
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Zahid Mumtaz | -- | 2387 | 2023-01-10 11:21:52 | | | |
| 2 | Jason Zhu | -4 word(s) | 2383 | 2023-01-11 03:25:20 | | | | |
| 3 | Jason Zhu | Meta information modification | 2383 | 2023-02-14 09:28:22 | | |
Video Upload Options
Rhizosphere bacterial inoculants are indisputably necessary for the augmentation of plant growth and maintenance of soil output. As reported, rhizosphere bacterial inoculants benefit plants through various mechanisms, although some studies indicate adverse effects. In this entry, the pros and cons of rhizosphere bacterial biofertilizers are compared, and a comparison of such biofertilizers is presented in and demonstrated in.
1. Introduction
Recently, crop production has been facing serious threats due to various biotic and abiotic stresses. Feeding the growing population and enhancing agricultural production on limited land are significant challenges for researchers and farmers in the current era [1]. In addition, present agricultural practices, such as the use of fertilizers, pesticides, herbicides, and irrigation with untreated wastewater, pose serious threats to the environment and cause soil degradation [2]. Moreover, urbanization and industrialization have caused a significant reduction in the agricultural area, which has further motivated scientists to develop sustainable strategies to increase crop yields from the already shrinking cropped area [3]. Increasing the area available for crop production has proven difficult; therefore, strategies should be developed to increase the crop yield per unit area in a way that prevents the further degradation of natural resources [4]. Therefore, adopting alternative approaches in today’s agriculture is necessary for ensuring environmental sustainability and future food security.
Several technical strategies suggested in the past involve enhancing agricultural production by reducing agricultural inputs such as fertilizers and pesticides. In this context, the use of rhizobacteria has been increasingly gaining momentum. Rhizobacteria reside in the rhizosphere, and those having beneficial effects on plants are termed plant growth-promoting rhizobacteria [5][6]. These rhizobacteria are equipped with a number of mechanisms (both direct and indirect) through which they improve plant growth in diverse agricultural settings. Several previous studies have reported the natural enhancement of plant growth of field crops by applying plant growth-promoting rhizobacteria (PGPR). The mechanisms for plant growth promotion used by rhizobacteria, which inhabit the rhizosphere, include metabolic adjustments, adjustments in phytohormone levels, production of exopolysaccharides, root colonization, and enhancement of nutrient availability [7][8][9]. These rhizobacteria also indirectly improve plant growth by inducing plant resistance to various biotic and abiotic stresses, such as pathogen attack and heavy metal contamination, using such mechanisms as the production of antibiotics, induction of induced systemic resistance, rhizosphere competence, and production of antagonistic substances for biocontrol [7][10][11][12][13]. Moreover, the mechanisms used by rhizobacteria for the bioremediation of contaminated soils include the production of biosurfactants and siderophores, biosorption, ACC deaminase activity, and production of polymeric substances [14][15][16].
In this entry, the key knowledge of plant growth promotion resulting from rhizosphere bacterial application in diverse agricultural settings is summarized. Here, researchers synthesize the main findings and highlight the in-depth analysis of mechanisms used by rhizobacteria for plant growth promotion, biocontrol, and bioremediation of contaminated sites ( Figure 1 ) in a comprehensive manner. then the pros and cons of rhizobacterial application in modern agriculture for improving plant growth are discussed, and then technical suggestions are evaluated for the future use of rhizobacteria in agriculture.
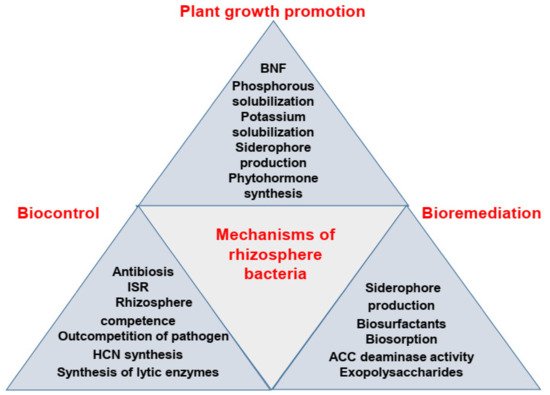
2. Plant Growth Promotion by Plant Growth-Promoting Rhizobacteria (PGPR): An Overview
During their development, plants are in intimate and continuous contact with microorganisms present in the root vicinity, known as the rhizosphere. Microbes living in the rhizosphere of several plants and having several positive effects on the host plant through various mechanisms are usually termed plant growth-promoting rhizobacteria (PGPR) [17][18]. In the rhizosphere, plant roots secrete a number of exudates that act as attractants for microbes, which eventually improve the physicochemical properties of the surrounding soil. On the other hand, these exudates maintain the function and structure of microbial communities near plant roots [9][19]. Plants and bacteria form symbiotic associations to alleviate abiotic stresses [20][21][22][23]. PGPR can assist plants in their growth by fixation of atmospheric nitrogen, producing siderophores, generating phytohormones (auxins, gibberellins, cytokinins), solubilizing phosphorus (P), or synthesizing stress-relieving enzymes [24]. Moreover, certain bacteria improve the accessibility of essential nutrients, improve root progression, and lessen stress-induced damage by modifying plant defense systems [25][26]. Furthermore, PGPR indirectly help plant symbionts by initiating induced systemic resistance, exerting an antibiosis effect, and potentially improving the content of plant cell metabolites [25][27]. PGPR can withstand hostile natural conditions such as shortage of water, salt stress, weed invasion, lack of nutrients, and heavy metal pollution [28]. The use of PGPR could help to enhance and improve sustainable agriculture and natural stability. These PGPR can be found in association with roots (in the rhizosphere), which enhance plant growth in the absence of pathogens or lessen the harmful effects of pathogens on crop yield by antibiosis, competition, induced systemic resistance, and siderophore production [29][30][31]. Several mechanisms used by PGPR in plant growth promotion are described in detail in the following section.
3. Plant Growth-Promoting Rhizobacteria (PGPR) as Biocontrol Agents: An Overview
Plant disease outbreaks are a major cause of decreased crop yield, deteriorating production quality, and causing the contamination of food grains. Pesticides have been developed in response to the ever-increasing range and complexity of plant diseases [32][33]. Unfortunately, continued use of these pesticides has resulted in phytopathogen resistance, which raises a number of environmental concerns. Biological control is being considered as an alternative to pesticides for phytopathogen control. [34]. Plant growth and health are assisted by the use of PGPB as biological agents. PGPB have a number of benefits over traditional pest control methods. The use of PGPB in agriculture is both environmentally friendly and non-toxic. PGPB work through a variety of mechanisms to reduce or avoid harm caused by phytopathogens [24].
Plant growth is influenced by PGPR in two ways: indirectly and directly. Direct plant growth promotion by PGPR involves either providing the plant with bacterium-produced compounds, such as phytohormones, or promoting the absorption of certain nutrients from the environment [35]. As PGPR reduce or prevent the negative effects of one or more phytopathogenic species, they indirectly promote plant growth. This can be accomplished by generating antagonistic compounds or inducing pathogen resistance [35]. One or more of these mechanisms can be used by a specific PGPR to influence plant growth and development.
PGPR may function as biocontrol agents via a variety of mechanisms ( Table 1 ) irrespective of their position in plant growth enhancement, such as the establishment of auxin phytohormone development [36], reduction in plant ethylene levels [37], or nitrogen fixation [38]. Plant– PGPR interactions are commercially exploited [39], and they hold great promise for long-term agriculture. A number of commercial and food crops have been studied in relation to these associations [40].
| Biocontrol Agent | Plant Pathogen | Host Plant | Proposed Mechanism(s) | Reference |
|---|---|---|---|---|
| Pseudomonas fluorescens | Fusarium culmorum | Rye | Fe(III)-chelating compounds (including siderophores) | [41] |
| Acinetobacter, Pseudomonas, Staphylococcus, Bacillus, Enterobacter, Pantoea, Alcaligenes | Fusarium oxysporum, Alternaria alternate, F. culmorum, F. solani, Botrytis cinerea, Pythium ultimum, Phytophthora cryptogea | Wheat | Antagonism and growth promotion | [37] |
| Bacillus sp. L324-92 | Gaeumannomyces graminis var tritici, Rhizoctonia root rot, R. solani AG8, Pythium root rot, Pythium irregulare P. ultimum. | Wheat | Not specified | [42] |
| Bacillus sp., Pseudomonas fluorescens | R. oryzae, P. ultimum, G. graminis, R. solani | Wheat | Not specified | [43] |
| Pseudomonas fluorescens | Microconidium nivale/ Fusarium nivale | Wheat | Growth promotion, siderophore production, in vitro antibiosis | [44] |
| Bacillus subtilis and B. cereus | Take all (G. graminis var tritici) Rhizoctonia root rot (R. solaniAG8) | Wheat | Growth promotion | [45] |
| Bacillus subtilis CE1 | Fusarium verticillioides | Maize | Not specified | [46] |
| Pseudomonas chlororaphis | Macrophomina phaseolina (charcoal rot of sorghum) | Sorghum | Extracellular antibiotics, production of volatiles, siderophores, effective root colonization | [47] |
| Pseudomonas fluorescens MKB 100 and MKB 249, P. frederiksbergensis 202, Pseudomonas spp. MKB 158 | Fusarium culmorum | Wheat and barley | Induced resistance, antibiotic production, pathogenesis-related proteins (induced resistance) in wheat | [165 |
The use of bacteriophage as a biocontrol agent has been a promising yet uncommon technique in recent years. Phages have the inherent ability to address phage resistance or new bacterial strains and are compatible with a variety of other biocontrol agents. Because of their sensitivity to UV light, they must be sprayed on the plant in the evening [48]. They can be used in phage-based diagnostics of phytopathogenic bacteria in addition to being used as biocontrol agents.
4. Pros and Cons of Rhizosphere Bacteria for Agricultural Sustainability
Rhizosphere bacterial inoculants are indisputably necessary for the augmentation of plant growth and maintenance of soil output. As reported in the above sections, rhizosphere bacterial inoculants benefit plants through various mechanisms, although some studies indicate adverse effects [5]. In this section, the pros and cons of rhizosphere bacterial biofertilizers are compared, and a comparison of such biofertilizers is presented in Table 2 and demonstrated in Figure 2 .

Numerous commercially available microbial biofertilizers are sold as dried or liquid cultures under a variety of trade names, as listed in Table 2. The application of such rhizosphere bacterial biofertilizers could have an impact on agricultural sustainability and phytopathogen biocontrol and sustain soil and plant production by improving nutrient availability and reducing the application of chemical fertilizers and pesticides. The bioremediation and biodegradation of hazardous substances of biological or anthropogenic origin could also be improved [55]. Various beneficial rhizosphere bacterial inoculants could be formulated in the form of products, including biofertilizers. Such products are viable sources of nutrients that could act as alternatives to chemical fertilizers, stimulate plant growth, remediate heavy metal-contaminated environments, and mitigate environmental stresses [56][57].
| Pro’s Types | Product (Bacterial Composition) | References |
|---|---|---|
| Nitrogen fixers | AgriLife NitroFix (A. chroococcum, A. vinelandii, A. diazotrophicus, A. lipoferum, R. japonicum), Ajay Azospirillum (Azospirillum sp.), Azofer (A. brasilense), Azo-N (A. brasilense + A. lipoferum), Azo-N Plus (A. brasilense + A. lipoferum + A. chroococcum), Azoter (A. chroococcum, A. brasilense, B. megaterium), Azotobacterin (A. brasilense B-4485), BactoFil A10 (B. Megaterium + A. brasilense, A. vinelandii), BactoFil Soya (B. japonicum), BiAgro 10 (B. japonicum), Bioboots (Bradyrhizobium sp. + D. acidovorans), Biofix (Rhizobia), BioGro (C. freundii, K. pneumoniae, P. fluorescens), Bio-N (Azospirillum spp.), Cell-Tech (Rhizobia), Custom N2 (P. polymyxa), Dimargon (A. chroococcum), Legume Fix (B. japonicum + Rhizobium sp.), Mamezo (Rhizobia), Nitragin Gold (Rhizobia), Nitrasec (Rhizobium sp.), Nitrofix (Azospirillum sp.), Nodulator (B. Japonicum), Nodulator PRO (B. subtilis + B. Japonicum), Nodulest 10 (B. japonicum), Nodumax (Bradyrhizobium spp.), Phylazonit M (A. chroococcum + B. megaterium), Rhizofer (R. etli), Rhizosum Aqua (Azospirillum sp.), Rhizosum N (A. vinelandii + R. irregularis), Rizo-Liq (Bradyrhizobium sp., + M. ciceri, + Rhizobium spp.), Rizo-Liq Top (B. japonicum), Symbion N (Azospirillum sp. + Rhizobium sp. + Acetobacter sp. + Azotobacter sp.), TagTeam (P. bilaii + Rhizobia), TwinN and TripleN (Azorhizobium sp. + Azoarcus sp. + Azospirillum sp.), Zadspirillum (A. brasilense), | [11][49][50][58][59][60][61][62][63] |
| Nutrient solubilizers | Bio Phos (B. megaterium), Biozink (PGPR consortia), CataPult (Bacillus spp. + G. intraradices), CBF (B. mucilaginosus, + B. subtilis), Fosforina (P. Fluorescens), K Sol B (F. aurantia), P Sol B (P. striata, + B. polymyxa, B. megaterium), Phosphobacterin (B. megaterium), Rhizosum K (F. aurantia), Rhizosum PK (B. megaterium, + F. aurantia, + R. irregularis), Symbion van Plus (B. megaterium), Zn Sol B (T. thiooxidans), | [50][59][60][63][64] |
| Biopesticides | Biobit, Dipel, and Delfin (Bacillus thuringiensis var. kurstaki), Certan (Bacillus thuringiensis var. aizawai), Acrobe, Skectal, Vectobac (Bacillus thuringiensis var. israelensis), Trident, Novodor (Bacillus thuringiensis var. tenebrionis), Ciba-Foil, Agree, Cutlass (Bacillus thuringiensis var. conjugates), MVP, M-Trak (Pseudomonas fluorescens (Bt toxin), Doom (Bacillus papilliae), Invade (Serratia entomophila) | [65][66][67] |
| Other biofertilizers | Amase (P. azotoformans), Bioativo (PGPR consortia), EVL Coating (PGPR consortia), Biotilis (B. subtilis), Cedomon (P. chlororaphis), Cedress (P. chlororaphis) | [50][59] |
The shelf life of biofertilizers and the commercialization of a successful rhizosphere bacterial inoculant remain as major challenges [68]. Biofertilizers with a short shelf life need to be recycled before expiration, which results in financial losses for the associated firm. Their storage and transportation require additional caution because biofertilizers are composed of live bacterial cells and can deteriorate under harsh environmental conditions [64]. A mutation in a bioinoculant may create a serious problem if it decreases its efficiency and thus raises the cost of production. To increase the shelf life of biofertilizers, a suitable carrier is needed for field application. The lack of a suitable carrier is a significant restriction on its widespread usage in fields. Peat, charcoal, and lignite are considered excellent carriers for biofertilizer processing; however, the majority are in short supply in developing countries, and mining of these carriers has been downscaled in developed countries. A potential carrier should be inexpensive, nearly sterile, and free from moisture and toxic substances in addition to having both high organic matter content and water-holding capacity [52]. At the moment, there are no quality control procedures for biofertilizers. It is necessary to develop quality control standards for biofertilizers to demonstrate their efficacy in promoting plant growth on a field scale [64].
Farmers are skeptical of biofertilizers due to the extremely slow and frequently unsuccessful crop responses to applied biofertilizers, as the inoculum requires a longer time to colonize the roots and for the effective concentration to be established. Biofertilizer efficacy is reduced in the field due to the residual properties of harmful chemicals [64]. Environmental stresses contribute significantly to the reduction in biological activity in some areas. Several other factors contributing to the poor performance of biofertilizers include nutrient availability, soil acidity and alkalinity, high and low temperatures, pesticide application, radiation, and high nitrate concentrations in the soil, which limit the bioinoculants’ ability to fix atmospheric N, solubilize nutrients, and interact with indigenous soil microbiota, which influences the presence and survivability of rhizosphere bacteria and the host plant [69]. Numerous soils are contaminated with heavy metals as well as deficient in other critical nutrients, which reduce the biological potential of rhizosphere bacterial inoculants in biofertilizers [70]. Region-specific rhizosphere bacterial biofertilizers should be identified to optimize the effectiveness of the used strains. Soil fumigation with broad-spectrum biocidal fumigants has a deleterious effect on the soil microbial community [71]. The inconsistent application of biofertilizer limits the presence of viable bacterial populations, which results in their inefficiency in promoting the growth of agronomic crops. Typically, farmers expect rapid, visible outcomes from a single application of biofertilizers, which represents another serious limitation to their wide-scale application. The limited application of biofertilizer could be due to the lack of awareness in farmers about the concentration, time, and method of biofertilizer application. Repeated applications of biofertilizer are needed to maintain the bacterial numbers and ensure a viable PGP rhizobacterial population in soil for alleviating various environmental stresses. However, the literature is limited in specifying the required biofertilizer dosage. Therefore, extensive research is required to evaluate the optimal dose of biofertilizers and their effects on crop productivity and stress alleviation.
References
- Bharti, N.; Barnawal, D. Amelioration of salinity stress by PGPR: ACC deaminase and ROS scavenging enzymes activity. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 85–106.
- Barrow, C. Biochar: Potential for countering land degradation and for improving agriculture. Appl. Geogr. 2012, 34, 21–28.
- Niamat, B.; Naveed, M.; Ahmad, Z.; Yaseen, M.; Ditta, A.; Mustafa, A.; Rafique, M.; Bibi, R.; Sun, N.; Xu, M. Calcium-Enriched Animal Manure Alleviates the Adverse Effects of Salt Stress on Growth, Physiology and Nutrients Homeostasis of Zea mays L. Plants 2019, 8, 480.
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818.
- Saharan, B.S.; Nehra, V. Plant growth promoting rhizobacteria: A critical review. Life Sci. Med. Res. 2011, 21, 1–30.
- Danish, S.; Zafar-Ul-Hye, M.; Mohsin, F.; Hussain, M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS ONE 2020, 15, e0230615.
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The good, the bad, and the ugly of rhizosphere microbiome. In Probiotics and Plant Health; Springer: Singapore, 2017; pp. 253–290.
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.D.; Mahgoub, H.A.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059.
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the Interactions among Roots, Rhizosphere, and Rhizobacteria for Improving Plant Growth and Tolerance to Abiotic Stresses: A Review. Cells 2021, 10, 1551.
- Mustafa, A.; Naveed, M.; Saeed, Q.; Ashraf, M.N.; Hussain, A.; Abbas, T.; Kamran, M.; Minggang, X. Application potentials of plant growth promoting rhizobacteria and fungi as an alternative to conventional weed control methods. In Sustainable Crop Production; IntechOpen: London, UK, 2019.
- Mustafa, A.; Naveed, M.; Abbas, T.; Saeed, Q.; Hussain, A.; Ashraf, M.N.; Minggang, X. Growth response of wheat and associated weeds to plant antagonistic rhizobacteria and fungi. Ital. J. Agron. 2019, 14, 191–198.
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Abbas, S.; Alwahibi, M.S.; Elshikh, M.S.; Mustafa, A. Large scale screening of rhizospheric allelopathic bacteria and their potential for the biocontrol of wheat-associated weeds. Agronomy 2020, 10, 1469.
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to microbiome: A paradigm shift in the application of microorganisms for sustainable agriculture. Front. Microbiol. 2020, 11, 3323.
- Ahmad, M.; Naseer, I.; Hussain, A.; Zahid Mumtaz, M.; Mustafa, A.; Hilger, T.H.; Ahmad Zahir, Z.; Minggang, X. Appraising endophyte–plant symbiosis for improved growth, nodulation, nitrogen fixation and abiotic stress tolerance: An experimental investigation with chickpea (Cicer arietinum L.). Agronomy 2019, 9, 621.
- Nazli, F.; Mustafa, A.; Ahmad, M.; Hussain, A.; Jamil, M.; Wang, X.; Shakeel, Q.; Imtiaz, M.; El-Esawi, M.A. A Review on Practical Application and Potentials of Phytohormone-Producing Plant Growth-Promoting Rhizobacteria for Inducing Heavy Metal Tolerance in Crops. Sustainability 2020, 12, 9056.
- Haider, F.U.; Ejaz, M.; Cheema, S.A.; Khan, M.I.; Zhao, B.; Cai, L.; Salim, M.A.; Naveed, M.; Khan, N.; Núñez-Delgado, A.; et al. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies. Environ. Res. 2021, 197, 111031.
- Goudaa, S.; Kerryb, R.G.; Dasc, G.; Paramithiotisd, S.; Shine, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140.
- Umar, W.; Ayub, M.A.; ur Rehman, M.Z.; Ahmad, H.R.; Farooqi, Z.U.R.; Shahzad, A.; Rehman, U.; Mustafa, A.; Nadeem, M. Nitrogen and phosphorus use efficiency in agroecosystems. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 213–257.
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537.
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856.
- Raza, W.; Yousaf, S.; Rajer, F.U. Plant growth promoting activity of volatile organic compounds produced by Bio-control strains. Sci. Lett. 2016, 4, 40–43.
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzianatronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768.
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown gold of marginal soil: Plant growth promoting bacteria to overcome plant abiotic stress for agriculture, biofuels and carbon sequestration. Sci. Total Environ. 2020, 711, 135062.
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197.
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.A. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in Chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097.
- Gupta, A.; Gopal, M.; Thomas, G.V.; Manikandan, V.; Gajewski, J.; Thomas, G.; Seshagiri, S.; Schuster, S.C.; Rajesh, P.; Gupta, R. Whole genome sequencing and analysis of plant growth promoting bacteria isolated from the rhizosphere of plantation crops coconut, cocoa and arecanut. PLoS ONE 2014, 9, e104259.
- Verma, P.P.; Shelake, R.M.; Das, S.; Sharma, P.; Kim, J.Y. Plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF): Potential biological control agents of diseases and pests. In Microbial Interventions in Agriculture and Environment; Springer: Singapore, 2019; pp. 281–311.
- Kumari, A.; Kumar, R. Exploring phyllosphere bacteria for growth promotion and yield of potato (Solanum tuberosum L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1065–1071.
- Shivakumar, B. Biocontrol Potential and Plant Growth Promotional Activity of Fluorescent Pseudomonads of Western Ghats. Ph.D. Dissertation, UAS, Dharwad, India, 2007.
- Mohammed, A.F.; Oloyede, A.R.; Odeseye, A.O. Biological control of bacterial wilt of tomato caused by Ralstonia solanacearum using Pseudomonas species isolated from the rhizosphere of tomato plants. Arch. Phytopathol. Plant Prot. 2020, 53, 1–16.
- Larkin, R.P. Biological control of soil borne diseases in organic potato production using hypovirulent strains of Rhizoctonia solani. Biol. Agric. Hortic. 2020, 36, 1–11.
- Guo, R.F.; Yuan, G.F.; Wang, Q.M. Effect of NaCl treatments on glucosinolate metabolism in broccoli sprouts. J. Zhejiang Univ. Sci. B 2013, 14, 124.
- He, D.C.; Zhan, J.S.; Xie, L.H. Problems, challenges and future of plant disease management: From an ecological point of view. J. Integr. Agric. 2016, 15, 705–715.
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Barka, E.A. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693.
- Glick, B.R. The enhancement of plant growth by free living bacteria. Can. J. Microbiol. 1995, 41, 109114.
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801.
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-containing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339.
- Dobereiner, J. Review article History and New Perspectives of Diazotrophs in Association with Non-Leguminous Plants. Symbiosis 1992, 13, 1–13.
- Podile, A.R.; Kishore, G.K. Plant growth-promoting rhizobacteria. In Plant-Associated Bacteria; Springer: Dordrecht, The Netherlands, 2007; pp. 195–230.
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412.
- Cook, R.J.; Weller, D.M.; El-Banna, A.Y.; Vakoch, D.; Zhang, H. Yield responses of direct-seeded wheat to rhizobacteria and fungicide seed treatments. Plant Dis. 2002, 86, 780–784.
- Kim, J.; Rees, D.C. Nitrogenase and biological nitrogen fixation. Biochemistry 1994, 33, 389–397.
- Amein, T.; Omer, Z.; Welch, C. Application and evaluation of Pseudomonas strains for biocontrol of wheat seedling blight. Crop Prot. 2008, 27, 532–536.
- Ryder, M.H.; Yan, Z.; Terrace, T.E.; Rovira, A.D.; Tang, W.; Correll, R.L. Use of strains of Bacillus isolated in China to suppress take-all and rhizoctonia root rot, and promote seedling growth of glasshouse-grown wheat in Australian soils. Soil Biol. Biochem. 1999, 31, 19–29.
- Cavaglieri, L.; Orlando, J.R.M.I.; Rodríguez, M.I.; Chulze, S.; Etcheverry, M. Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Res. Microbiol. 2005, 156, 748–754.
- Moustafa, H.E.; Abo-Zaid, G.A.; Abd-Elsalam, H.E.; Hafez, E.E. Antagonistic and inhibitory effect of Bacillus subtilis against certain plant pathogenic fungi, I. Biotechnology 2009, 8, 53–61.
- Das, A.J.; Lal, S.; Kumar, R.; Verma, C. Bacterial biosurfactants can be an ecofriendly and advanced technology for remediation of heavy metals and co-contaminated soil. Int. J. Environ. Sci. Technol. 2016, 14, 1343–1354.
- Khan, M.R.; Fischer, S.; Egan, D.; Doohan, F.M. Biological control of Fusarium seedling blight disease of wheat and barley. Phytopathology 2006, 96, 386–394.
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and bacterial plant diseases. Front. Microbiol. 2017, 8, 34.
- Artyszak, A.; Gozdowski, D. The effect of growth activators and plant growth-promoting rhizobacteria (PGPR) on the soil properties, root yield, and technological quality of sugar beet. Agronomy 2020, 10, 1262.
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 162, pp. 31–87.
- Cardinale, M.; Ratering, S.; Suarez, C.; Zapata Montoya, A.M.; Geissler-Plaum, R.; Schnell, S. Paradox of plant growth promotion potential of rhizobacteria and their actual promotion effect on growth of barley (Hordeum vulgare L.) under salt stress. Microbiol. Res. 2015, 181, 22–32.
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33.
- Peiffer, J.A.; Spor, O.; Koren, A.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553.
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evolut. 2016, 31, 440–452.
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118.
- Kumar, S.S.; Kadier, A.; Malyan, S.K.; Ahmad, A.; Bishnoi, N.R. Phytoremediation and rhizoremediation: Uptake, mobilization and sequestration of heavy metals by plants. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: New Delhi, India, 2017; pp. 367–394.
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487.
- Uribe, D.; Sánchez-Nieves, J.; Vanegas, J. Role of microbial biofertilizers in the development of a sustainable agriculture in the Tropics. In Soil Biology and Agriculture in the Tropics; Dion, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 235–250.
- Mehnaz, S. An overview of globally available bioformulations. In Bioformulations: For Sustainable Agriculture; Arora, N., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 268–281.
- García-Fraile, P.; Menéndez, E.; Celador-Lera, L.; Díez-Méndez, A.; Jiménez-Gómez, A.; Marcos-García, M.; Cruz-González, X.A.; Martínez-Hidalgo, P.; Mateos, P.F.; Rivas, R. Bacterial probiotics: A truly green revolution. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 131–162.
- Adeleke, R.A.; Raimi, A.R.; Roopnarain, A.; Mokubedi, S.M. Status and prospects of bacterial inoculants for sustainable management of agroecosystems. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.-S., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 137–172.
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. Plant growth promoting rhizobacterial biofertilizers for sustainable crop production: The past, present, and future. Preprints 2020, 2020090650.
- Dal Cortivo, C.; Ferrari, M.; Visioli, G.; Lauro, M.; Fornasier, F.; Barion, G.; Panozzo, A.; Vamerali, T. Effects of seed-applied biofertilizers on rhizosphere biodiversity and growth of common wheat (Triticum aestivum L.) in the field. Front. Plant Sci. 2020, 11, 72.
- Mahajan, A.; Gupta, R.D. Bio-fertilizers: Their kinds and requirement in India. In Integrated Nutrient Management (INM) in a Sustainable Rice—Wheat Cropping System; Mahajan, A., Gupta, R.D., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 75–100.
- Malathi, S.; Sriramulu, M. Laboratory efficacy of biotic insecticides against lepidopterous pests fed on treated cabbage leaves. Shashpa 2000, 7, 63–66.
- Apurva, K.; Singh, C.; Kumar, V.; Jha, V.B. Bacterial biopesticides and their use in agricultural production. In Biofertilizers and Biopesticides in Sustainable Agriculture; Apple Academic Press: London, UK, 2019; pp. 23–42.
- Schwenk, V.; Riegg, J.; Lacroix, M.; Märtlbauer, E.; Jessberger, N. Enteropathogenic potential of Bacillus thuringiensis isolates from soil, animals, food and biopesticides. Foods 2020, 9, 1484.
- Zandi, P.; Basu, S.K. Role of plant growth-promoting rhizobacteria (PGPR) as biofertilizers in stabilizing agricultural ecosystems. Org. Farming Sustain. Agric. 2016, 71–87.
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335.
- Aka, R.J.N.; Babalola, O.O. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int. J. Phytoremediat. 2016, 18, 200–209.
- Dangi, S.R.; Tirado-Corbalá, R.; Gerik, J.; Hanson, B.D. Effect of long-term continuous fumigation on soil microbial communities. Agronomy 2017, 7, 37.




