in actual practice due to the expense and logistical challenges of wide genotyping. It was when Carey et al. performed a study regarding the safety and efficacy of reduced versus standard dose efavirenz (EFV) plus two nucleotide reverse transcriptase inhibitors in antiretroviral-naive HIV-infected individuals (ENCORE1 study, NCT01011413) found that the standard dose of efavirenz could be scaled back without losing effectiveness. The 400 mg dose maintained similar viral suppression, reduced side effects, and made this drug more affordable [6]. ENCORE 1 established that the efficacy and tolerance of efavirenz 400 mg daily (for viral control at week 48) was noninferior to efavirenz 600 mg daily as initial therapy for HIV treatment [6][7]. Moreover, ENCORE1 allowed researchers to investigate these variables in a geographically and genetically varied patient population and to investigate connections between efficacy and safety outcomes with low dose efavirenz.
1.1. Pharmacodynamics
Efavirenz inhibits the activity of viral RNA-directed DNA polymerase (i.e., reverse transcriptase) [8]. The antiviral activity of efavirenz is dependent on intracellular conversion to the active triphosphorylated form. The rate of efavirenz phosphorylation varies, depending on cell type. It is believed that inhibition of reverse transcriptase interferes with the generation of DNA copies of viral RNA, which, in turn, are necessary for the synthesis of new virions. Intracellular enzymes subsequently eliminate the HIV particle (or even the intact particles) that previously had been uncoated and left unprotected during entry into the host cell. Thus, reverse transcriptase inhibitors are virustatic and do not eliminate HIV from the body. Even though human DNA polymerase is less susceptible to the pharmacologic effects of triphosphorylated efavirenz, this action may nevertheless account for some of the drug’s toxicity [9].
1.1.1. Anti-HIV Effects
Efavirenz falls in the NNRTI class of antiretrovirals. Both nucleoside and non-nucleoside RTIs inhibit the same target, the reverse transcriptase enzyme, an essential viral enzyme that transcribes viral RNA into DNA. Unlike nucleoside RTIs (NRTIs), which bind at the enzyme’s active site, NNRTIs act allosterically by binding to a distinct site away from the active site known as the NNRTI pocket [10]. Efavirenz is ineffective against HIV-2, as the pocket of the HIV-2 reverse transcriptase has a different structure, which confers intrinsic resistance to the NNRTI class [11].
As most NNRTIs bind within the same pocket, viral strains which are resistant to efavirenz are usually also resistant to the other NNRTIs. The most common mutation observed after efavirenz treatment is K103N. NRTIs and efavirenz have different binding targets, so cross-resistance is unlikely; the same is true about efavirenz and protease inhibitors [12].
1.1.2. Neuropsychiatric Effects
Efavirenz appears to cause neuropsychiatric side effects in approximately 50% of patients. These effects typically start soon after the beginning of medication and frequently peak two weeks later. They can range from depression, anxiety, and sleep issues to more aggressive behavior, paranoia, and psychosis [13]. Neuropsychiatric effects frequently have a detrimental effect on treatment adherence. It is well known that some factors can raise the possibility of neuropsychiatric side effects in HIV-positive patients, such as weight, gender and CYP450 2B6 [14]. The behavioral effects of efavirenz seem to be dose-dependent, mainly mediated by the 5-HT2A receptor, which is the lysergic acid diethylamide main site of action (LSD) [15]. Furthermore, it may be challenging to discern between the neuropsychiatric side effects of efavirenz and those caused by substance abuse, preexisting mental illness, or HIV-related neuropsychiatric symptoms. Although the adverse events are dose-dependent, they are often reversible [16].
1.2. Pharmacokinetics
Peak plasma concentrations take between 3 and 5 h to reach. Following a 600 mg average adult oral dose, EFV is easily absorbed and reaches a peak serum concentration (Cmax) of 4.07 mcg/quantities or doses of 200, 400, and 600 mg EFV, increases in Cmax and the area under the plasma concentration-time curve (AUC) are dosage proportional [17]. EFV has a lengthy serum half-life of 45 h and takes 6 to 10 days to attain steady-state plasma concentrations. When compared to fasting, a reduced-fat/normal-calorie meal (EFV capsules) and, in particular, a high-fat/high-calorie meal (EFV capsules and tablets), both increase the bioavailability of EFV.
The cytochrome P450 system principally metabolizes efavirenz to hydroxylated metabolites with subsequent glucuronidation of these hydroxylated metabolites. Metabolized efavirenz forms 7-hydroxy and 8-hydroxy efavirenz (8-OH-efavirenz is the main metabolite), and the formation rate of this metabolism has been proven to have different variability between human microsome samples [18]. However, these metabolites are essentially inactive against HIV-1. The main metabolite of EFV detected in urine is 8-hydroxy-EFV-gluc various UDP-glucuronosyltransferase (UGT) isoforms can process the hydroxylated EFV metabolites processed by various UDP-glucuronosyltransferase (UGT) isoforms to create glucuronide form [19]. The rate at which EFV-N—glucuronide forms varies greatly among human microsome samples as well.
The efavirenz pharmacokinetics are characterized by significant between-subject variability, which influences both therapeutic response and adverse effects. Genetic variation in cytochrome P450 genes, especially in CYP2A6, has been linked to some of the variability in efavirenz pharmacokinetics. After stopping an EFV-based regimen, the CYP2B6 G516T polymorphism has also been linked to a prolonged elimination serum half-life and an increased risk of developing drug resistance [20]. The likelihood ratio of having very high EFV plasma levels was 35 (95% CI, 11–110) in people with a poor metabolizer genotype. The genotypes of CYP2B6 poor metabolizers can identify those who are at risk of having high plasma concentrations of EFV. High EFV plasma concentrations and effective EFV dosage reduction based on genotype. The high prevalence of EFV-related CYP2B6 polymorphisms in individuals of African origin, who today make up the bulk of HIV-infected people globally, is highly significant.
2. Development
2.1. A Comprehensive Evaluation and a Change in the Paradigm of Efavirenz 400 and 600 mg Once Daily
Clinical data suggested that the standard dose of efavirenz could be reduced without compromising its effectiveness, resulting in a reduction in side effects, and making the drug more affordable. Therefore, a randomized, double-blind, placebo-controlled clinical trial was performed to compare the efficiency and safeness of a reduced dose of efavirenz (400 mg) with the standard dose (600 mg) plus two NRTI in antiretroviral naïve HIV infected individuals who have not received any treatment, over 96 weeks [21].
ENCORE1 assessed the effectiveness and safety of tenofovir/emtricitabine (TDF/FTC) and reduced versus regular dose efavirenz (EFV) as first-line HIV therapy. At 48 weeks, the initial study revealed that 400 mg of EFV was safe and virologically superior to 600 mg [22]. The persistence of efficacy and safety is examined over 96 weeks in this investigation. At week 96, non-inferiority between EFV 400 mg and EFV 600 mg when used in conjunction with TDF/FTC as the first HIV therapy was established. Both doses showed comparable safety characteristics. These findings support the regular use of a lower EFV dose in HIV care. It also assessed patient demographics and genetic polymorphisms (CYP2B6, CYP2A6, CYP3A4, NR1I3) [23] as covariates, evaluating EFV400 and EFV600 pharmacokinetics (NONMEM v. 7.2) and examining relationships with efficacy (plasma HIV-RNA (pVL) 200 copies/mL) and safety outcomes at 48 weeks in 606 randomized ENCORE1 patients [21][24]. EFV400 was linked to a better safety profile among adults who had not received ART and to a lower rate of discontinuation while remaining effective in pregnant patients and patients with tuberculosis without adjusting the dose.
Up to June 2018, the World Health Organization’s preferred first-line therapy for HIV-1 infection was an efavirenz-based regimen (with a 400 mg dose of efavirenz), see Figure 2. The dolutegravir-based regimen is the preferred first-line treatment, and low-dose efavirenz-based is an alternative for HIV-1 [25]. Dolutegravir is an integrase inhibitor with a better profile regarding sustained viral suppression and immunologic recovery than the EFV600-based regimen [26]. Comparative efficacy, tolerability, and safety between dolutegravir and EFV400-based regimen argue in favor of low-dose efavirenz-based regimens as an alternative to dolutegravir in combination with lamivudine/emtricitabine and tenofovir disoproxil fumarate as the preferred first-line treatment [27].
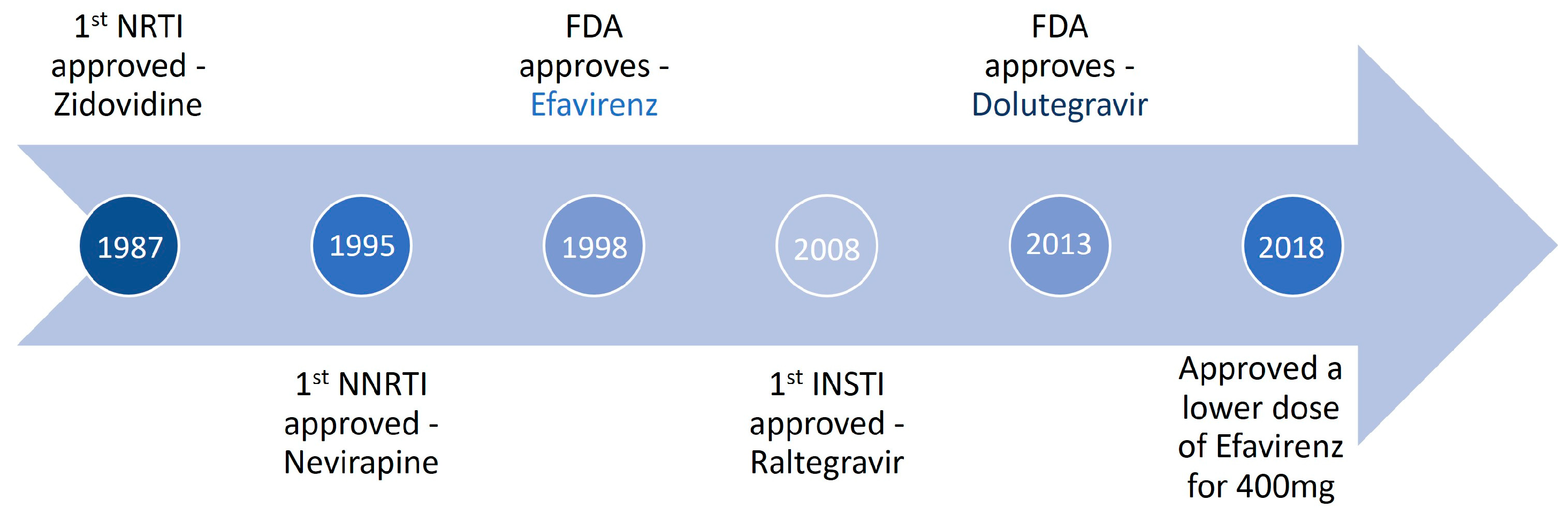
Figure 2. Timeline of efavirenz use to treat HIV infection.
Due to adverse neurosensory effects, EFV600 has now been reduced in the most recent international recommendations. Additionally, the low genetic barrier of efavirenz can lead to the accumulation of drug-resistance mutations without comedication administration, particularly in the context of recurrent drug shortages and restricted access to routine HIV-1 RNA monitoring [28][29]. Increased mortality, the propagation of treatment-resistant mutations in HIV-1, and a rise in the prevalence of primary drug resistance are consequences of a high dose of efavirenz.
2.2. Human Immunodeficiency Virus Treatment Regimens Paradigm
The incidence of NNRTI resistance mutations in antiretroviral-naive individuals and the low genetic barrier of NNRTIs for developing drug resistance are both significant drawbacks of currently available NNRTIs [30][31]. Treatment failure of first-line NNRTI-based regimens can have severe repercussions, such as the further accumulation of NNRTI and nucleoside reverse transcriptase inhibitor resistance mutations [32]. This may lead to cross-resistance to second-generation NNRTIs (such as etravirine and rilpivirine) and decreased efficacy of the nucleoside “backbone” of subsequent treatment regimens, respectively [33]. Subpar immunological recovery and increased morbidity and mortality linked to virological control are possible additional effects, particularly in patients presenting advanced HIV infection [34].
Some academics even consider that it is time to stop using efavirenz as a first-line treatment on a global scale due to the emergence of integrase strand transfer inhibitors (INSTIs) that the standard of care for the management of HIV infection in some parts of the world. On the other parts, NNRTIs keep on being used as a first-line treatment due to cost and availability. Efavirenz has been a primary first-line antiviral drug for more than 15 years. Due to the increased degree of transferred NNRTI resistance in treatment-naive patients with HIV and the frequency of adverse events, efavirenz is no longer recommended as a first-line HIV treatment option in the majority of resource-rich nations. However, due to its superior safety profile compared to standard dose EFV (600 mg), low-dose EFV (400 mg) continues to be one of the first-line options for WHO guidelines. TDF/FTC/longevity EFVs of effectiveness and its comparison to INSTI regimens demonstrate the potential utility of this regimen.
The primary justifications for switching from EFV-based regimens to INSTI-based regimens in settings with limited resources are an emergent virologic failure or a contraindication to EFV (such as an increased risk of coronary heart disease or a history of neuropsychiatric conditions). However, if the world is to achieve the target of reducing HIV transmission and lowering the number of HIV-positive people by 2030, quick virologic control cannot be understated. In conclusion, the mounting data points to INSTI as the initial therapy option with the best long-term clinical efficacy, virologic control, and safety profile [27][35]
References
- Dong, B.J. Efavirenz DuPont Pharmaceuticals Co. IDrugs 1998, 1, 700–711.
- WHO. Model List of Essential Medicines—22nd List. 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 5 December 2022).
- Best, B.M.; Goicoechea, M. Efavirenz—Still First Line King? Expert Opin. Drug Metab. Toxicol. 2008, 4, 965.
- Clay, P.G.; Taylor, T.A.; Glaros, A.G.; McRae, M.; Williams, C.; McCandless, D.; Oelklaus, M. “One Pill, Once Daily”: What Clinicians Need to Know about AtriplaTM. Ther. Clin. Risk Manag. 2008, 4, 291.
- Wang, P.F.; Neiner, A.; Kharasch, E.D. Efavirenz Metabolism: Influence of Polymorphic CYP2B6 Variants and Stereochemistry. Drug Metab. Dispos. 2019, 47, 1195.
- Amin, J.; Becker, S.; Belloso, W.; Boffito, M.; Cooper, D.; Crabtree-Ramirez, B.; Duncombe, C.; Foulkes, S.; Hill, A.; Jessen, H.; et al. Efficacy and Safety of Efavirenz 400 Mg Daily versus 600 Mg Daily: 96-Week Data from the Randomised, Double-Blind, Placebo-Controlled, Non-Inferiority ENCORE1 Study. Lancet Infect. Dis. 2015, 15, 793–802.
- Carey, D. Efavirenz 400 Mg Daily Remains Non-Inferior to 600 Mg: 96 Week Data from the Double-Blind, Placebo-Controlled ENCORE1 Study. J. Int. AIDS Soc. 2014, 17, 19523.
- Arts, E.J.; Hazuda, D.J. HIV-1 Antiretroviral Drug Therapy. Cold Spring Harb. Perspect. Med. 2012, 2, a007161.
- McDonagh, E.M.; Lau, J.L.; Alvarellos, M.L.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Efavirenz Pathway, Pharmacokinetics. Pharmacogenet. Genom. 2015, 25, 363–376.
- Sharaf, N.G.; Ishima, R.; Gronenborn, A.M. Conformational Plasticity of the NNRTI-Binding Pocket in HIV-1 Reverse Transcriptase—A Fluorine NMR Study. Biochemistry 2016, 55, 3864.
- Ren, J.; Bird, L.E.; Chamberlain, P.P.; Stewart-Jones, G.B.; Stuart, D.I.; Stammers, D.K. Structure of HIV-2 Reverse Transcriptase at 2.35-Å Resolution and the Mechanism of Resistance to Non-Nucleoside Inhibitors. Proc. Natl. Acad. Sci. USA 2002, 99, 14410.
- Koval, C.E.; Dykes, C.; Wang, J.; Demeter, L.M. Relative Replication Fitness of Efavirenz-Resistant Mutants of HIV-1: Correlation with Frequency during Clinical Therapy and Evidence of Compensation for the Reduced Fitness of K103N + L100I by the Nucleoside Resistance Mutation L74V. Virology 2006, 353, 184.
- Dheda, M. Efavirenz and Neuropsychiatric Effects. S. Afr. J. HIV Med. 2017, 18, 741.
- Nemaura, T.; Nhachi, C.; Masimirembwa, C. Impact of Gender, Weight and CYP2B6 Genotype on Efavirenz Exposure in Patients on HIV/AIDS and TB Treatment: Implications for Individualising Therapy. Afr. J. Pharm. Pharmacol. 2012, 6, 2188–2193.
- Gatch, M.B.; Kozlenkov, A.; Huang, R.Q.; Yang, W.; Nguyen, J.D.; González-Maeso, J.; Rice, K.C.; France, C.P.; Dillon, G.H.; Forster, M.J.; et al. The HIV Antiretroviral Drug Efavirenz Has LSD-Like Properties. Neuropsychopharmacology 2013, 38, 2373.
- Zareifopoulos, N.; Lagadinou, M.; Karela, A.; Kyriakopoulou, O.; Velissaris, D. Neuropsychiatric Effects of Antiviral Drugs. Cureus 2020, 12, e9536.
- Rakhmanina, N.Y.; van den Anker, J.N. Efavirenz in the Therapy of HIV Infection. Expert Opin. Drug Metab. Toxicol. 2010, 6, 95.
- Ogburn, E.T.; Jones, D.R.; Masters, A.R.; Xu, C.; Guo, Y.; Desta, Z. Efavirenz Primary and Secondary Metabolism In Vitro and In Vivo: Identification of Novel Metabolic Pathways and Cytochrome P450 2A6 as the Principal Catalyst of Efavirenz 7-Hydroxylation. Drug Metab. Dispos. 2010, 38, 1218–1229.
- Ward, B.A.; Gorski, J.C.; Jones, D.R.; Hall, S.D.; Flockhart, D.A.; Desta, Z. The Cytochrome P450 2B6 (CYP2B6) Is the Main Catalyst of Efavirenz Primary and Secondary Metabolism: Implication for HIV/AIDS Therapy and Utility of Efavirenz as a Substrate Marker of CYP2B6 Catalytic Activity. J. Pharmacol. Exp. Ther. 2003, 306, 287–300.
- Rotger, M.; Tegude, H.; Colombo, S.; Cavassini, M.; Furrer, H.; Décosterd, L.; Blievernicht, J.; Saussele, T.; Günthard, H.F.; Schwab, M.; et al. Predictive Value of Known and Novel Alleles of CYP2B6 for Efavirenz Plasma Concentrations in HIV-Infected Individuals. Clin. Pharmacol. Ther. 2007, 81, 557–566.
- Dickinson, L.; Amin, J.; Else, L.; Boffito, M.; Egan, D.; Owen, A.; Khoo, S.; Back, D.; Orrell, C.; Clarke, A.; et al. Pharmacokinetic and Pharmacodynamic Comparison of Once-Daily Efavirenz (400 Mg vs. 600 Mg) in Treatment-Naïve HIV-Infected Patients: Results of the ENCORE1 Study. Clin. Pharmacol. Ther. 2015, 98, 406.
- Amin, J.; Becker, S.; Belloso, W.; Boffito, M.; Cooper, D.; Crabtree-Ramirez, B.; Duncombe, C.; Emery, S.; Foulkes, S.; Hill, A.; et al. Efficacy of 400 Mg Efavirenz versus Standard 600 Mg Dose in HIV-Infected, Antiretroviral-Naive Adults (ENCORE1): A Randomised, Double-Blind, Placebo-Controlled, Non-Inferiority Trial. Lancet 2014, 383, 1474–1482.
- Kwara, A.; Lartey, M.; Sagoe, K.W.; Rzek, N.L.; Court, M.H. CYP2B6 (c.516G-->T) and CYP2A6 (*9B and/or *17) Polymorphisms Are Independent Predictors of Efavirenz Plasma Concentrations in HIV-Infected Patients. Br. J. Clin. Pharmacol. 2009, 67, 427–436.
- Arab-Alameddine, M.; di Iulio, J.; Buclin, T.; Rotger, M.; Lubomirov, R.; Cavassini, M.; Fayet, A.; Décosterd, L.A.; Eap, C.B.; Biollaz, J.; et al. Pharmacogenetics-Based Population Pharmacokinetic Analysis of Efavirenz in HIV-1-Infected Individuals. Clin. Pharmacol. Ther. 2009, 85, 485–494.
- Calmy, A.; Tovar Sanchez, T.; Kouanfack, C.; Mpoudi-Etame, M.; Leroy, S.; Perrineau, S.; Lantche Wandji, M.; Tetsa Tata, D.; Omgba Bassega, P.; Abong Bwenda, T.; et al. Dolutegravir-Based and Low-Dose Efavirenz-Based Regimen for the Initial Treatment of HIV-1 Infection (NAMSAL): Week 96 Results from a Two-Group, Multicentre, Randomised, Open Label, Phase 3 Non-Inferiority Trial in Cameroon. Lancet HIV 2020, 7, e677–e687.
- Dolutegravir-Based or Low-Dose Efavirenz–Based Regimen for the Treatment of HIV-1. N. Engl. J. Med. 2019, 381, 816–826.
- Kanters, S.; Vitoria, M.; Zoratti, M.; Doherty, M.; Penazzato, M.; Rangaraj, A.; Ford, N.; Thorlund, K.; Anis, P.A.H.; Karim, M.E.; et al. Comparative Efficacy, Tolerability and Safety of Dolutegravir and Efavirenz 400mg among Antiretroviral Therapies for First-Line HIV Treatment: A Systematic Literature Review and Network Meta-Analysis. EClinicalMedicine 2020, 28, 100573.
- Ajibola, G.; Rowley, C.; Maruapula, D.; Leidner, J.; Bennett, K.; Powis, K.; Shapiro, R.L.; Lockman, S. Drug Resistance after Cessation of Efavirenz-Based Antiretroviral Treatment Started in Pregnancy. S. Afr. J. HIV Med. 2020, 21, 1023.
- Arnedo, M.; Alonso, E.; Eisenberg, N.; Ibáñez, L.; Ferreyra, C.; Jaén, A.; Flevaud, L.; Khamadi, S.; Roddy, P.; Gatell, J.M.; et al. Monitoring HIV Viral Load in Resource Limited Settings: Still a Matter of Debate? PLoS ONE 2012, 7, e47391.
- Cheng, C.Y.; Tsai, M.S.; Yang, C.J.; Cheng, S.H.; Sun, H.Y.; Chang, S.F.; Su, L.H.; Su, Y.C.; Hung, C.C.; Chang, S.Y. Patterns of Emergent Resistance-Associated Mutations after Initiation of Non-Nucleoside Reverse-Transcriptase Inhibitor-Containing Antiretroviral Regimens in Taiwan: A Multicenter Cohort Study. Infect. Drug Resist. 2018, 11, 849.
- Mackie, N. Resistance to non-nucleoside reverse transcriptase inhibitors. In Antiretroviral Resistance in Clinical Practice; Mediscript: London, UK, 2006.
- Nanfuka, M.; Forrest, J.I.; Zhang, W.; Okoboi, S.; Birungi, J.; Kaleebu, P.; Zhu, J.; Tibenganas, S.; Moore, D.M. Durability of Non-Nucleotide Reverse Transcriptase Inhibitor-Based First-Line ART Regimens after 7 Years of Treatment in Rural Uganda: A Prospective Cohort Study. Medicine 2021, 100, e25763.
- Sluis-Cremer, N. The Emerging Profile of Cross-Resistance among the Nonnucleoside HIV-1 Reverse Transcriptase Inhibitors. Viruses 2014, 6, 2960.
- Boyd, A.T.; Oboho, I.; Paulin, H.; Ali, H.; Godfrey, C.; Date, A.; Sean Cavanaugh, J. Addressing Advanced HIV Disease and Mortality in Global HIV Programming. AIDS Res. Ther. 2020, 17, 40.
- Cole, S.R.; Edwards, J.K.; Hall, H.I.; Brookhart, M.A.; Mathews, W.C.; Moore, R.D.; Crane, H.M.; Kitahata, M.M.; Mugavero, M.J.; Saag, M.S.; et al. Incident AIDS or Death After Initiation of Human Immunodeficiency Virus Treatment Regimens Including Raltegravir or Efavirenz Among Adults in the United States. Clin. Infect. Dis. 2017, 64, 1591–1596.
More
Information
Subjects:
Virology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
11 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




