Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Duygu Harmancı | -- | 3896 | 2023-01-09 10:43:32 | | | |
| 2 | Rita Xu | Meta information modification | 3896 | 2023-01-09 10:53:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hanoglu, S.B.; Harmanci, D.; Ucar, N.; Evran, S.; Timur, S. Magnetic Nanoparticles for Detection of miRNA. Encyclopedia. Available online: https://encyclopedia.pub/entry/39903 (accessed on 07 February 2026).
Hanoglu SB, Harmanci D, Ucar N, Evran S, Timur S. Magnetic Nanoparticles for Detection of miRNA. Encyclopedia. Available at: https://encyclopedia.pub/entry/39903. Accessed February 07, 2026.
Hanoglu, Simge Balaban, Duygu Harmanci, Nursima Ucar, Serap Evran, Suna Timur. "Magnetic Nanoparticles for Detection of miRNA" Encyclopedia, https://encyclopedia.pub/entry/39903 (accessed February 07, 2026).
Hanoglu, S.B., Harmanci, D., Ucar, N., Evran, S., & Timur, S. (2023, January 09). Magnetic Nanoparticles for Detection of miRNA. In Encyclopedia. https://encyclopedia.pub/entry/39903
Hanoglu, Simge Balaban, et al. "Magnetic Nanoparticles for Detection of miRNA." Encyclopedia. Web. 09 January, 2023.
Copy Citation
Magnetic nanoparticles (MNPs) have been widely used in many fields due to their advantageous properties, such as biocompatibility, easy modifiability, and high chemical stability. One of these areas is the detection of cancer. It is essential to use existing biomarkers, such as microRNAs (miRNAs), for the early diagnosis of this disease. miRNAs are challenging to distinguish and detect in biological samples because they are small, circulating molecules.
magnetic nanoparticles (MNPs)
magnetic sensing
biosensors
1. Introduction
Magnetic nanoparticles (MNPs) can be manipulated by a magnetic field [1]. They can generate responses in magnetism, and their small size is related to this response. They have many superior properties to large-scale materials with the same features because they have the chemical, mechanical, and magnetic capabilities that both nanomaterials and magnetism offer. These capabilities, which vary depending on their properties, have paved the way for them to attract attention and be used for various purposes in many fields [2].
MNPs can help isolate biomolecules that are difficult to separate from biological materials due to their complex matrices. They can also be used in water purification by increasing the sensitivity of existing systems, enabling the development of diagnostic support systems, and even improving and targeting treatments [3]. Especially in biomedical processes, they can immobilize biomolecules such as antibodies, proteins, enzymes, and DNA by binding to them, enabling their separation from complex mixtures with high efficiency. In this way, they can differentiate and determine biomolecules that can potentially be biomarkers but not easily detected in various biological samples [1][4]. One of these biomolecules is microRNA (miRNA).
After the Human Genome Project, findings on the non-coding part of the genome and the acceleration of studies in this field led to the discovery of miRNAs in 1993. miRNAs are small non-coding RNAs [5][6]. The evidence of their involvement in physiological and pathological processes is increasing day by day. Hence, their potential as biomarkers of various diseases indicates that researchers can also evaluate them as diagnostic molecules [7][8]. The main challenge is getting standardized and pure miRNA from biological samples, which could be obtained by non-invasive or minimally invasive methods [1][9]. The ability of MNPs to bind these nucleic acid fragments and release them in a reusable form can be used for this purpose. The content of miRNA in these biological samples is relatively low [3][10][11]. It is possible to use the superior capabilities of MNPs to overcome the difficulties in isolating total miRNA, which is scarce anyway, from the existing sample and to detect miRNA with biomarker potential from this total miRNA. There are methods such as quantitative real-time polymerase chain reaction (qRT-PCR), microarray, northern blot, and modified invader test that researchers still use to detect miRNA today [12]. Besides these methods, biosensing systems are also being developed to detect specific miRNAs. MNPs can also be used in biosensor systems developed for miRNA detection. MNPs, along with other electrochemical, optical, plasmonic, and fluorescent sensing technologies, enable the development of systems for miRNA detection. Their adaptation to these systems is also related to the simple functionalization properties of MNPs. MNP-based sensing systems for miRNA detection are prevalent [1][4][13].
2. MNPs for Detection of miRNA
2.1. miRNA
miRNAs are non-coding RNAs of 18–24 nucleotides in length and play essential roles in gene expression. miRNA biogenesis mainly follows the canonical biogenesis pathway [14]. Briefly, miRNA is transcribed by RNA polymerase II, and a microprocessor complex processes the primary transcript (pri-miRNAs). The resulting precursor-miRNA (pre-miRNA) is exported to the cytoplasm, where the ribonuclease Dicer processes it to generate an miRNA duplex. The miRNA duplex is then unwound, and the RNA strand that remains on the AGO protein acts as the mature miRNA [15].
Since their first discovery [16] thousands of miRNAs have been identified in different organisms, ranging from viruses [17] to fungi [18], and to higher eukaryotes [19][20]. With the advances in next-generation sequencing technologies, novel miRNAs could be identified [21][22].
miRNAs were found to be linked to several biological mechanisms, such as thermal stress response [23], regulation of immune cells in sepsis [24], atherosclerosis [25], and B cell receptor signaling [26]. The expression levels of miRNAs are usually changed in several pathological conditions such as multiple myeloma [27], non-small cell lung cancer [28], leukemia [29], Parkinson’s disease [30], and diabetes-induced cardiomyopathy [31]. For instance, miRNA-937 was found to be overexpressed in colon cancer [32] and hepatocellular carcinoma [33]. The decrease in the level of miRNA-149-3p was shown to be associated with poor prognosis in oral squamous cell carcinoma [34]. Considering the correlation between changes in miRNA levels and diseases, miRNAs hold great promise as novel biomarkers. Thereby, miRNAs offer opportunities for early detection [35][36][37]. In particular, circulating miRNAs in biological fluids are significant for non-invasive diagnosis. The intracellularly produced miRNAs were found to be released in the extracellular environment through incorporation into the exosomes or by forming a complex with proteins [38][39]. Moreover, miRNA content in the extracellular vesicles was found to be affected by the disease state [40]. Thus, it was proposed that the miRNA derived from the extracellular vesicles could be used as a biomarker [41][42]. Interestingly, miRNA was found to be more stable than messenger RNA (mRNA) [43][44]. As stable biomarkers, miRNAs were detected in different types of extracellular fluids, such as urine [45], plasma [46], serum [47], cerebrospinal fluid [48], and saliva [49]. As a non-invasive approach, miRNA-based liquid biopsy was proven to be successful at detecting melanoma [50] and urothelial carcinoma of the bladder [51]. Given that the disease regulation mechanisms of miRNAs continue to be discovered [52][53][54][55][56], miRNAs attract much interest as non-invasive diagnostic and prognostic biomarkers.
2.2. Traditional Methods for miRNA Detection
Specificity and sensitivity are the two critical parameters for miRNA detection. Sequence similarity among miRNAs [57], and the need for detection at very low concentrations [58] are the challenging issues that should be considered. Northern blotting, RT-qPCR, and microarrays are the conventional methods with distinct advantages and disadvantages in terms of those two parameters [10][28][59].
2.2.1. Northern Blotting
Northern blotting, which is also known as Northern hybridization, is a classical technique to analyze RNA molecules [60][61]. RNA is first subjected to denaturing gel electrophoresis and then transferred onto a positively charged nylon membrane. RNA is fixed on the membrane by UV-mediated cross-linking. Then, it can be visualized after hybridization with a probe, which could be either radioactive or non-radioactive. Northern blotting is advantageous due to the fact that it is simple and reliable due to the sample being directly used after the isolation step. It also allows discrimination between miRNAs and their precursors [62]. However, it is time-consuming and not suitable for the analysis of different miRNAs having the same molecular weight. Low sensitivity and the requirement for large amounts of RNA are the other disadvantages.
Regarding miRNA analysis, several modifications to the original method were adopted to overcome those limitations. As an alternative to UV, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) was proposed to cross-link RNA to the nylon membrane, which resulted in improved sensitivity [63]. Locked nucleic acid (LNA) modified oligonucleotide probes were successfully used against the sensitivity and specificity problems of DNA oligonucleotides [64]. In order to avoid the safety problems of radioactively labeled probes, digoxigenin (DIG)-labeled oligonucleotide probes containing locked nucleic acids [65], and biotin-labeled probes [65] were proposed. The protocol was also optimized for the detection of viral miRNAs [66]. In another study, liquid hybridization consisting of pre-hybridization of target RNA with oligonucleotide probes, Exo-1 digestion, and non-denaturing gel electrophoresis achieved greater sensitivity than Northern blot [67].
2.2.2. RT-qPCR
RT-qPCR is a gold standard for miRNA detection, enabling specific, sensitive, and quantitative results. For RT-qPCR analysis, target miRNAs are reverse-transcribed to complementary DNA (cDNA), which is then amplified by qPCR. Using intercalating dye or hydrolysis-based probes allows real-time fluorescence detection of the amplified products. Quantification is then achieved by using the relationship between the threshold value and the starting copy number of miRNA [68].
Although RT-qPCR is a well-established method, primer design and reaction conditions may need optimization for every new miRNA analysis [69][70]. Regarding the primer design issue in miRNA analysis, the tools such as miRprimer [71] and miPrimer [72] were proposed. A modified form of RT-qPCR was named the stem-loop RT-qPCR, which consisted of two steps [73]. The stem-loop primer was first hybridized in this technique to the miRNA molecule. Then, it was reverse-transcribed and used as a template in conventional qPCR, including the fluorescent-based probe. In a further study [74], a universal stem-loop primer was designed and shown to save 75% of the cost of primers and 60% of the test time compared to the stem-loop primer. Later, a universal hairpin primer system was proposed to eliminate the need for designing miRNA-specific hairpin primers, thereby reducing the cost [75]. RT-qPCR was used for the analysis of miRNA profile in embryonic stem cell differentiation [76] and detection of extracellular vesicle miRNAs [77], as well as to distinguish miRNA editing isoforms [78]. As RT-qPCR is sensitive and specific, it is still widely used for miRNA detection despite the challenges with optimization. In recent years, compared to conventional RT-qPCR, droplet digital PCR [79][80][81] has also been popular due to its better sensitivity and diagnostic potential.
2.2.3. Microarrays
Microarray technology relies on hybridization between the oligonucleotide probe immobilized on a solid surface and the target miRNA. Before hybridization, the target miRNA is reverse-transcribed to cDNA and simultaneously labeled. Then, the arrays are scanned for the hybridization signal, depending on the labeling strategy. Labeling can be achieved by the incorporation of fluorescence dyes [82][83] or radioisotopes [84]. Alternatively, miRNAs can be directly labeled with biotin, and the hybridization can be monitored by a fluorescent signal that results from the binding between biotin-labeled miRNAs and streptavidin-conjugated quantum dots [85]. The protocols differ in terms of probe design, probe immobilization strategy, sample labeling, and signal detection [86]. For instance, instead of oligonucleotides, peptide nucleic acids (PNA) can be used as probes [87]. Furthermore, miRNA microarray data analysis [88] and data submission [89] issues need to be considered.
The major advantage of microarrays is that they enable a high-throughput screen for comparing miRNA expression profiles in different organs or tissues. For instance, miRNA microarray profiling allowed the identification of miRNAs that could be associated with liver cancer [90], attention deficit hyperactivity disorder in children [91], epithelial ovarian cancer [92], and rheumatoid arthritis [93]. Although miRNA microarray analysis is very useful for comparing miRNA expression levels between two states (e.g., control tissue and cancer tissue), it suffers from low sensitivity and low specificity. Similar to Northern blot analysis, it is not a suitable method for the discovery of novel miRNAs.
2.3. MNP-Based Biosensors for miRNA Detection
As described above, there are many conventional methods for detecting miRNA. These complex techniques require specially trained personnel and can give false positive results [10]. Therefore, new methods are needed to detect abnormal miRNA expression, which is critical for early diagnosis. With this approach, biosensor studies based on nanomaterials can be an alternative to conventional analytical devices due to their small sample size, low cost, fast response time, and ease of use. Biosensors provide measurable signals depending on the concentration of the target analyte. They are defined as analytical devices consisting of two main parts, a detection element and a transducer [94]. According to the type of signal generation (electrochemical, optical, thermal, etc.), it is possible to classify biosensors [95]. In recent years, MNP-based biosensor systems have been used for the detection of miRNA.
2.3.1. Optical Biosensor Systems
There are advantages to using optical biosensor systems in terms of noise-free, stable, and sensitive properties compared to other biosensor systems [96]. These biosensors are a good alternative for the detection of cancer markers, as they provide a non-invasive approach [97]. The optical sensing systems based on different signal conversion principles such as colorimetry, fluorescence, chemo/bioluminescence, and scattering-based biosensing, can be used to detect microRNA from biological samples such as plasma, serum, and blood [98].
The colorimetric optical sensor is an analytical system that measures the emitted or absorbed light intensity resulting from the recognition of the target molecule by the bioreceptor [99]. The biosensing that occurs in these systems is converted into a color change. Nanomaterials such as magnetic nanoparticles and gold nanoparticles have been widely used for this purpose. These systems can be described as simple, practical, and cheap because they can be read visually and without any tools [100]. In MNP-based colorimetric biosensor systems developed for miRNA detection, hemin chemistry [101], colorimetric TMB-H2O2 systems [102], and aggregation of gold nanoparticles [103] in salt were used and the color changes were monitored with UV-Vis absorption spectrum. Colorimetric sensor platforms were achieved by converting a colorless substrate to a green product in the plate for the detection of miR-21 (Figure 1a) [101]. Gold decorated MNPs (GMNP) were used in the sensor platform based on the catalytic hairpin assembly (CHA) reaction. In the presence of the cofactor hemin, oligonucleotides were labeled with GMNP to form a colored product. As a result of the reaction in the presence of H2O2, the LOD was determined to be 1 aM and the reaction time was reduced to less than four hours. Another colorimetric sensor system was developed for the detection of Lethal-7 (let-7a) miRNAs in gastric cancer [102]. In this system, Fe3O4 nanosheets were functionalized with target miRNA (let-7a), and hairpins H1 and H2. Thanks to the hybridization chain reaction, a double-stranded DNA (dsDNA) structure was formed on the nanosheet. After removing the excess Fe3O4/dsDNAs in the medium with a magnet, H2O2 and TMB were added. LOD was determined to be 13 aM as a result of the isothermal process without enzyme. In the colorimetric sensing system developed by Wang et al. [103], the ability of MNPs to isolate the target analyte from the sample with a single magnet was exploited. Subsequently, the miRNA-155 functionalized with AuNP was precipitated by the salt-induced aggregation method. A two-part colorimetric sensor system was developed. First, Fe3O4 nanoparticles were coated with gold, then functionalized with DNAzyme. Single-stranded DNAs (ssDNAs) were formed in the presence of miRNA-155. ssDNAs were separated with a magnet and quantified by precipitation with NaCl in AuNP. It was possible to detect miRNA within 2 h at room temperature using the LOD in the fM level.
Fluorescence-based optical sensors are widely used for the detection of small biomarkers such as DNA due to their advantageous properties, such as low cost, high efficiency, and easy processing steps. In these platforms, the light emitted from the target sample is measured as a result of the excitation. In fluorescence-based biosensor systems developed with MNPs for miRNA detection, dyes (fluorescein (FAM) [104][105][106]) and substrate (tyramine) [107] are used for fluorescent labeling. Measurements are simplified with fluorescence resonance energy transfer (FRET) [106] or quenching [108]. Two different fluorescence systems have been developed for the potential prostate cancer biomarker miRNA-141. One study by Sun et al. [104] was based on duplex-specific nuclease (DSN), while the other study by Wang et al. [107] was based on the toehold-mediated strand displacement reaction (TSDR) using the horseradish peroxidase (HRP) enzyme. In the study by Sun et al., Fe3O4 was used as the MNP, and the surfaces of the MNPs were coated with polydopamine [104]. In the presence of the target miRNA, it hybridized with 6-carboxy fluorescence (FAM)-labeled single-stranded DNAs. Due to duplex-specific nuclease (DSN) in the medium, the FAM-labeled DNA was separated from the RNA and fragmented into small fragments. When the remaining DNA was absorbed on the Ca2+ surface of the MNPs, a new cycle began in which the miRNA hybridized with a new DNA. The released small particles FAM-DNA caused strong fluorescence intensity, and the miRNA could be quantified based on the changes in fluorescence. For this method, LOD was determined as 0.42 pM. It is quite remarkable that it is used for the detection of miRNA from human cell lines. According to the study by Wang et al. [107], the LOD of exosomal miRNA-141 could be reduced to fM by detecting it with MNP-based HRP-catalyzed TSDR (Figure 1b). For this purpose, 4 different probes were immobilized on the surface of carboxyl terminated MNPs. The miRNA-141 sequence recognized the toehold domain complementary part of the probe1, and as a result of the TSDR reaction, P2 was removed from the environment while a new toehold part was formed. Upon the addition of biotin-tipped probe 4, probe 4 and probe 1 formed a double chain. miRNA-141 released thus initiated a new cyclic amplification. After the addition of streptavidin-HRP (SA-HRP), MNP was collected from the medium with a magnet. Then, tyramine, one of the fluorescent substrates, was added to the MNP. The color change that occurred under H2O2 catalysis was used to calculate the miRNA concentration. In addition, a fluorescence biosensor using MNP and Clustered Regularly Spaced Short Palindromic Repeats (CRISPR)/CRISPR-associated proteins system family (Cas12a) was developed for the first time to detect exosomal miRNA [108]. Cas12 increased target specificity because it could bind ssDNA or double-stranded DNA (dsDNA) without the contiguous protospacer motif, and thanks to MNPs, the trans-splicing activity of CRISPR/Cas12a was abolished in the absence of the target miRNA. This is a remarkable study in terms of the detectability of exosomal miR-21 from lung cancer plasma with high sensitivity and specificity.
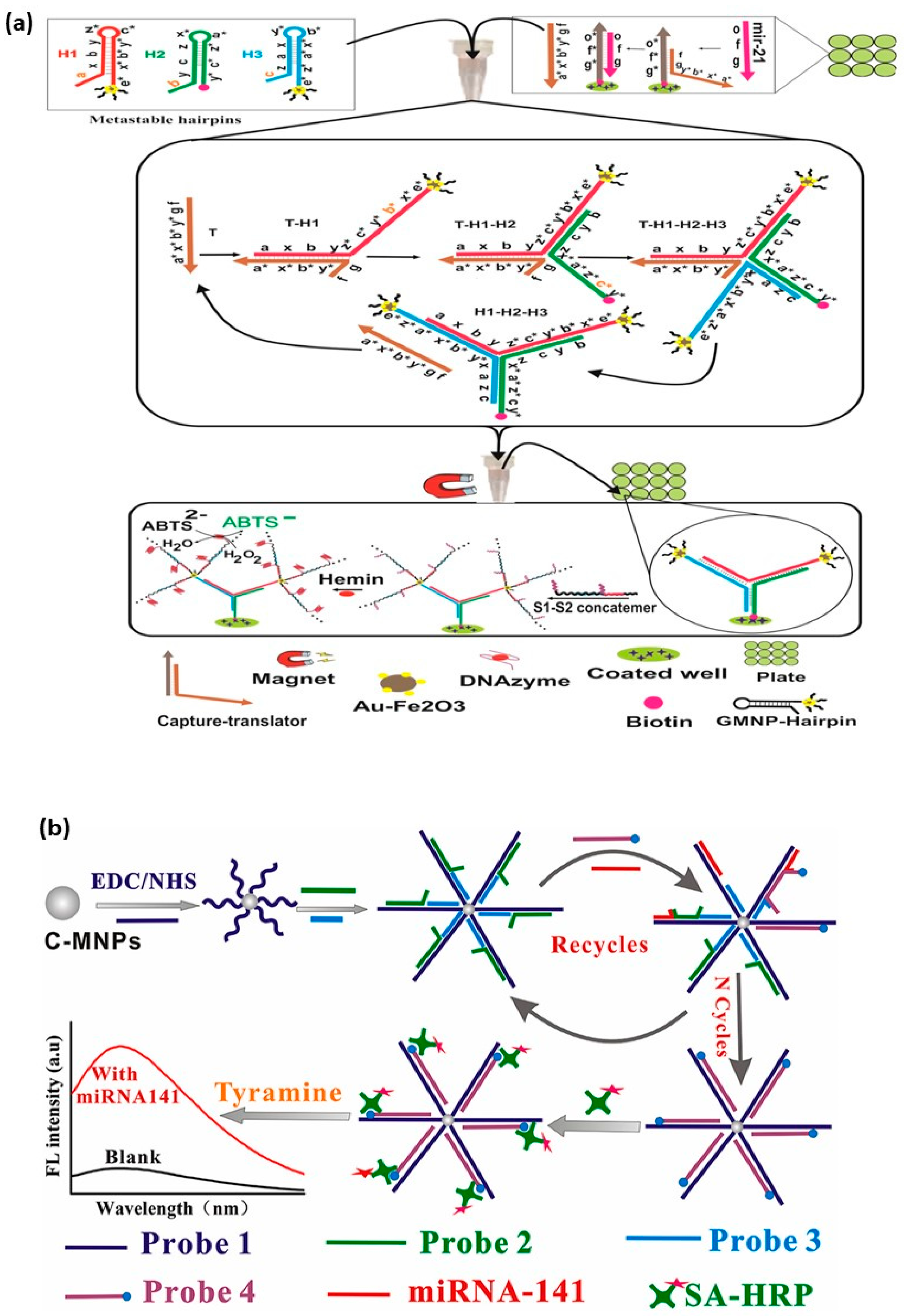
Figure 1. MNP-based (a) Colorimetric-based optical biosensor platform. H1 and H3 are GMNP-labeled, while H2 is biotinylated. The segments that are exposed and involved in the serial reactions required for the formation of the targeted nanostructure are shown with “*”. [Reproduced with the permission of [101] Copyright 2019 Published by Elsevier B.V.]; (b) Fluorescence-based platform optical biosensor platform [Reproduced with the permission of [107] Copyright 2019 Published by Elsevier B.V.].
Electrogenerated chemiluminescence sensing is a detection method that originated from a combination of electrochemical and chemiluminescent sensors. Basically, an electron transfer reaction takes place on the electrode surface [109]. This strategy could be used for clinical detection of miRNA [110][111][112] because of its rapid response, high sensitivity, and low cost. Wang et al. [110] designed a multichannel paper-based electrochemiluminescence microfluidic platform that focused on the detection of two different miRNAs 155 and 126. After subjecting the paper to a wax printing process to enable the fabrication of bipolar electrodes, the electrode sites were prepared with AuNP. Two different surfaces were designed for miRNA detection. Signal probes with CdTe quantum dots (QD) and Au@g-C3N4 nanosheets were used for the detection of miRNA-155 and miRNA-126, respectively. In this system, K2S2O8 was used as a co-reactant, while Fe3O4 MNP was assigned as a carrier. It is important to note that the luminescent light on both platforms is stronger than the single electroluminescent signal. In addition, the use of K2S2O8 was found to improve the selectivity, sensitivity, response speed, and signal intensity of the sensor. The use of nanosheets in electrochemiluminescence systems also attracts attention. These structures were used in a sensor system for miRNA-210, a breast cancer marker. Due to their optoelectronic properties, ease of synthesis, biocompatibility, and ability to absorb ssDNA structures, analysis from serum was achieved [111]. In addition, SiO2-coated Fe3O4-NPs were functionalized with cholesterol-linked aptamers and were immobilized on the surface of the magnetic carbon electrode. The MoS2 nanosheet-DNA probe was attached to the surface. The LOD value was determined as 0.3 fM using the luminescence change.
Another interesting system in the development of MNP-based optical platforms is the surface-enhanced Raman spectroscopy (SERS) based sensor system. This is a surface-sensitive technique used for specific detection that measures the interaction between nanostructures and light by Raman scattering [113]. Recently, the method has also been used for the detection of miRNA [114][115][116][117]. SERS tags are used for optical measurement. With these tags, a single target can be analyzed in a single system, while it can be prepared in multiplexed systems. By Zhang et al., two different systems have been developed for miRNA-141 alone [117] as well as a multiplex system for simultaneous analysis of miRNA-141, -429, and -200b [116] sing the same SERS-nanotag and its substrate. In both systems, silica-coated, analyte-embedded Au nanoparticles (SA@GNPs) and Au-coated paramagnetic nanoparticles (Au@MNPs) were used as SERS-nanotag and substrate, respectively. For the detection of miRNA-141 alone, 5,5′-dithiobis(succinimidyl-2-nitrobenzoate) (DSNB) was used as SERS-tag, whereas DSNB, methylene blue, and Nile blue were used as SERS-tag for the determination of miRNA-141, -429, and -200b in the multiplex system, respectively.
In addition to all these optical sensors, optomagnetic sensor systems were developed for the detection of miRNA. These systems consist of a photodetector, a light source, and a magnetic actuator to generate a magnetic field. It is a system that has become a trend in recent years due to advantages such as the suppression of noise, low cost, and the increase in the reaction rate to the molecules colliding with the action of the magnetic force [118]. Several optomagnetic sensing systems have been developed for let-7b miRNA, a member of the let7 family [119][120]. In both systems, MNPs released as a result of the recognition of the target miRNA by the designed surface could be measured by a laser-based optomagnetic sensor. By analyzing serum in both systems, it offers potential for use in clinical applications.
2.3.2. Electrochemical Biosensor Systems
Electrochemical systems are one of the most commonly used sensors for the detection of miRNA. A reaction between the target analyte and the biological recognition element is converted into an electrical signal in these systems. Since this reaction and the flow of electrodes occur in the electrode system, the electrode is one of the essential components in these systems [97]. The counter, reference, and working electrodes are used. Generally, Ag/AgCl is used as the reference electrode, and Pt is used as the counter electrode. The working electrode can be made of materials such as gold, carbon, or graphene and can be used specifically [121]. Electrochemical platforms categorized according to the measurement principles of amperometric, potentiometric, voltammetric, and impedimetric are in great demand due to their advantageous properties such as ease of operation, miniaturization and fabrication, and low cost [122].
Voltammetric systems based on measuring the change in current with a change in potential are common electrochemical sensors. These techniques in various forms, such as chronoamperometry [18], cyclic voltammetry (CV) [123][124], square wave voltammetry (SWV) [125][126][127], and differential pulse voltammetry (DPV) [124][128][129][130], are also commonly used for miRNA detection due to their sensitivity and wide linear range. In addition, electrochemical biosensors are functionalized or combined with MNPs because they are easy to functionalize, inexpensive, and have the ability to specifically detect target miRNAs from the sample under the magnetic field. In this regard, MNPs can also play a role in the sensing system by capturing miRNAs from body fluids such as serum and plasma and simply connecting them to the electrode surface using a magnet. By Shen et al. [125], MNPs were functionalized with gold nanoparticles and gold stir bars in the electrochemical sensing system designed for the simultaneous detection of miRNA-21 and miRNA-155 (Figure 2). They were both used to capture miRNAs from serum samples and electrochemical measurements. In this system, Fe3O4 nanoparticles were functionalized with electrochemical labeled and complementary DNAs after gold plating and immobilized on the SPCE surface with magnets. SWN measurements were performed on target miRNAs captured by DNA/RNA hybridization. The method has the advantage of being easy to use and increasing amplification efficiency, not requiring enzymes. Using a similar strategy, another electrochemical sensor system was developed by Tavallay et al. [126]. It is also reported to be the first system that can detect miRNA from unprocessed blood samples. For the analysis of miR-21, gold-coated magnetic nanoparticles were used and immobilized on the surface of the gold microelectrode using a magnet. Direct analysis of miR-21 in untreated blood from a lung cancer mouse model was achieved. The sensitivity was reported to be better than other sensors with a LOD of 10 aM. It was also much faster than conventional PCR, providing the analysis result in less than 30 min.
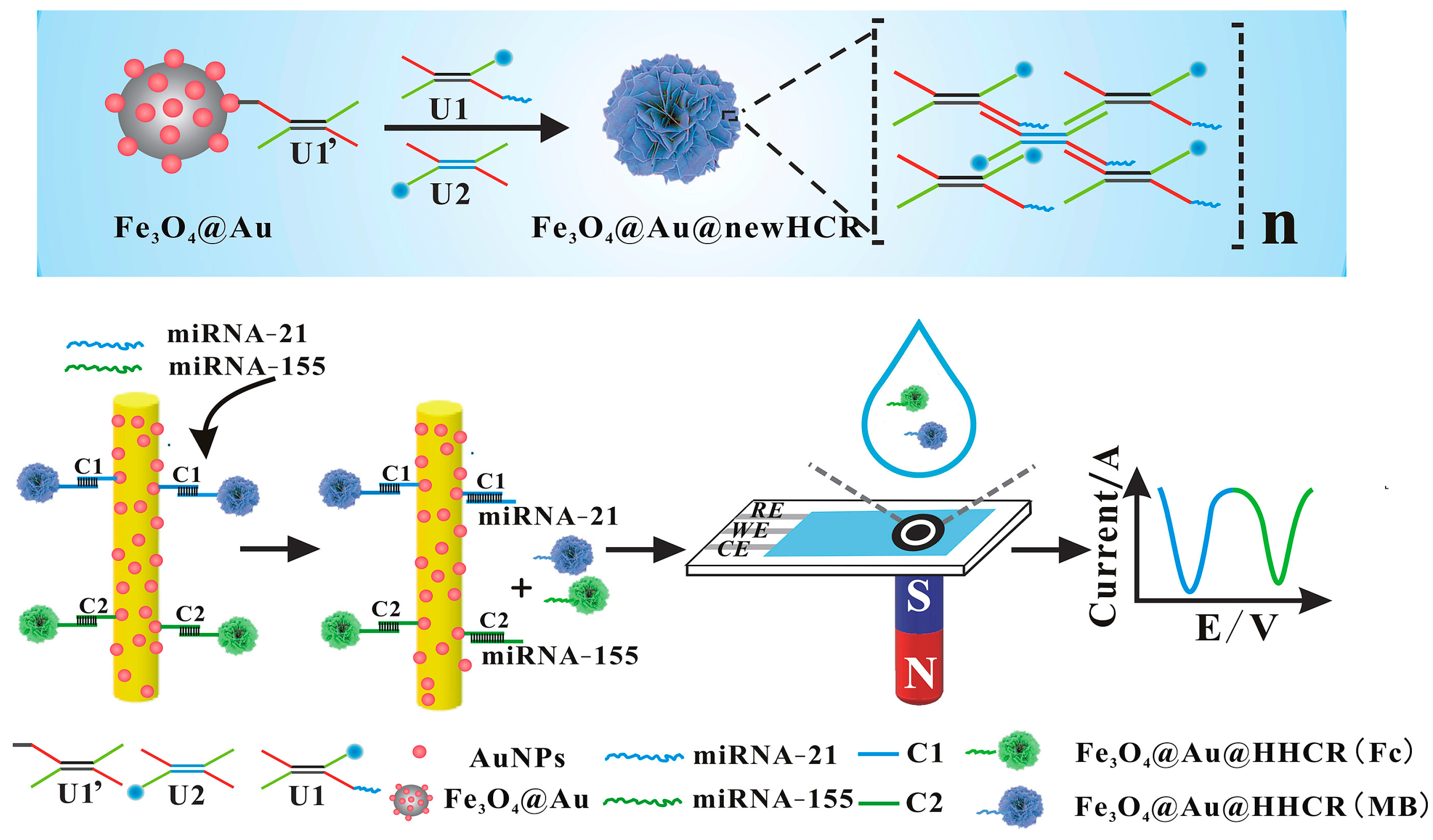
Figure 2. MNP-based electrochemical biosensor platform for miRNA-21 and miRNA-155. [Reproduced with the permission of [125] Copyright 2019 Published by Elsevier B.V.].
References
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of Magnetic Nanoparticles in Nucleic Acid Detection. J. Nanobiotechnol. 2020, 18, 62.
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic Nanoparticles: Preparation, Physical Properties, and Applications in Biomedicine. Nanoscale Res. Lett. 2012, 7, 144.
- Gessner, I.; Fries, J.W.U.; Brune, V.; Mathur, S. Magnetic Nanoparticle-Based Amplification of MicroRNA Detection in Body Fluids for Early Disease Diagnosis. J. Mater. Chem. B 2021, 9, 9–22.
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the Application of Magnetic Nanoparticles for Sensing. Adv. Mater. 2019, 31, e1904385.
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, Biogenesis, and Their Evolving Role in Animal Development and Disease. Vet. Pathol. 2014, 51, 759–774.
- Harmanci, D.; Erkan, E.P.; Kocak, A.; Akdogan, G.G. Role of the MicroRNA-29 Family in Fibrotic Skin Diseases. Biomed. Rep. 2017, 6, 599–604.
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276.
- Filipów, S.; Łaczmański, Ł. Blood Circulating MiRNAs as Cancer Biomarkers for Diagnosis and Surgical Treatment Response. Front. Genet. 2019, 10, 169.
- Schade, A.; Delyagina, E.; Scharfenberg, D.; Skorska, A.; Lux, C.; David, R.; Steinhoff, G. Innovative Strategy for MicroRNA Delivery in Human Mesenchymal Stem Cells via Magnetic Nanoparticles. Int. J. Mol. Sci. 2013, 14, 10710–10726.
- Ye, J.; Xu, M.; Tian, X.; Cai, S.; Zeng, S. Research Advances in the Detection of MiRNA. J. Pharm. Anal. 2019, 9, 217–226.
- Chaudhary, V.; Jangra, S.; Yadav, N.R. Nanotechnology Based Approaches for Detection and Delivery of MicroRNA in Healthcare and Crop Protection. J. Nanobiotechnol. 2018, 16, 40.
- Ye, Y.; Lin, Y.; Chi, Z.; Zhang, J.; Cai, F.; Zhu, Y.; Tang, D.; Lin, Q. Rolling Circle Amplification (RCA) -Based Biosensor System for the Fluorescent Detection of MiR-129-2-3p MiRNA. PeerJ 2022, 10, e14257.
- Meng, T.; Zhao, D.; Ye, H.; Feng, Y.; Wang, H.; Zhang, Y. Construction of an Ultrasensitive Electrochemical Sensing Platform for MicroRNA-21 Based on Interface Impedance Spectroscopy. J. Colloid Interface Sci. 2020, 578, 164–170.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402.
- Yoshida, T.; Asano, Y.; Ui-Tei, K. Modulation of MicroRNA Processing by Dicer via Its Associated DsRNA Binding Proteins. Non-Coding RNA 2021, 7, 57.
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854.
- Mishra, R.; Kumar, A.; Ingle, H.; Kumar, H. The Interplay Between Viral-Derived MiRNAs and Host Immunity During Infection. Front. Immunol. 2020, 10, 3079.
- Wong-Bajracharya, J.; Singan, V.R.; Monti, R.; Plett, K.L.; Ng, V.; Grigoriev, I.V.; Martin, F.M.; Anderson, I.C.; Plett, J.M. The Ectomycorrhizal Fungus Pisolithus Microcarpus Encodes a MicroRNA Involved in Cross-Kingdom Gene Silencing during Symbiosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2103527119.
- Millar, A.A. The Function of MiRNAs in Plants. Plants 2020, 9, 198.
- The Roles of MicroRNAs in Mouse Development|Nature Reviews Genetics. Available online: https://www.nature.com/articles/s41576-020-00309-5 (accessed on 21 November 2022).
- Lee, L.W.; Zhang, S.; Etheridge, A.; Ma, L.; Martin, D.; Galas, D.; Wang, K. Complexity of the MicroRNA Repertoire Revealed by Next-Generation Sequencing. RNA 2010, 16, 2170–2180.
- Galluzzo, A.; Gallo, S.; Pardini, B.; Birolo, G.; Fariselli, P.; Boretto, P.; Vitacolonna, A.; Peraldo-Neia, C.; Spilinga, M.; Volpe, A.; et al. Identification of Novel Circulating MicroRNAs in Advanced Heart Failure by Next-Generation Sequencing. ESC Heart Fail. 2021, 8, 2907–2919.
- Raza, S.H.A.; Abdelnour, S.A.; Dhshan, A.I.M.; Hassanin, A.A.; Noreldin, A.E.; Albadrani, G.M.; Abdel-Daim, M.M.; Cheng, G.; Zan, L. Potential Role of Specific MicroRNAs in the Regulation of Thermal Stress Response in Livestock. J. Therm. Biol. 2021, 96, 102859.
- Chen, M.; Wang, F.; Xia, H.; Yao, S. MicroRNA-155: Regulation of Immune Cells in Sepsis. Mediat. Inflamm. 2021, 2021, 8874854.
- Tabaei, S.; Tabaee, S.S. Implications for MicroRNA Involvement in the Prognosis and Treatment of Atherosclerosis. Mol. Cell. Biochem. 2021, 476, 1327–1336.
- Borbet, T.C.; Hines, M.J.; Koralov, S.B. MicroRNA Regulation of B Cell Receptor Signaling. Immunol. Rev. 2021, 304, 111–125.
- Chen, D.; Yang, X.; Liu, M.; Zhang, Z.; Xing, E. Roles of MiRNA Dysregulation in the Pathogenesis of Multiple Myeloma. Cancer Gene Ther. 2021, 28, 1256–1268.
- Siddika, T.; Heinemann, I.U. Bringing MicroRNAs to Light: Methods for MicroRNA Quantification and Visualization in Live Cells. Front. Bioeng. Biotechnol. 2021, 8, 619583.
- Anelli, L.; Zagaria, A.; Specchia, G.; Musto, P.; Albano, F. Dysregulation of MiRNA in Leukemia: Exploiting MiRNA Expression Profiles as Biomarkers. Int. J. Mol. Sci. 2021, 22, 7156.
- Nies, Y.H.; Najib, N.H.M.; Lim, W.L.; Kamaruzzaman, M.A.; Yahaya, M.F.; Teoh, S.L. MicroRNA Dysregulation in Parkinson’s Disease: A Narrative Review. Front. Neurosci. 2021, 15, 660379.
- Ahmed, U.; Ashfaq, U.A.; Qasim, M.; Ahmad, I.; Ahmad, H.U.; Tariq, M.; Masoud, M.S.; Khaliq, S. Dysregulation of Circulating MiRNAs Promotes the Pathogenesis of Diabetes-Induced Cardiomyopathy. PLoS ONE 2021, 16, e0250773.
- Liu, H.; Ma, L.; Wang, L.; Yang, Y. MicroRNA-937 Is Overexpressed and Predicts Poor Prognosis in Patients with Colon Cancer. Diagn. Pathol. 2019, 14, 136.
- Chen, Z.; Zhang, Y. Upregulation of MicroRNA-937 Predicts a Poor Prognosis and Promotes Hepatocellular Carcinoma Cell Proliferation, Migration, and Invasion. Mol. Biotechnol. 2022, 64, 33–41.
- Shen, Q.; Xiong, P.; Yang, D.; Chen, L. Downregulated MicroRNA-149-3p Triggers Malignant Development and Predicts Worse Prognosis in Oral Squamous Cell Carcinoma. Arch. Oral Biol. 2022, 134, 105336.
- Tribolet, L.; Kerr, E.; Cowled, C.; Bean, A.G.; Stewart, C.R.; Dearnley, M.; Farr, R.J. MicroRNA Biomarkers for Infectious Diseases: From Basic Research to Biosensing. Front. Microbiol. 2020, 11, 1197.
- Liu, X.; Li, Y.; Zhu, X.; Jiang, C. MicroRNA as an Early Diagnostic Biomarker for Contrast-Induced Acute Kidney Injury. Drug Chem. Toxicol. 2022, 45, 1552–1557.
- Vykoukal, J.; Fahrmann, J.F.; Patel, N.; Shimizu, M.; Ostrin, E.J.; Dennison, J.B.; Ivan, C.; Goodman, G.E.; Thornquist, M.D.; Barnett, M.J.; et al. Contributions of Circulating MicroRNAs for Early Detection of Lung Cancer. Cancers 2022, 14, 4221.
- Iguchi, H.; Kosaka, N.; Ochiya, T. Secretory MicroRNAs as a Versatile Communication Tool. Commun. Integr. Biol. 2010, 3, 478–481.
- Sohel, M.H. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev. Life Sci. 2016, 10, 175–186.
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044.
- Durur, D.Y.; Tastan, B.; Tufekci, K.U.; Olcum, M.; Uzuner, H.; Karakülah, G.; Yener, G.; Genc, S. Alteration of MiRNAs in Small Neuron-Derived Extracellular Vesicles of Alzheimer’s Disease Patients and the Effect of Extracellular Vesicles on Microglial Immune Responses. J. Mol. Neurosci. 2022, 72, 1182–1194.
- Panvongsa, W.; Pegtel, D.M.; Voortman, J. More than a Bubble: Extracellular Vesicle MicroRNAs in Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 1160.
- Aryani, A.; Denecke, B. In Vitro Application of Ribonucleases: Comparison of the Effects on MRNA and MiRNA Stability. BMC Res. Notes 2015, 8, 164.
- Li, Z.; Chen, D.; Wang, Q.; Tian, H.; Tan, M.; Peng, D.; Tan, Y.; Zhu, J.; Liang, W.; Zhang, L. MRNA and MicroRNA Stability Validation of Blood Samples under Different Environmental Conditions. Forensic Sci. Int. Genet. 2021, 55, 102567.
- Shihana, F.; Wong, W.K.; Joglekar, M.V.; Mohamed, F.; Gawarammana, I.B.; Isbister, G.K.; Hardikar, A.A.; Seth, D.; Buckley, N.A. Urinary MicroRNAs as Non-Invasive Biomarkers for Toxic Acute Kidney Injury in Humans. Sci. Rep. 2021, 11, 9165.
- Ban, E.; Kwon, H.; Seo, H.S.; Yoo, Y.S.; Song, E.J. Screening of MiRNAs in Plasma as a Diagnostic Biomarker for Cardiac Disease Based on Optimization of Extraction and QRT-PCR Condition Assay through Amplification Efficiency. BMC Biotechnol. 2021, 21, 50.
- Demiray, A.; Sarı, T.; Çalışkan, A.; Nar, R.; Aksoy, L.; Akbubak, İ.H. Serum MicroRNA Signature Is Capable of Predictive and Prognostic Factor for SARS-COV-2 Virulence. Turk. J. Biochem. 2021, 46, 245–253.
- Kopkova, A.; Sana, J.; Machackova, T.; Vecera, M.; Radova, L.; Trachtova, K.; Vybihal, V.; Smrcka, M.; Kazda, T.; Slaby, O.; et al. Cerebrospinal Fluid MicroRNA Signatures as Diagnostic Biomarkers in Brain Tumors. Cancers 2019, 11, 1546.
- Bendifallah, S.; Suisse, S.; Puchar, A.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J. Clin. Med. 2022, 11, 612.
- Sabato, C.; Noviello, T.M.R.; Covre, A.; Coral, S.; Caruso, F.P.; Besharat, Z.M.; Splendiani, E.; Masuelli, L.; Battistelli, C.; Vacca, A.; et al. A Novel MicroRNA Signature for the Detection of Melanoma by Liquid Biopsy. J. Transl. Med. 2022, 20, 469.
- Singh, P.; Singh, A.; Gupta, N.; Raja, K.D.; Singh, P.; Agarwal, S.; Sharma, A. Non-Invasive Diagnostic Potential of MicroRNA-203 in Liquid Biopsy of Urothelial Carcinoma of Bladder. Mol. Cell. Biochem. 2022, 477, 2173–2182.
- Hussein, Z. Leading to Intention: The Role of Attitude in Relation to Technology Acceptance Model in E-Learning. Procedia Comput. Sci. 2017, 105, 159–164.
- Huang, F.; Xiang, Y.; Li, T.; Huang, Y.; Wang, J.; Zhang, H.-M.; Li, H.-H.; Dai, Z.-T.; Li, J.-P.; Li, H.; et al. Metformin and MiR-365 Synergistically Promote the Apoptosis of Gastric Cancer Cells via MiR-365-PTEN-AMPK Axis. Pathol. Res. Pract. 2022, 230, 153740.
- Zhang, W.; Liu, Z. MiRNA-139-3p inhibits malignant progression in urothelial carcinoma of the bladder via targeting KIF18B and inactivating Wnt/beta-catenin pathway. Pharm. Genom. 2022, 33, 1–9.
- Pan, B.; Han, B.; Zhu, X.; Wang, Y.; Ji, H.; Weng, J.; Liu, Y. Dysfunctional MicroRNA-144-3p/ZBTB20/ERK/CREB1 Signalling Pathway Is Associated with MK-801-Induced Schizophrenia-like Abnormalities. Brain Res. 2023, 1798, 148153.
- Liu, L.; Xiao, C.; Sun, Q. MiRNA-375 Inhibits Retinoblastoma Progression through Targeting ERBB2 and Inhibiting MAPK1/MAPK3 Signalling Pathway. Cutan. Ocul. Toxicol. 2021, 41, 1–10.
- Kehl, T.; Backes, C.; Kern, F.; Fehlmann, T.; Ludwig, N.; Meese, E.; Lenhof, H.-P.; Keller, A. About MiRNAs, MiRNA Seeds, Target Genes and Target Pathways. Oncotarget 2017, 8, 107167.
- Špringer, T.; Krejčík, Z.; Homola, J. Detecting Attomolar Concentrations of MicroRNA Related to Myelodysplastic Syndromes in Blood Plasma Using a Novel Sandwich Assay with Nanoparticle Release. Biosens. Bioelectron. 2021, 194, 113613.
- Ouyang, T.; Liu, Z.; Han, Z.; Ge, Q. MicroRNA Detection Specificity: Recent Advances and Future Perspective. Anal. Chem. 2019, 91, 3179–3186.
- Streit, S.; Michalski, C.W.; Erkan, M.; Kleeff, J.; Friess, H. Northern Blot Analysis for Detection and Quantification of RNA in Pancreatic Cancer Cells and Tissues. Nat. Protoc. 2009, 4, 37–43.
- He, S.L.; Green, R. Chapter Three—Northern Blotting. In Methods in Enzymology; Laboratory Methods in Enzymology: RNA; Lorsch, J., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 530, pp. 75–87.
- Koscianska, E.; Starega-Roslan, J.; Sznajder, L.J.; Olejniczak, M.; Galka-Marciniak, P.; Krzyzosiak, W.J. Northern Blotting Analysis of MicroRNAs, Their Precursors and RNA Interference Triggers. BMC Mol. Biol. 2011, 12, 14.
- Pall, G.S.; Codony-Servat, C.; Byrne, J.; Ritchie, L.; Hamilton, A. Carbodiimide-Mediated Cross-Linking of RNA to Nylon Membranes Improves the Detection of SiRNA, MiRNA and PiRNA by Northern Blot. Nucleic Acids Res. 2007, 35, e60.
- Várallyay, E.; Burgyán, J.; Havelda, Z. MicroRNA Detection by Northern Blotting Using Locked Nucleic Acid Probes. Nat. Protoc. 2008, 3, 190–196.
- Kim, S.W.; Li, Z.; Moore, P.S.; Monaghan, A.P.; Chang, Y.; Nichols, M.; John, B. A Sensitive Non-Radioactive Northern Blot Method to Detect Small RNAs. Nucleic Acids Res. 2010, 38, e98.
- McClure, L.V.; Lin, Y.-T.; Sullivan, C.S. Detection of Viral MicroRNAs by Northern Blot Analysis. Methods Mol. Biol. 2011, 721, 153–171.
- Ahmad, W.; Gull, B.; Baby, J.; Mustafa, F. A Comprehensive Analysis of Northern versus Liquid Hybridization Assays for MRNAs, Small RNAs, and MiRNAs Using a Non-Radiolabeled Approach. Curr. Issues Mol. Biol. 2021, 43, 457–484.
- Chen, C.; Tan, R.; Wong, L.; Fekete, R.; Halsey, J. Quantitation of MicroRNAs by Real-Time RT-QPCR. PCR Protoc. 2011, 687, 113–134.
- Dellett, M.; Simpson, D.A. Considerations for Optimization of MicroRNA PCR Assays for Molecular Diagnosis. Expert Rev. Mol. Diagn. 2016, 16, 407–414.
- Kotlarek, M.; Kubiak, A.; Jażdżewski, K.; Wójcicka, A. MicroRNA Analysis Using the Quantitative Real-Time PCR Reaction. Methods Mol. Biol. 2018, 1823, 69–85.
- Busk, P.K. A Tool for Design of Primers for MicroRNA-Specific Quantitative RT-QPCR. BMC Bioinform. 2014, 15, 29.
- Kang, S.-T.; Hsieh, Y.-S.; Feng, C.-T.; Chen, Y.-T.; Yang, P.E.; Chen, W.-M. MiPrimer: An Empirical-Based QPCR Primer Design Method for Small Noncoding MicroRNA. RNA 2018, 24, 304–312.
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-Time Quantification of MicroRNAs by Stem-Loop RT-PCR. Nucleic Acids Res. 2005, 33, e179.
- Yang, L.; Wang, S.; Tang, L.; Liu, B.; Ye, W.; Wang, L.; Wang, Z.; Zhou, M.; Chen, B. Universal Stem-Loop Primer Method for Screening and Quantification of MicroRNA. PLoS ONE 2014, 9, e115293.
- He, F.; Ni, N.; Wang, H.; Zeng, Z.; Zhao, P.; Shi, D.; Xia, Y.; Chen, C.; Hu, D.A.; Qin, K.H.; et al. OUHP: An Optimized Universal Hairpin Primer System for Cost-Effective and High-Throughput RT-QPCR-Based Quantification of MicroRNA (MiRNA) Expression. Nucleic Acids Res. 2022, 50, e22.
- Pan, X.; Murashov, A.K.; Stellwag, E.J.; Zhang, B. Using Quantitative Real-Time PCR to Detect MicroRNA Expression Profile During Embryonic Stem Cell Differentiation. In RNAi and Small Regulatory RNAs in Stem Cells; Humana Press: New York, NY, USA, 2017; pp. 255–265.
- Lee, H.; He, X.; Le, T.; Carnino, J.M.; Jin, Y. Single-Step RT-QPCR for Detection of Extracellular Vesicle MicroRNAs in Vivo: A Time- and Cost-Effective Method. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L742–L749.
- Voss, G.; Edsjö, A.; Bjartell, A.; Ceder, Y. Quantification of MicroRNA Editing Using Two-Tailed RT-QPCR for Improved Biomarker Discovery. RNA 2021, 27, 1412–1424.
- Robinson, S.; Follo, M.; Haenel, D.; Mauler, M.; Stallmann, D.; Tewari, M.; Duerschmied, D.; Peter, K.; Bode, C.; Ahrens, I.; et al. Droplet Digital PCR as a Novel Detection Method for Quantifying MicroRNAs in Acute Myocardial Infarction. Int. J. Cardiol. 2018, 257, 247–254.
- Wang, C.; Ding, Q.; Plant, P.; Basheer, M.; Yang, C.; Tawedrous, E.; Krizova, A.; Boulos, C.; Farag, M.; Cheng, Y.; et al. Droplet Digital PCR Improves Urinary Exosomal MiRNA Detection Compared to Real-Time PCR. Clin. Biochem. 2019, 67, 54–59.
- Laprovitera, N.; Riefolo, M.; Porcellini, E.; Durante, G.; Garajova, I.; Vasuri, F.; Aigelsreiter, A.; Dandachi, N.; Benvenuto, G.; Agostinis, F.; et al. MicroRNA Expression Profiling with a Droplet Digital PCR Assay Enables Molecular Diagnosis and Prognosis of Cancers of Unknown Primary. Mol. Oncol. 2021, 15, 2732–2751.
- Yan, N.; Lu, Y.; Sun, H.; Tao, D.; Zhang, S.; Liu, W.; Ma, Y. A Microarray for MicroRNA Profiling in Mouse Testis Tissues. Reproduction 2007, 134, 73–79.
- Castoldi, M.; Schmidt, S.; Benes, V.; Hentze, M.W.; Muckenthaler, M.U. MiChip: An Array-Based Method for MicroRNA Expression Profiling Using Locked Nucleic Acid Capture Probes. Nat. Protoc. 2008, 3, 321–329.
- Krichevsky, A.M.; King, K.S.; Donahue, C.P.; Khrapko, K.; Kosik, K.S. A MicroRNA Array Reveals Extensive Regulation of MicroRNAs during Brain Development. RNA 2003, 9, 1274–1281.
- Liang, R.-Q.; Li, W.; Li, Y.; Tan, C.; Li, J.-X.; Jin, Y.-X.; Ruan, K.-C. An Oligonucleotide Microarray for MicroRNA Expression Analysis Based on Labeling RNA with Quantum Dot and Nanogold Probe. Nucleic Acids Res. 2005, 33, e17.
- Liu, C.-G.; Calin, G.A.; Volinia, S.; Croce, C.M. MicroRNA Expression Profiling Using Microarrays. Nat. Protoc. 2008, 3, 563–578.
- Forte, G.; Ventimiglia, G.; Pesaturo, M.; Petralia, S. A Highly Sensitive PNA-Microarray System for MiRNA122 Recognition. Biotechnol. J. 2022, 17, 2100587.
- Mastriani, E.; Zhai, R.; Zhu, S. Microarray-Based MicroRNA Expression Data Analysis with Bioconductor. In Transcriptome Data Analysis: Methods and Protocols; Methods in Molecular, Biology; Wang, Y., Sun, M., Eds.; Springer: New York, NY, USA, 2018; pp. 127–138. ISBN 978-1-4939-7710-9.
- Witwer, K.W. Data Submission and Quality in Microarray-Based MicroRNA Profiling. Clin. Chem. 2013, 59, 392–400.
- Lin, L.; Lin, Y.; Jin, Y.; Zheng, C. RETRACTED: Microarray Analysis of MicroRNA Expression in Liver Cancer Tissues and Normal Control. Gene 2013, 523, 158–160.
- Zhu, P.; Pan, J.; Cai, Q.Q.; Zhang, F.; Peng, M.; Fan, X.L.; Ji, H.; Dong, Y.W.; Wu, X.Z.; Wu, L.H. MicroRNA Profile as Potential Molecular Signature for Attention Deficit Hyperactivity Disorder in Children. Biomarkers 2022, 27, 230–239.
- Prahm, K.P.; Høgdall, C.K.; Karlsen, M.A.; Christensen, I.J.; Novotny, G.W.; Høgdall, E. MicroRNA Characteristics in Epithelial Ovarian Cancer. PLoS ONE 2021, 16, e0252401.
- Wu, L.-F.; Zhang, Q.; Mo, X.-B.; Lin, J.; Wu, Y.-L.; Lu, X.; He, P.; Wu, J.; Guo, Y.-F.; Wang, M.-J.; et al. Identification of Novel Rheumatoid Arthritis-Associated MiRNA-204-5p from Plasma Exosomes. Exp. Mol. Med. 2022, 54, 334–345.
- Silva, S.M.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold Coated Magnetic Nanoparticles: From Preparation to Surface Modification for Analytical and Biomedical Applications. Chem. Commun. 2016, 52, 7528–7540.
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109.
- Chen, C.; Wang, J. Optical Biosensors: An Exhaustive and Comprehensive Review. Analyst 2020, 145, 1605–1628.
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent Advancements in Optical Biosensors for Cancer Detection. Biosens. Bioelectron. 2022, 197, 113805.
- McCracken, K.E.; Yoon, J.-Y. Recent Approaches for Optical Smartphone Sensing in Resource-Limited Settings: A Brief Review. Anal. Methods 2016, 8, 6591–6601.
- Aydindogan, E.; Ceylan, A.E.; Timur, S. Paper-Based Colorimetric Spot Test Utilizing Smartphone Sensing for Detection of Biomarkers. Talanta 2020, 208, 120446.
- Song, Y.; Wei, W.; Qu, X. Colorimetric Biosensing Using Smart Materials. Adv. Mater. 2011, 23, 4215–4236.
- Hosseinzadeh, E.; Ravan, H.; Mohammadi, A.; Mohammad-rezaei, R.; Norouzi, A.; Hosseinzadeh, H. Target-Triggered Three-Way Junction in Conjugation with Catalytic Concatemers-Functionalized Nanocomposites Provides a Highly Sensitive Colorimetric Method for MiR-21 Detection. Biosens. Bioelectron. 2018, 117, 567–574.
- Tang, S.; Li, Y.; Zhu, A.; Yao, Y.; Sun, J.; Zheng, F.; Lin, Z.; Shen, W. A Triple-Amplification Strategy Based on the Formation of Peroxidase-like Two-Dimensional DNA/Fe3O4 Networks Initiated by the Hybridization Chain Reaction for Highly Sensitive Detection of MicroRNA. Chem. Commun. 2019, 55, 8386–8389.
- Wang, L.; Liu, Z.-J.; Cao, H.-X.; Liang, G.-X. Ultrasensitive Colorimetric MiRNA Detection Based on Magnetic 3D DNA Walker and Unmodified AuNPs. Sens. Actuators B Chem. 2021, 337, 129813.
- Sun, Y.; Wang, C.; Tang, L.; Zhang, Y.; Zhang, G.-J. Magnetic-Enhanced Fluorescence Sensing of Tumor MiRNA by Combination of with Duplex Specific Nuclease. RSC Adv. 2021, 11, 2968–2975.
- Tian, H.; Yuan, C.; Liu, Y.; Li, Z.; Xia, K.; Li, M.; Xie, F.; Chen, Q.; Chen, M.; Fu, W.; et al. A Novel Quantification Platform for Point-of-Care Testing of Circulating MicroRNAs Based on Allosteric Spherical Nanoprobe. J. Nanobiotechnol. 2020, 18, 158.
- Fan, Y.; Liu, Y.; Zhou, Q.; Du, H.; Zhao, X.; Ye, F.; Zhao, H. Catalytic Hairpin Assembly Indirectly Covalent on Fe3O4@C Nanoparticles with Signal Amplification for Intracellular Detection of MiRNA. Talanta 2021, 223, 121675.
- Wang, Q.; Liu, J.; Zeng, J.; Yang, Z.; Ran, F.; Wu, L.; Yang, G.; Mei, Q.; Wang, X.; Chen, Q. Determination of MiRNA Derived from Exosomes of Prostate Cancer via Toehold-Aided Cyclic Amplification Combined with HRP Enzyme Catalysis and Magnetic Nanoparticles. Anal. Biochem. 2021, 630, 114336.
- Liu, Q.; Liu, J.; He, N.; Zhang, M.; Wu, L.; Chen, X.; Zhu, J.; Ran, F.; Chen, Q.; Zhang, H. CRISPR/Cas12a Coupling with Magnetic Nanoparticles and Cascaded Strand Displacement Reaction for Ultrasensitive Fluorescence Determination of Exosomal MiR-21. Molecules 2022, 27, 5338.
- Shao, B.; Xiao, Z. Recent Achievements in Exosomal Biomarkers Detection by Nanomaterials-Based Optical Biosensors—A Review. Anal. Chim. Acta 2020, 1114, 74–84.
- Wang, F.; Liu, Y.; Fu, C.; Li, N.; Du, M.; Zhang, L.; Ge, S.; Yu, J. Paper-Based Bipolar Electrode Electrochemiluminescence Platform for Detection of Multiple MiRNAs. Anal. Chem. 2021, 93, 1702–1708.
- Shi, J.; Zhang, Y.; Wang, P.; Nie, Y.; Ma, Q. Luminous MoS2 Nanosheet-Based Electrochemiluminescence Biosensor with Biomimetic Vesicle for MiRNA-210 Detection. Talanta 2022, 237, 122969.
- Shao, H.; Lu, J.; Zhang, Q.; Hu, Y.; Wang, S.; Guo, Z. Ruthenium-Based Metal Organic Framework (Ru-MOF)-Derived Novel Faraday-Cage Electrochemiluminescence Biosensor for Ultrasensitive Detection of MiRNA-141. Sens. Actuators B Chem. 2018, 268, 39–46.
- Üzek, R.; Sari, E.; Merkoçi, A. Optical-Based (Bio) Sensing Systems Using Magnetic Nanoparticles. Magnetochemistry 2019, 5, 59.
- He, Y.; Zeng, Z.; Cao, Y.; Zhang, X.; Wu, C.; Luo, X. Ultrasenstive SERS Biosensor Based on Zn2+ from ZnO Nanoparticle Assisted DNA Enzyme Amplification for Detection of MiRNA. Anal. Chim. Acta 2022, 1228, 340340.
- Wu, J.; Zhou, X.; Li, P.; Lin, X.; Wang, J.; Hu, Z.; Zhang, P.; Chen, D.; Cai, H.; Niessner, R.; et al. Ultrasensitive and Simultaneous SERS Detection of Multiplex MicroRNA Using Fractal Gold Nanotags for Early Diagnosis and Prognosis of Hepatocellular Carcinoma. Anal. Chem. 2021, 93, 8799–8809.
- Zhang, H.; Fu, C.; Wu, S.; Shen, Y.; Zhou, C.; Neng, J.; Yi, Y.; Jin, Y.; Zhu, Y. Magnetic-Capture-Based SERS Detection of Multiple Serum MicroRNA Biomarkers for Cancer Diagnosis. Anal. Methods 2019, 11, 783–793.
- Zhang, H.; Fu, C.; Yi, Y.; Zhou, X.; Zhou, C.; Ying, G.; Shen, Y.; Zhu, Y. A Magnetic-Based SERS Approach for Highly Sensitive and Reproducible Detection of Cancer-Related Serum MicroRNAs. Anal. Methods 2018, 10, 624–633.
- Xiao, X.; Yuan, C.; Li, T.; Fock, J.; Svedlindh, P.; Tian, B. Optomagnetic Biosensors: Volumetric Sensing Based on Magnetic Actuation-Induced Optical Modulations. Biosens. Bioelectron. 2022, 215, 114560.
- Tian, B.; Han, Y.; Wetterskog, E.; Donolato, M.; Hansen, M.F.; Svedlindh, P.; Strömberg, M. MicroRNA Detection through DNAzyme-Mediated Disintegration of Magnetic Nanoparticle Assemblies. ACS Sens. 2018, 3, 1884–1891.
- Tian, B.; Qiu, Z.; Ma, J.; Donolato, M.; Hansen, M.F.; Svedlindh, P.; Strömberg, M. On-Particle Rolling Circle Amplification-Based Core–Satellite Magnetic Superstructures for MicroRNA Detection. ACS Appl. Mater. Interfaces 2018, 10, 2957–2964.
- Celikbas, E.; Balaban, S.; Evran, S.; Coskunol, H.; Timur, S. A Bottom-Up Approach for Developing Aptasensors for Abused Drugs: Biosensors in Forensics. Biosensors 2019, 9, 118.
- Cui, F.; Zhou, Z.; Zhou, H.S. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J. Electrochem. Soc. 2019, 167, 037525.
- Islam, N.; Gorgannezhad, L.; Masud, M.K.; Tanaka, S.; Hossain, S.A.; Yamauchi, Y.; Nguyen, N.-T.; Shiddiky, M.J.A. Graphene-Oxide-Loaded Superparamagnetic Iron Oxide Nanoparticles for Ultrasensitive Electrocatalytic Detection of MicroRNA. ChemElectroChem 2018, 5, 2488–2495.
- Daneshpour, M.; Karimi, B.; Omidfar, K. Simultaneous Detection of Gastric Cancer-Involved MiR-106a and Let-7a through a Dual-Signal-Marked Electrochemical Nanobiosensor. Biosens. Bioelectron. 2018, 109, 197–205.
- Shen, Z.; He, L.; Wang, W.; Tan, L.; Gan, N. Highly Sensitive and Simultaneous Detection of MicroRNAs in Serum Using Stir-Bar Assisted Magnetic DNA Nanospheres-Encoded Probes. Biosens. Bioelectron. 2020, 148, 111831.
- Tavallaie, R.; McCarroll, J.; Le Grand, M.; Ariotti, N.; Schuhmann, W.; Bakker, E.; Tilley, R.D.; Hibbert, D.B.; Kavallaris, M.; Gooding, J.J. Nucleic Acid Hybridization on an Electrically Reconfigurable Network of Gold-Coated Magnetic Nanoparticles Enables MicroRNA Detection in Blood. Nat. Nanotechnol. 2018, 13, 1066–1071.
- Yu, L.; He, P.; Xu, Y.; Kou, X.; Yu, Z.; Xie, X.; Miao, P. Manipulations of DNA Four-Way Junction Architecture and DNA Modified Nanomaterials for the Detection of MiRNA. Sens. Actuators B Chem. 2020, 313, 128015.
- Liu, S.; Yang, Z.; Chang, Y.; Chai, Y.; Yuan, R. An Enzyme-Free Electrochemical Biosensor Combining Target Recycling with Fe3O4/ Nanocatalysts for MicroRNA-21 Detection. Biosens. Bioelectron. 2018, 119, 170–175.
- Lin, X.; Jiang, J.; Wang, J.; Xia, J.; Wang, R.; Diao, G. Competitive Host-Guest Recognition Initiated by DNAzyme-Cleavage Cycling for Novel Ratiometric Electrochemical Assay of MiRNA-21. Sens. Actuators B Chem. 2021, 333, 129556.
- Amoshahi, H.; Shafiee, M.R.M.; Kermani, S.; Mirmohammadi, M. A Biosensor for Detection of MiR-106 a by Using Duplex-Specific Nuclease, Assisted Target, Magnetic Nanoparticles, Gold Nanoparticles and Enzymatic Signal Amplification. ChemistrySelect 2022, 7, e202103115.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
09 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




