Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joseph Maria Kumar Irudayaraj | -- | 1627 | 2023-01-08 02:43:44 | | | |
| 2 | Camila Xu | Meta information modification | 1627 | 2023-01-09 02:11:49 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, X.; Flaws, J.A.; Spinella, M.J.; Irudayaraj, J. Dioxin in Kidney Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/39870 (accessed on 13 January 2026).
Zhang X, Flaws JA, Spinella MJ, Irudayaraj J. Dioxin in Kidney Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/39870. Accessed January 13, 2026.
Zhang, Xing, Jodi A. Flaws, Michael J. Spinella, Joseph Irudayaraj. "Dioxin in Kidney Disease" Encyclopedia, https://encyclopedia.pub/entry/39870 (accessed January 13, 2026).
Zhang, X., Flaws, J.A., Spinella, M.J., & Irudayaraj, J. (2023, January 08). Dioxin in Kidney Disease. In Encyclopedia. https://encyclopedia.pub/entry/39870
Zhang, Xing, et al. "Dioxin in Kidney Disease." Encyclopedia. Web. 08 January, 2023.
Copy Citation
Endocrine disrupting chemicals (EDCs) are a class of hormone-like chemicals that exist in the environment and interfere with the production, transport, metabolism, regulation, degradation, and/or action of hormones. The kidney is one of the most important organs in the urinary system and an accumulation point. Dioxins were identified as toxic compounds in the 1960s. Dioxins are a group of structurally related chemicals composed of two coplanar benzene rings.
endocrine disrupting chemicals
kidney diseases
renal cell carcinoma
1. Introduction
Endocrine disrupting chemicals (EDCs) are a class of hormone-like chemicals that exist in the environment and interfere with the production, transport, metabolism, regulation, degradation, and/or action of hormones. The ability of EDCs to interfere with endogenous hormones can lead to adverse effects on development, reproduction, immune, endocrine, and nervous systems of the organism. The cascade of events can also result in endocrine and metabolic imbalances in offspring as well [1][2]. EDCs are present in wastewater, textiles, cosmetics, waste residues produced by industrial and agricultural processes and in several domestic and household goods [3][4][5], and ultimately end up in landfills. Common contaminants include per- and polyfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), dioxins, bisphenol A (BPA), and phthalates, as well as heavy metals such as Cd, Pb, Hg [6]. Upon entry into the human system, EDCs often indirectly interact to cause endocrine imbalance in the organism by affecting the formation, secretion, transport, and metabolism of hormones in the organism, and thus, affecting growth, development, and reproduction.
Due to the environmental persistence of EDCs and their mobility in water bodies, these chemicals can migrate for a long distance along with water bodies [7][8][9]. At present, EDCs such as PFAS, BPA, PCBs and PAHs have been found in main water bodies worldwide [10][11]. Due to the limitations in existing wastewater treatment technologies, EDCs from livestock and poultry as well as domestic and industrial wastewater enter natural water bodies with wastewater discharge and continue to migrate and transform. EDCs in the environment can also be enriched through the food chain and accumulate in organisms at all trophic levels. The higher the trophic level, the higher the accumulation, eventually leading to toxicity [12][13][14]. There are several standard methods for extracting and estimating the concentration of EDCs in biological and environmental samples, including solid-phase extraction (SPE) [15], liquid chromatography-mass spectrometry (LC-MS) [16], gas chromatography-mass spectrometry (GC-MS) [17][18] and high-performance liquid chromatography (HPLC) [15][19]. It is important to use appropriate sample extraction and estimation methods for accurate and reliable detection of the levels of EDCs in different types of samples. With extensive advances in the chemical industry and the ubiquitous presence of plastics, EDCs entering the environment are on the rise both in terms of quantity and variety (new materials), and consequently, their hazards to humans and animals have attracted increased attention by societal and regulatory agencies (Figure 1).
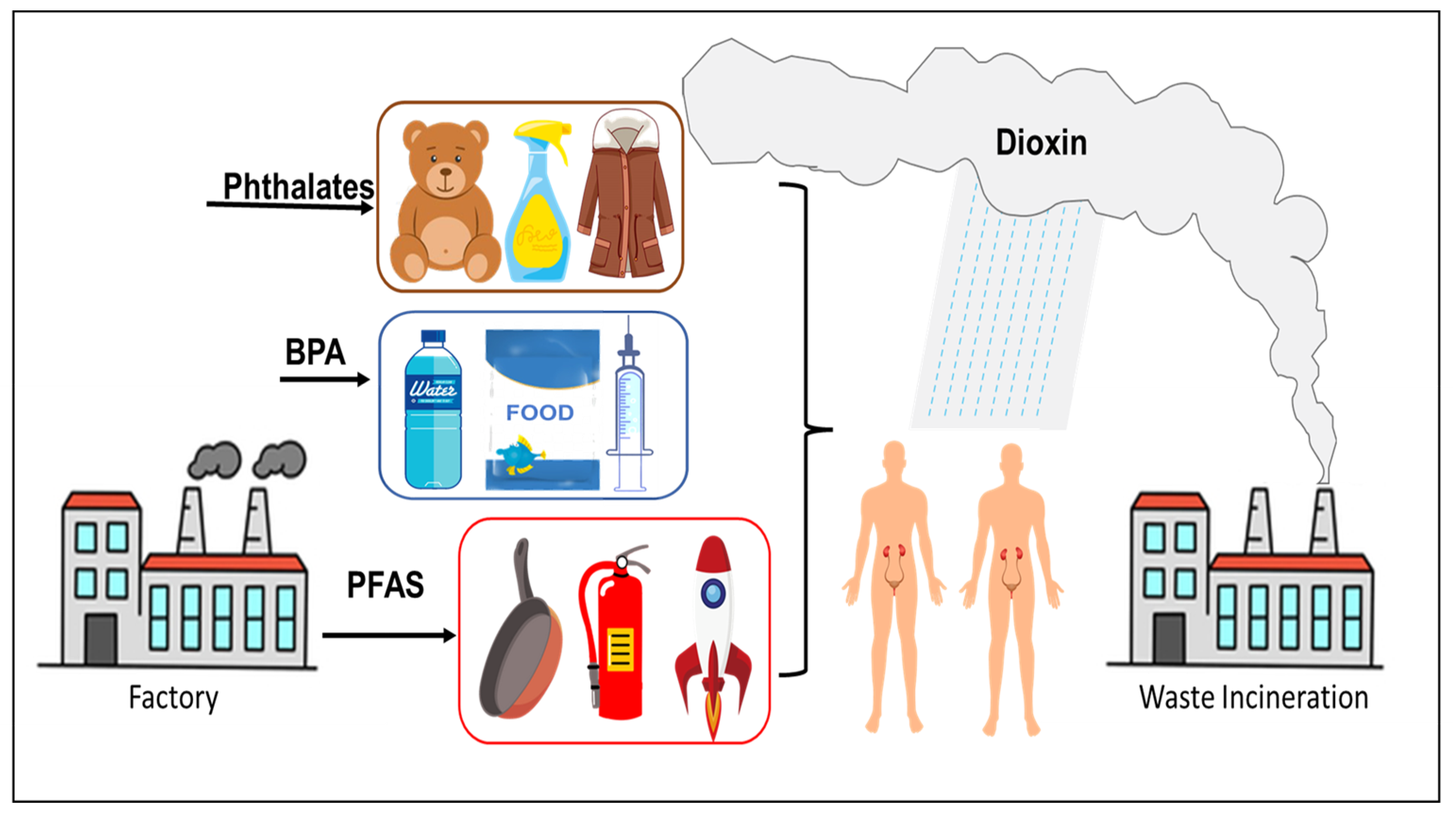
Figure 1. Common sources of EDCs.
The kidney is one of the main target organs of EDCs for accumulation. Patients with kidney disease usually show decreased renal function and proteinuria, which affects recovery from the disease [20]. Common renal diseases include chronic nephritis, renal calculi, renal failure, and renal cysts. These diseases are usually associated with the glomerular filtration rate (GFR) of the kidney, pathological damage, and abnormal blood or urine composition [21][22].
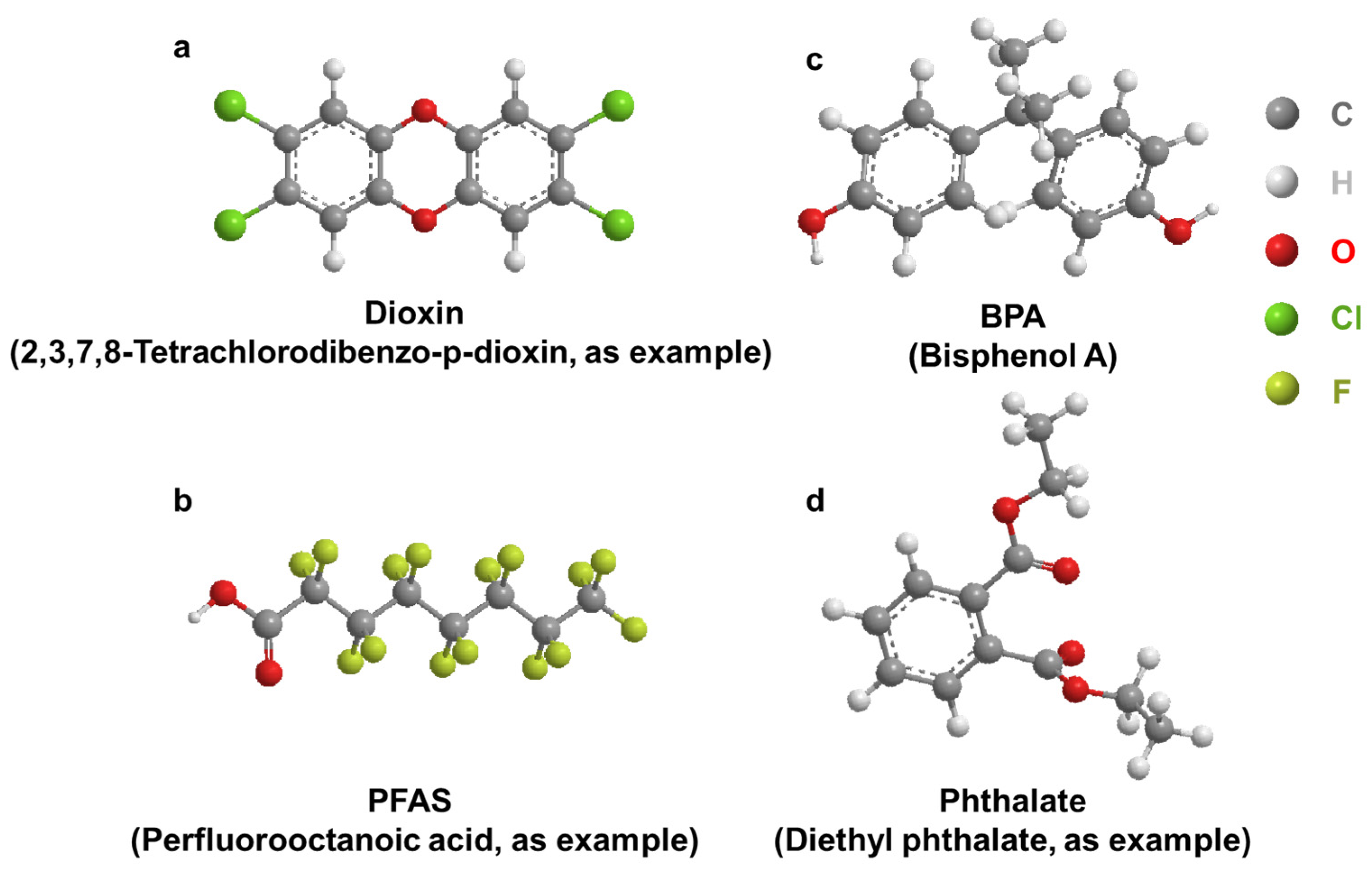
2. Dioxin
Dioxins were identified as toxic compounds in the 1960s. Dioxins are a group of structurally related chemicals composed of two coplanar benzene rings (Figure 2a). These compounds induce a similar spectrum of toxic phenotypes, with a wide range of potency. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is one of the most toxic compounds in this group of chemicals [23]. Incineration is the largest source of dioxin emissions in the environment [24][25]. Dioxins enter the ambient air through chimneys, spread continuously, and accumulate in the surrounding area of the incineration plant. Routes of exposure to dioxins include inhalation, dust ingestion, and skin contact. Dioxins accumulate in the tissues of various animals [26][27][28][29][30][31] and cause chloracne, embryotoxicity [32][33] and nephrotoxicity [34].

Figure 2. General structure of select EDCs. (a): Dioxin; (b): BPA; (c): PFAS; (d): Phthalate.
2.1. Accumulation of Dioxins in the Kidney
Animal studies: Several studies, but not all, suggest that TCDD may accumulate in the kidney to impart toxicity. Examination of aquatic organisms by analyzing ten-year-old fish in heavily polluted lakes in China showed that a large amount of dioxin accumulated in the kidneys [35]. Numerous studies have indicated that the accumulation of TCDD can cause the ballooning degeneration or even necrosis of the renal tubules of zebrafish [36]. Polybrominated diphenyl ethers (PBDEs), which have similar structures to PCB, have also been associated with renal histopathological changes [37] and significantly reduced catalase activity [38]. Exposure of infant mice to PCB caused hyperuricemia in adults, leading to secondary nephrotoxicity such as renal hypertrophy and fibrosis [39]. After 12 days of exposure, the combined exposure to TCDD and PCB was more likely to induce nephrotoxicity through high expression of CYP1A1 (Cytochrome P450 Family 1 Subfamily A Member 1), compared with the control group (equivalent volume of olive oil). TCDD and PCB exposure also significantly increased serum creatinine and blood urea nitrogen levels, renal oxidative stress and histopathological changes compared to control in rats [40]. Further studies will shed light on TCDD and PCDD mediated carcinogenesis [41].
Human studies: Shalat et al. [42] reported that three young male utility workers developed kidney cancer after chronic exposure to PCB-containing transformers. Residents from an e-waste dismantling area showed increased accumulations of PCB, which could have contributed to abnormal changes in markers of kidney injury [43]. The screening of environmental chemicals in the soil of an e-waste recycling area and human cancer risk assessment calculations showed that dioxins have the highest potential cancer risk to residents, followed by PCBs [44]. Through a multiple linear regression model analysis of 150 pregnant women, it was found that exposure to environmental pollutants may have negative effects, while exposure to greenspace may have positive effects on fetal renal function during pregnancy [45]. Epidemiological evidence indicated that the development of diabetes and chronic kidney disease was also associated with long-term exposure and accumulation of dioxins [46][47][48]. Jain [48] analyzed data from US adults from 1999 to 2004 to investigate concentration changes of four dioxin homologs and four separate furan homologs at various stages of renal function decline and found that renal dysfunction was associated with high dioxin/furan concentrations.
2.2. Effect of Dioxin on AHR Regulation/Activity and RCC
The AHR is a ligand activated transcription factor that mediates the toxic effects of TCDD. The AHR is mostly expressed in the nucleus of advanced clear cell renal cell carcinoma (RCC) and tumor infiltrating lymphocytes, and its expression is related to the stage and histological grade of pathological tumors [49]. Numerous studies have shown a complex association between the AHR and cancer characteristics, including increased malignant cell invasion, migration, metastasis, and survival [50][51][52]. The primary structure of the AHR is considered to be critical to determining the sensitivity and specificity of animal responses to dioxins.
Animal studies: In a constructed adenine diet model of chronic kidney disease, female AHR knockout mice showed inflammatory and pro-fibrotic gene expression and acute tubular injury [53]. After exposure to TCDD, 9 renal function was significantly reduced in wild-type male mice, indicating that the AHR plays a major role in mouse kidney development [54]. Due to poor renal excretion in patients with chronic kidney disease, the accumulation of toxic substances was found to increase CYP1A1 expression. Because of the higher inducibility of polymorphic genotypes, the pathway may become more deleterious in individuals with homozygous mutant alleles [55].
TCDD-induced fetal hydronephrosis (TiFH) is a type of obstructive hydronephrosis characterized by the presence of dilated ureter or ureteral effusion. The relationship between TiFH and AHR was investigated in both rats and mice. In mice, Cox-2 (cyclooxygenase-2) plays a key role in TiFH [56][57]. The induction effect of TCDD in the mouse kidney does not require translocation of AHR to the nucleus. TCDD induction of Cox-2 in mouse kidney is primarily mediated by a non-genomic pathway that activates AHR. In rats, TiFH is also induced and may be an endogenous ligand for AHR and/or a protein interacting with AHR. In contrast to rats, mice lacking AHR did not develop hydronephrosis or hydronephrosis in the absence of TCDD [58]. To better explore the role of AHR in normal development and chemical response, AHR knockout (AHR-KO) models were created in rats and mice, respectively. However, in AHR-KO rats, hydronephrosis and hydroureter were observed and AHR was found to play significantly different roles in tissue development and virulence in rodent species [59].
Human studies: In a study of more than 300 chronic kidney disease (CKD) patients and healthy controls, overexpression of polymorphic variants of CYP1A1 were associated with free radical production related enzymes after exposure to environmental pollutants, and with induction of renal dysfunction. Because of the different effects of AHR in rats and mice, it was not possible to directly use animal models to verify the effect of TCDD in the human kidney. To better detect the effects of dioxins on human health and reduce the differences between species, the mouse AHR was replaced with human AHR cDNA by knock-in strategy. Human AHR can be expressed in mice to mediate the development of TCDD-induced hydronephrosis [60]. Transcriptional analysis of human AHR was performed and compared in the liver and kidney, but dioxin exposure in the kidney altered only 17 genes, including many AHR target genes [61].
Overall, the relationship between CYP1A1, TCDD, and the AHR is complex and involves the metabolism of TCDD by CYP1A1 and the activation of the AHR by TCDD. The activation of the AHR by TCDD may lead to the expression of various genes that could contribute to nephrotoxicity, including genes involved in inflammation and oxidative stress. CYP1A1 is involved in the metabolism of TCDD, and the activation of the AHR by TCDD may also lead to the expression of CYP1A1. More research is needed to fully understand the mechanisms by which TCDD causes adverse health effects.
References
- Cabana, H.; Jones, J.P.; Agathos, S.N. Elimination of Endocrine Disrupting Chemicals using White Rot Fungi and their Lignin Modifying Enzymes: A Review. Eng. Life Sci. 2007, 7, 429–456.
- Tri, T.M.; Anh, D.H.; Hoai, P.M.; Minh, N.H.; Nam, V.D.; Viet, P.H.; Minh, T.B. Emerging Endocrine Disrupting Chemicals and Pharmaceuticals in Vietnam: A Review of Environmental Occurrence and Fate in Aquatic and Indoor Environments. In Persistent Organic Chemicals in the Environment: Status and Trends in the Pacific Basin Countries II Temporal Trends; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2016; Volume 1244, pp. 223–253.
- Pinar, E.; Belma, K.-G. Environmental Effects of Endocrine-Disrupting Chemicals: A Special Focus on Phthalates and Bisphenol A. In Environmental Health Risk; Marcelo, L.L., Sonia, S., Eds.; IntechOpen: London, UK, 2016; pp. 155–190.
- Hsu, C.N.; Tain, Y.L. Adverse Impact of Environmental Chemicals on Developmental Origins of Kidney Disease and Hypertension. Front. Endocrinol. 2021, 12, 745716.
- Singh, R.D.; Koshta, K.; Tiwari, R.; Khan, H.; Sharma, V.; Srivastava, V. Developmental Exposure to Endocrine Disrupting Chemicals and Its Impact on Cardio-Metabolic-Renal Health. Front. Toxicol. 2021, 28, 663372.
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify plastic waste as hazardous. Nature 2013, 494, 169–171.
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838.
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene Spherules in Coastal Waters. Science 1972, 178, 749–750.
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257.
- Kwan, C.S.; Takada, H. Release of Additives and Monomers from Plastic Wastes. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Takada, H., Karapanagioti, H.K., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 51–70.
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
- Peng, X.; Zheng, K.; Liu, J.; Fan, Y.; Tang, C.; Xiong, S. Body size–dependent bioaccumulation, tissue distribution, and trophic and maternal transfer of phenolic endocrine-disrupting contaminants in a freshwater ecosystem. Environ. Toxicol. Chem. 2018, 37, 1811–1823.
- Ruhí, A.; Acuña, V.; Barceló, D.; Huerta, B.; Mor, J.-R.; Rodríguez-Mozaz, S.; Sabater, S. Bioaccumulation and trophic magnification of pharmaceuticals and endocrine disruptors in a Mediterranean river food web. Sci. Total Environ. 2016, 540, 250–259.
- Windsor, F.M.; Ormerod, S.J.; Tyler, C.R. Endocrine disruption in aquatic systems: Up-scaling research to address ecological consequences. Biol. Rev. 2018, 93, 626–641.
- Li, Y.; Taggart, M.A.; McKenzie, C.; Zhang, Z.; Lu, Y.; Pap, S.; Gibb, S.W. A SPE-HPLC-MS/MS method for the simultaneous determination of prioritised pharmaceuticals and EDCs with high environmental risk potential in freshwater. J. Environ. Sci. 2021, 100, 18–27.
- Sosa-Ferrera, Z.; Mahugo-Santana, C.; Santana-Rodríguez, J.J. Analytical Methodologies for the Determination of Endocrine Disrupting Compounds in Biological and Environmental Samples. BioMed. Res. Int. 2013, 2013, 674838.
- Liu, Y.; Guan, Y.; Mizuno, T.; Tsuno, H.; Zhu, W. A Pretreatment Method for GC–MS Determination of Endocrine Disrupting Chemicals in Mollusk Tissues. Chromatographia 2009, 69, 65–71.
- Azzouz, A.; Rascón, A.J.; Ballesteros, E. Determination of free and conjugated forms of endocrine-disrupting chemicals in human biological fluids by GC−MS. Bioanalysis 2016, 8, 1145–1158.
- Deng, Z.-H.; Li, N.; Jiang, H.-L.; Lin, J.-M.; Zhao, R.-S. Pretreatment techniques and analytical methods for phenolic endocrine disrupting chemicals in food and environmental samples. TrAC Trends Anal. Chem. 2019, 119, 115592.
- Kitazawa, T.; Sato, T.; Nishiyama, K.; Asai, R.; Arima, Y.; Uchijima, Y.; Kurihara, Y.; Kurihara, H. Identification and developmental analysis of endothelin receptor type-A expressing cells in the mouse kidney. Gene Expr. Patterns 2011, 11, 371–377.
- Lehrke, I.; Waldherr, R.; Ritz, E.; Wagner, J. Renal Endothelin-1 and Endothelin Receptor Type B Expression in Glomerular Diseases with Proteinuria. J. Am. Soc. Nephrol. 2001, 12, 2321.
- Provenzano, M.; Andreucci, M.; Garofalo, C.; Minutolo, R.; Serra, R.; De Nicola, L. Selective endothelin A receptor antagonism in patients with proteinuric chronic kidney disease. Expert Opin. Investig. Drugs 2021, 30, 253–262.
- Higginbotham, G.R.; Huang, A.; Firestone, D.; Verrett, J.; Ress, J.; Campbell, A.D. Chemical and Toxicological Evaluations of Isolated and Synthetic Chloro Derivatives of Dibenzo-p-dioxin. Nature 1968, 220, 702–703.
- Kulkarni, P.S.; Crespo, J.G.; Afonso, C.A.M. Dioxins sources and current remediation technologies—A review. Environ. Int. 2008, 34, 139–153.
- Olie, K.; Addink, R.; Schoonenboom, M. Metals as Catalysts during the Formation and Decomposition of Chlorinated Dioxins and Furans in Incineration Processes. J. Air Waste Manag. Assoc. 1998, 48, 101–105.
- Fernandes, A.; Mortimer, D.; Rose, M.; Gem, M. Dioxins (PCDD/Fs) and PCBs in offal: Occurrence and dietary exposure. Chemosphere 2010, 81, 536–540.
- Rose, M.; Fernandes, A.; Foxall, C.; Dowding, A. Transfer and uptake of polychlorinated dibenzo-p-dioxins and furans (PCDD/Fs) and polychlorinated biphenyls (PCBs) into meat and organs of indoor and outdoor reared pigs. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2012, 29, 431–448.
- Fernández-González, R.; Yebra-Pimentel, I.; Martínez-Carballo, E.; Simal-Gándara, J. A Critical Review about Human Exposure to Polychlorinated Dibenzo-p-Dioxins (PCDDs), Polychlorinated Dibenzofurans (PCDFs) and Polychlorinated Biphenyls (PCBs) through Foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 1590–1617.
- Chen, Y.P.; Liu, Q.; Ma, Q.Y.; Maltby, L.; Ellison, A.M.; Zhao, Y. Environmental toxicants impair liver and kidney function and sperm quality of captive pandas. Ecotoxicol. Environ. Saf. 2018, 162, 218–224.
- Amutova, F.; Delannoy, M.; Baubekova, A.; Konuspayeva, G.; Jurjanz, S. Transfer of persistent organic pollutants in food of animal origin—Meta-analysis of published data. Chemosphere 2021, 262, 128351.
- Driesen, C.; Zennegg, M.; Rothacher, M.; Dubois, S.; Wyss, U.; Nowack, B.; Lerch, S. Transgenerational mass balance and tissue distribution of PCBs and PCDD/Fs from grass silage and soil into cow-calf continuum. Chemosphere 2022, 307, 135745.
- Roy, M.A.; Sant, K.E.; Venezia, O.L.; Shipman, A.B.; McCormick, S.D.; Saktrakulkla, P.; Hornbuckle, K.C.; Timme-Laragy, A.R. The emerging contaminant 3,3′-dichlorobiphenyl (PCB-11) impedes Ahr activation and Cyp1a activity to modify embryotoxicity of Ahr ligands in the zebrafish embryo model (Danio rerio). Environ. Pollut. 2019, 254, 113027.
- Ji, C.; Yan, L.; Chen, Y.; Yue, S.; Dong, Q.; Chen, J.; Zhao, M. Evaluation of the developmental toxicity of 2,7-dibromocarbazole to zebrafish based on transcriptomics assay. J. Hazard. Mater. 2019, 368, 514–522.
- Erdemli, M.E.; Yigitcan, B.; Erdemli, Z.; Gul, M.; Bag, H.G.; Gul, S. Thymoquinone protection against 2,3,7,8-tetrachlorodibenzo-p-dioxin induced nephrotoxicity in rats. Biotech. Histochem. 2020, 95, 567–574.
- Wu, W.Z.; Zhang, Q.H.; Schramm, K.W.; Xu, Y.; Kettrup, A. Distribution, transformation, and long-term accumulation of polychlorinated dibenzo-p-dioxins and dibenzofurans in different tissues of fish and piscivorous birds. Ecotoxicol. Environ. Saf. 2000, 46, 252–257.
- Henry, T.R.; Spitsbergen, J.M.; Hornung, M.W.; Abnet, C.C.; Peterson, R.E. Early Life Stage Toxicity of 2,3,7,8-Tetrachlorodibenzo-p-dioxin in Zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 1997, 142, 56–68.
- Raldúa, D.; Padrós, F.; Solé, M.; Eljarrat, E.; Barceló, D.; Riva, M.C.; Barata, C. First evidence of polybrominated diphenyl ether (flame retardants) effects in feral barbel from the Ebro River basin (NE, Spain). Chemosphere 2008, 73, 56–64.
- Albina, M.L.; Alonso, V.; Linares, V.; Bellés, M.; Sirvent, J.J.; Domingo, J.L.; Sánchez, D.J. Effects of exposure to BDE-99 on oxidative status of liver and kidney in adult rats. Toxicology 2010, 271, 51–56.
- Ruan, F.; Liu, C.; Hu, W.; Ruan, J.; Ding, X.; Zhang, L.; Yang, C.; Zuo, Z.; He, C.; Huang, J. Early life PCB138 exposure induces kidney injury secondary to hyperuricemia in male mice. Environ. Pollut. 2022, 301, 118977.
- Lu, C.-F.; Wang, Y.-M.; Peng, S.-Q.; Zou, L.-B.; Tan, D.-H.; Liu, G.; Fu, Z.; Wang, Q.-X.; Zhao, J. Combined Effects of Repeated Administration of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and Polychlorinated Biphenyls on Kidneys of Male Rats. Arch. Environ. Contam. Toxicol. 2009, 57, 767–776.
- Randerath, K.; Putman, K.L.; Randerath, E.; Mason, G.; Kelley, M.; Safe, S. Organ-specific effects of long term feeding of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 1,2,3,7,8-pentachlorodibenzo-p-dioxin on I-compounds in hepatic and renal DNA of female Sprague-Dawley rats. Carcinogenesis 1988, 9, 2285–2289.
- Shalat, S.L.; True, L.D.; Fleming, L.E.; Pace, P.E. Kidney cancer in utility workers exposed to polychlorinated biphenyls (PCBs). Br. J. Ind. Med. 1989, 46, 823–824.
- Xu, P.; Lou, X.; Ding, G.; Shen, H.; Wu, L.; Chen, Z.; Han, J.; Wang, X. Effects of PCBs and PBDEs on thyroid hormone, lymphocyte proliferation, hematology and kidney injury markers in residents of an e-waste dismantling area in Zhejiang, China. Sci. Total Environ. 2015, 536, 215–222.
- Niu, S.; Tao, W.; Chen, R.; Hageman, K.J.; Zhu, C.; Zheng, R.; Dong, L. Using Polychlorinated Naphthalene Concentrations in the Soil from a Southeast China E-Waste Recycling Area in a Novel Screening-Level Multipathway Human Cancer Risk Assessment. Environ. Sci. Technol. 2021, 55, 6773–6782.
- Rahmani Sani, A.; Abroudi, M.; Heydari, H.; Adli, A.; Miri, M.; Mehrabadi, S.; Pajohanfar, N.S.; Raoufinia, R.; Bazghandi, M.S.; Ghalenovi, M.; et al. Maternal exposure to ambient particulate matter and green spaces and fetal renal function. Environ. Res. 2020, 184, 109285.
- De Tata, V. Association of Dioxin and Other Persistent Organic Pollutants (POPs) with Diabetes: Epidemiological Evidence and New Mechanisms of Beta Cell Dysfunction. Int. J. Mol. Sci. 2014, 15, 7787–7811.
- Huang, C.Y.; Wu, C.L.; Wu, J.S.; Chang, J.W.; Cheng, Y.Y.; Kuo, Y.C.; Yang, Y.C.; Lee, C.C.; Guo, H.R. Association between Blood Dioxin Level and Chronic Kidney Disease in an Endemic Area of Exposure. PLoS ONE 2016, 11, e0150248.
- Jain, R.B. Trends in concentrations of selected dioxins and furans across various stages of kidney function for US adults. Environ. Sci. Pollut. Res. 2021, 28, 43763–43776.
- Ishida, M.; Mikami, S.; Shinojima, T.; Kosaka, T.; Mizuno, R.; Kikuchi, E.; Miyajima, A.; Okada, Y.; Oya, M. Activation of aryl hydrocarbon receptor promotes invasion of clear cell renal cell carcinoma and is associated with poor prognosis and cigarette smoke. Int. J. Cancer 2015, 137, 299–310.
- Wang, Z.; Snyder, M.; Kenison, J.E.; Yang, K.; Lara, B.; Lydell, E.; Bennani, K.; Novikov, O.; Federico, A.; Monti, S.; et al. How the AHR Became Important in Cancer: The Role of Chronically Active AHR in Cancer Aggression. Int. J. Mol. Sci. 2021, 22, 387.
- Zhao, H.; Chen, L.; Yang, T.; Feng, Y.-L.; Vaziri, N.D.; Liu, B.-L.; Liu, Q.-Q.; Guo, Y.; Zhao, Y.-Y. Aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. J. Transl. Med. 2019, 17, 302.
- Fiorito, F.; Ciarcia, R.; Granato, G.E.; Marfe, G.; Iovane, V.; Florio, S.; De Martino, L.; Pagnini, U. 2,3,7,8-tetrachlorodibenzo-p-dioxin induced autophagy in a bovine kidney cell line. Toxicology 2011, 290, 258–270.
- Makhloufi, C.; Nicolas, F.; McKay, N.; Fernandez, S.; Hache, G.; Garrigue, P.; Brunet, P.; Guillet, B.; Burtey, S.; Poitevin, S. Female AhR Knockout Mice Develop a Minor Renal Insufficiency in an Adenine-Diet Model of Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 2483.
- Esteban, J.; Sánchez-Pérez, I.; Hamscher, G.; Miettinen, H.M.; Korkalainen, M.; Viluksela, M.; Pohjanvirta, R.; Håkansson, H. Role of aryl hydrocarbon receptor (AHR) in overall retinoid metabolism: Response comparisons to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure between wild-type and AHR knockout mice. Reprod. Toxicol. 2021, 101, 33–49.
- Siddarth, M.; Datta, S.K.; Ahmed, R.S.; Banerjee, B.D.; Kalra, O.P.; Tripathi, A.K. Association of CYP1A1 gene polymorphism with chronic kidney disease: A case control study. Environ. Toxicol. Pharmacol. 2013, 36, 164–170.
- Dong, B.; Nishimura, N.; Vogel, C.F.; Tohyama, C.; Matsumura, F. TCDD-induced cyclooxygenase-2 expression is mediated by the nongenomic pathway in mouse MMDD1 macula densa cells and kidneys. Biochem. Pharmacol. 2010, 79, 487–497.
- Nebert, D.W.; Dalton, T.P.; Okey, A.B.; Gonzalez, F.J. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J. Biol. Chem. 2004, 279, 23847–23850.
- Yoshioka, W.; Tohyama, C. Mechanisms of Developmental Toxicity of Dioxins and Related Compounds. Int. J. Mol. Sci. 2019, 20, 617.
- Harrill, J.A.; Hukkanen, R.R.; Lawson, M.; Martin, G.; Gilger, B.; Soldatow, V.; LeCluyse, E.L.; Budinsky, R.A.; Rowlands, J.C.; Thomas, R.S. Knockout of the aryl hydrocarbon receptor results in distinct hepatic and renal phenotypes in rats and mice. Toxicol. Appl. Pharmacol. 2013, 272, 503–518.
- Moriguchi, T.; Motohashi, H.; Hosoya, T.; Nakajima, O.; Takahashi, S.; Ohsako, S.; Aoki, Y.; Nishimura, N.; Tohyama, C.; Fujii-Kuriyama, Y.; et al. Distinct response to dioxin in an arylhydrocarbon receptor (AHR)-humanized mouse. Proc. Natl. Acad. Sci. USA 2003, 100, 5652–5657.
- Boutros, P.C.; Bielefeld, K.A.; Pohjanvirta, R.; Harper, P.A. Dioxin-Dependent and Dioxin-Independent Gene Batteries: Comparison of Liver and Kidney in AHR-Null Mice. Toxicol. Sci. 2009, 112, 245–256.
More
Information
Subjects:
Developmental Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
09 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




