
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vytenis Babrauskas | -- | 5544 | 2023-01-08 01:46:26 | | | |
| 2 | Vytenis Babrauskas | Meta information modification | 5544 | 2023-08-09 18:08:03 | | | | |
| 3 | Vytenis Babrauskas | + 18 word(s) | 5562 | 2023-08-10 05:45:54 | | | | |
| 4 | Camila Xu | -872 word(s) | 4690 | 2023-08-10 07:50:05 | | | | |
| 5 | Camila Xu | -2 word(s) | 4688 | 2023-08-10 07:51:46 | | | | |
| 6 | Camila Xu | -3 word(s) | 4685 | 2023-08-10 08:00:33 | | |
Video Upload Options
In a number of countries, somewhere around 20% of reported building fires are due to electrical faults or failures. There can be a number of mechanisms responsible, but arcing in air and hot-surface ignitions of combustible materials are important causes. Details of these two mechanisms are reviewed. It is shown that even though arcing in air produces temperatures greatly higher than the ignition temperature of any ignitable solid, this does not always result in ignition. With regards to ignitions from hot surfaces or objects, it is shown that the area of the hot object presented to the ignitable material is a crucial variable.
1. Introduction
Since electric current involves the flow of energy, under some circumstances this flow of energy can result in an unwanted or accidental fire being ignited [1]. The US national fire statistics [2] have some serious problems [3], but it is estimated that around 20% of structure (building) fires are due to electrical causes [4]. In many other countries this fraction is also broadly similar [4]. This makes electrical causes one of the more significant fire causes, typically exceeded only by arson and cooking fires. Thus, understanding the nature of electrical fires is important for advancing the cause of fire safety. To understand in more detail how electric current can lead to the occurrence of fire, it is necessary to examine some of the physical mechanisms by which this happens. This entry will focus on two important causes: (1) arcing through air; and (2) hot-surface ignition of combustible materials. The latter can be gas, liquid, solid, or dust cloud, but the focus here will be limited to gases and solids.
2. Ignition Temperature
In studying the ignitions of materials, one needs to be guided by some knowledge of a criterion for ignition. In the most ambitious sense, it can be possible to run a complex numerical calculation of the physics and chemistry of the substances in the locale where the ignition will take place. Ignition can then be found to occur when a sizable jump in temperature occurs. Such an approach has seen some academic usage [5], but generally it is not cost-effective for practical safety problems. Instead, the concept of ignition temperature is introduced. This is often taken to be the temperature to which the sample has risen, when ignition takes place. For testing and standardization purposes, it is usually assumed that the whole sample has been raised to a uniformly elevated temperature. For solid materials, testing is often done according to the ASTM D1929 test [6]. This test, or equivalent ones, can be run under two different test conditions—with, or without a pilot. A pilot flame is a very small flame which does not raise the temperature of the specimen, but allows its evolved vapors to be more readily ignited. The reported variable is the piloted ignition temperature if a pilot flame is used, while if no pilot is used, the autoignition temperature (AIT) is reported. Gas mixtures which are potentially flammable can also be subjected to standardized testing. ASTM E659 [7] is commonly used for determining the AIT of gases. For gases, however, the determining of ignition potential with a small pilot is referred to as flash point testing, and a variety of tests exists for this purpose [5]. The exact analogue for gases of the piloted ignition temperature would be the fire point. This differs from the flash point in that the flaming needs to be sustained, as opposed to brief. But such testing is rarely used. The fire point is usually only a few degrees higher than the flash point, in any case.
3. Ignition of Gases, Vapors, and Dust Clouds
As noted above, standard test methods exist for measuring the AIT of gases, vapors, or dust clouds. Dust clouds behave more similarly to gaseous atmospheres than to solid matter. But since they are of interest to only a few sectors of industry, they will not be considered further here. Similarly, numerous flash point (FP) tests exist for measuring the minimum temperature at which ignition is possible, if there is a presence of a small pilot flame. These two measured temperatures are not related, however, as can be seen in Figure 1. One might ask why is this so? The answer is because the two variables characterize different properties. The AIT is a measure of the chemical reactivity of the fuel (the higher the AIT, the lower is the reactivity of the substance). Meanwhile, the FP assesses primarily the volatility of the fuel (more volatile fuels have a lower FP). And, on the whole, reactivity and volatility are unrelated chemical traits. The highest hazard generally corresponds to substances which show both a low FP and a low AIT.
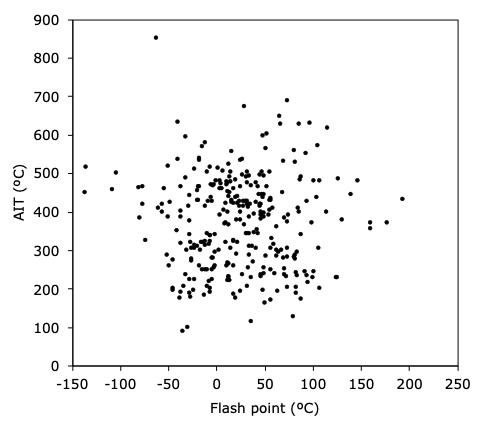
Figure 1. The lack of relation between the FP and the AIT for a number of pure gases and vapors (from Ref. [5]).
For gases, vapors, or dust clouds, in addition, there is a third chemical hazard variable of importance: the MIE, minimum ignition energy. If the energy available is too low, it should be expected that the ignition process will not be possible. The MIE refers to measurements made under standardized conditions, so it should not be taken as being directly relatable to an end-use environment. The units of MIE are energy, and since the values are small, quantities are generally reported as millijoules, mJ. The relationship is that the lower the MIE, the greater the hazard, since a lower external energy source will suffice to ignite the substance. Examples of MIE for some common hydrocarbons are shown in Figure 2.
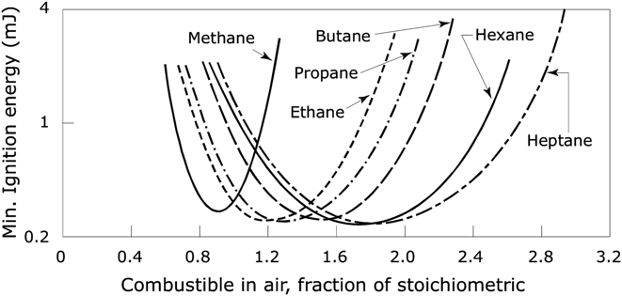
Figure 2. The effect of fuel/air mixture on the MIE for alkane-series hydrocarbons (from Refs. [4][8]).
4. Ignition from Electric Arcs
When studying conditions for arcing, it is important to understand that two different limits may be involved. Some research is undertaken to obtain the minimum values of voltage or current needed to establish an arc. In such experiments, ignition testing is not undertaken. But other research has focused on incendive arcs, i.e., conditions under which an arc can actually ignite something. These two types of findings are not necessarily related. But of course, one necessarily needs to create conditions where an arc is possible, before determining if it is incendive, or not.
4.1. Ignition of Gases or Vapors from Electric Arcs
Researchers studying electric arcs have long ago concluded that there exists a minimum voltage and a minimum current needed for an electric arc to be possible. These values depend on the material of the electrodes, and for copper electrodes the reported values [4] are 11.0 V and 0.45 A. At a lower voltage or current, a discharge can still be created, but it is generally classed as a spark [9], rather than an arc, due to the non-sustained nature.
But, in 1923, Nottingham [10] presented experimental results showing that steady arcs between copper electrodes can run at 6 V and hypothesized that the actual minimum voltage is 5 V. The disparity between these low values and present accepted values of 11.0 V and 0.45 A is presumably due to experimental differences, but this has not been satisfactorily elucidated. Thus, the question of what is the minimum voltage or current needed for an electric arc to be feasible is still unresolved.
Now turning to conditions under which an arc is not only present, but is incendive, already in in 1914, Wheeler [11] was able to ignite atmospheres of methane in air using a primitive break-flash apparatus running at 4.5 V. And in his 1915 report [12], he showed ignitions using only 0.30 A. But Zborovszky [13] conducted experiments showing that, for values below the arc minima, while spark discharges are possible, the energy delivery is so small that ignition will not occur. In addition, she further noted that, even above the minima values, for Imin < I < 2Imin and Vmin < V < 2Vmin, discharges tended to allow only low rates of power delivery. However, she noted that, under some circumstances, ignition could occur due to hot electrode surfaces, rather than the gas discharge. Her test rig, however, was not similar to most end-use conditions. And she noted that the limitations for operating below 2Vmin and 2Imin are due to specific heat losses in this test rig, and should not be taken as universal.
Some additional research points to the poorly-understood role of the experimental arrangements in determining minimum values. If one multiplies the Vmin times the Imin, this would indicate that, in a resistive circuit, 11.0 × 0.45 = 4.95 W would be the minimum power needed for an arc discharge. But Pugh [14] conducted some experiments where she directly measured the minimum power needed to sustain an arc. Using a 2000 – 5000 VAC power supply, she found that, typically, 1 W sufficed, but in some cases, depending on the circuit resistance, as little as 0.2 W was sufficient. Even the 1 W value is substantially below the 4.95 W.
The assessment has to be that neither the minimum voltage/current values for an arc to be possible, nor the minimum conditions needed for an arc to be incendive have been fully resolved. The values presented above, however, can give some rough guidance.
4.2. Ignition of Solids from Electric Arcs
An electrical arc exhibits extremely high temperatures, starting at 6500 K for low-current arcs, and rising for higher currents [4]. A value of 6500 K (6227ºC) is hugely above the ignition temperature of any ignitable material. Faults in 480 VAC buses or switchboards are likely to result in massive ignitions of nearby objects [4]. Yet, an electrical arc will not necessarily ignite nearby combustibles in every case. Research on such non-ignitions would be valuable, but only a limited amount of such information exists [4]. In the most common case, a non-ignition due to nearby arcing is likely to be due to rapid ablation of the material. For ignition of a solid to be possible, material must get pyrolyzed into the vapor phase, then present a local cloud of air/flammable vapor mixture which stays in place long enough to be heated to its ignition temperature. It is believed that non-ignitions from powerful arcs involve a situation where vaporized material is ejected out of the vicinity faster than it can be ignited. For 277 VAC, or lower voltages, a second effect needs to be considered. Such arcs may be self-extinguishing, rather than sustained [15]. Thus, limited exposure time may decrease the likelihood of ignition.
5. Hot Surface Ignition
5.1. Hot Surface Ignition of Gases or Vapors
The ignition-temperature tests discussed above all involve test conditions where the specimen is raised to a uniformly elevated temperature. This is necessary if a standardized test is to be established. But in some cases—and very commonly in the case of electrical fires—the material is only exposed to high temperatures over a small area, not in its entirety. This requires some understanding to be established of the hot-surface ignition concept. Babrauskas published extensive reviews of hot-surface ignition of gases, vapors, or liquids [16], and also of solids [17], with some additional research being presented in the Ignition Handbook [5].
Starting with gases or vapors, extensive research has shown that the hot-surface ignition temperature (HSIT) progressively decreases as the area of the hot surface increases (Figure 3). The figure also shows the relationship is often linear, if plotted on a semi-log graph. It is useful to note the actual HSIT values here. The AITv of JP-6 is 232 ºC, while its flash point is 36 – 43ºC. This can be contrasted to values above 500ºC for the HSIT (Figure 3), unless very large surface areas are presented.
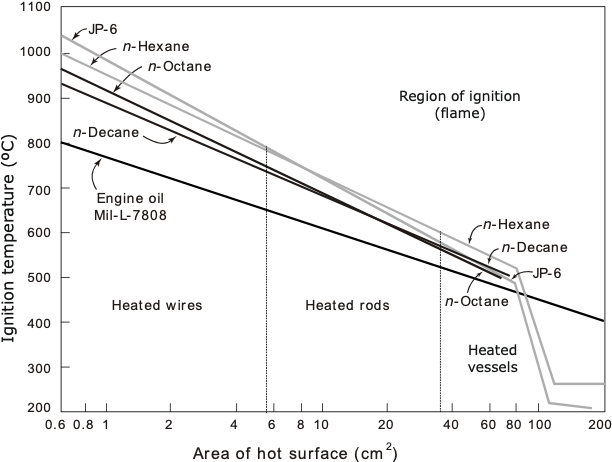
Figure 3. Effect of hot surface area on the HSIT of some gases and vapors (from Ref. [5])
5.2. Hot Surface Ignition of Solids
Solids are more likely to be ignited from electrical failures by hot-surface heating, than by heating from arcs. Ignition of solids by hot surfaces, of course, is a general mechanism and electricity does not necessarily need to get involved. But it is difficult to draw broad conclusions, since hot-surface heating involves more than a single variable. The temperature of the hot surface is the primary variable, but size and shape of the surface are important, as are the thermophysicochemical properties of the hot body.
A theory exists for treating the situation where a spherical hot body is immersed (not just touching) into a granular material [5]. This can cover the situation where ejecta from electrical arcing end up embedded into a granular material. Such incidents are not common, however. For a very few materials, e.g., steel balls in cellulose powder, sufficient data exist so that empirical guidance curves have been published [18]. These show that for a substance with an AIT = 250 ºC, a hot-spot temperature of around 1200 ºC would be needed for a 3 mm diameter body.
A small amount of research exists where square or rectangular hot plates were used to ignite plasticsv [17]. The main conclusion that can be drawn is that the hot surface needs to be at a temperature substantially greater than the AIT of the ignitable solid. Conceptually, one can expect that curves similar to those shown in Figure 3 for gases would govern. But detailed research is not available to actually create plots of this kind for solid materials. Most research on the ignitability of solids involve exposure of sizable areas, often [19] on the order of 100 × 100 mm. If the area of heat exposure is made progressively smaller, then increasing thermal attack (a higher heat flux) is needed to achieve ignitionv [17].
Perhaps the most important case of ignition of solid materials due to electrical failures involving hot surfaces is the ignition of wire insulation by excessive overcurrent flowing in wires. In Ref. [4] an equation is given for the overcurrent needed for ignition to occur, if thermophysical properties, along with the ignition temperature, are known. An example calculation indicates that a single AWG 14 (2.08 mm2) copper wire, insulated with PVC insulation, would ignite at 86 A. In view of a rated ampacity of 15 A, this represents an overcurrent of 5.7×. This situation was also explored in several experimental programs. Significant scatter exists, but ignition generally can be expected at 3× to 8× of the rated current. This is a broad enough range that focused experiments will generally be needed if reconstruction of an accident is necessary.
The Tokyo Fire Department [20] reported a case where a person’s pocket ignited when a Ni-Cd battery (1.2 V, 600 mAH) shorted out inside his pocket. Testing showed that, when short-circuited by an external metallic object, the battery was able to pass about 18 – 20 A through test clips and staples and heat them red-hot. This 1.2 V value is believed to be lowest reported voltage for the ignition of a solid substance.
6. Energy Criteria for Ignition of Solids
It was noted above that MIE, the minimum ignition energy, is a valid, often-used criterion for the ignition of gases or vapors. Is there an analogous criterion for solids? The question of whether an energy criterion for the ignition of solids is appropriate has been studied [5]. Since solids can be of indefinitely large size, it is clear that a pure energy (mJ) criterion cannot possibly be valid, since the same amount of energy distributed over progressively larger bodies would lead to progressively lower temperature rise. Meanwhile, as noted above, under many conditions it is found that a fixed temperature rise criterion does serve to characterize the ignition process. For some explosives [21], it has been found that energy fluence(defined as energy, divided by exposed face area, e.g., kJ/m2) can be a valid ignition criterion. However, this concept is not valid for ordinary solid combustibles, as illustrated in Figure 4. If the fluence concept were valid, the data would be situated along horizontal lines, not downward-sloping curves. Thus, there is generally no energy criterion which is applicable to quantify the ignition of solids.
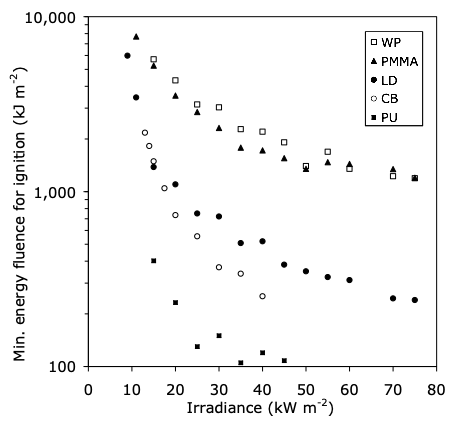
Figure 4. Minimum energy fluence for some thermally-thick solids [5] . (WP wood particleboard; PMMA polymethylmethacrylate; LD low-density fiberboard; CB cardboard; PU polyurethane foam)
In some cases, the minimum heat flux for ignition has been studied. This refers to an imposed heat flux in a test, where the surface area of the sample is uniformly exposed to a specified heat flux. This variable is reported in units of kW/m2. As an example, wood materials [4] do not ignite below 7.5 kW/m2. This variable is not often encountered, since there are no standards which require its reporting. A modest amount of compiled research data, however, is available [5].
7. Ignition of PVC and Wood
In many countries, poly(vinylchloride), PVC, is the most common material used for electrical insulation for wiring in buildings. Much of this wiring is run within walls. In North America and in the Nordic countries of Europe, walls of small buildings are most commonly built from wood materials. Thus, wood is a material whose ignition traits also need to be considered. Unfortunately, neither material is simple to characterize from an ignition point of view.
PVC
The Ignition Handbook [5] reports that the piloted ignition temperature of flexible PVC is in the range of 240 – 422 ºC, while the AIT is in the range of 263 – 441 ºC, when run under the ASTM D1929 test. It can readily be seen that both these temperature ranges are exceedingly broad. This is understood to be due to the nature of commercial formulations of PVC plastics for use as electrical insulation. Unlike some other plastics, which are often used in a nearly-pure state, with minimal additives, PVC does not make a useful wire/cable compound unless a sizable loading of various additives is incorporated [22]. Yet, there is no way of determining where a particular PVC insulation would fall in this range of ignition temperature values, without doing specific testing.
There is an additional complication. The ASTM D1929 test is run in a standardized tube furnace, where a small sample is immersed in a controlled hot-temperature environment. But this environment does not simulate electrical ignitions. There are two issues here: (1) the role of the flow of electric current can be very important for the ignition process, yet it is not used in the ASTM test; and (2) the actual ignition environment involves hot surfaces and not a hot-air tube furnace. There is another, even more important complication. Almost all practical grades of PVC plastic used for wire/cable insulation incorporate calcium carbonate as one of the additives. One of the failure mechanisms for electrical wiring is wet tracking. This is a complex phenomenon and readers are referred to Electrical Fires and Explosions [4] for details. Under most circumstances, this is a failure mechanism which, as the name implies, will occur only under wet conditions. But PVC is a material which, due to the presence of calcium carbonate, is uniquely susceptible to failure in a self-induced wet-tracking mode, even under dry atmospheric conditions [4][23]. In view of this, research has shown that PVC electrical insulation can fail at 120ºC for prolonged overheating conditions, or at 165ºC for short-term heating. These are failure temperatures for actual electrical wiring products. They are not ignition temperatures per se, but a prudent expectation is that, once electrical failure has taken place, ignition may occur shortly thereafter.
Wood
Chemically, wood is a complex substance, and it also shows complexities in its ignition process. Under ASTM D1929 test conditions, wood ignites at 250ºC [5][24], and this is irrespective of whether a pilot is or is not provided during the test. Since this is an unusual situation, some explanation is needed. Due to its specialized chemistry, wood can ignite and can burn under either flaming or smoldering (lack of flames) combustion conditions. But at the lowest temperature at which ignition can be achieved in the ASTM test, ignition is always in the smoldering mode. The presence of a pilot flame has no effect, then, since the burning is a surface-glowing condition, and not burning with flames.
There are two additional complications to this: (1) If wood is heated with a thermal exposure greater than the minimum needed to achieve ignition, the ignition temperature actually rises. (2) If wood is subjected to long-term (months-to-years), low-temperature heating conditions, then ignition may take place at temperatures substantially below 250 ºC [25].
Implications for fire investigation
The above discussion indicates that neither the ignition of PVC nor wood in an electrical fire can be calculated from some kind of equation or formula. Instead, the facts have to be assessed empirically. For this purpose, the guidance of NFPA 921 [26] must be relied upon, which is the only standard of care for conducting a fire investigation. If not done properly, the fire investigation process can be subverted by bias, thus, it is important that the guidance of NFPA 921 be followed. This is especially important with regards to the order of the steps to be completed in the fire investigation. There are three main steps: (1) determine the fire area of origin; (2) determine the fire cause; and (3) assign responsibility (which is not required in all cases). What is crucial is that the investigation be done in the order: 1, 2, 3. Neither the cause nor the responsibility must be pre-judged before the area of origin is determined. Identifying the fire cause consists of examining all the heat sources which were, or there is reason to believe, could have been in the area of origin. The fire cause then corresponds to the circumstances which led the source of heat to impinge upon a fuel in such a way that ignition occurs.
8. Preventive Measures
There are three primary safety measures that can be used to prevent electrical fires:
- Good wiring practice and adherence to relevant electrical codes and standards. This is a massive topic and differs in different countries, depending on their national provisions. It will not be covered here.
- Intrinsic safety.
- Power-limited circuits.
8.1. Intrinsic Safety
In 1913, there was a large life-loss explosion at the Universal Colliery, in Senghenyddin, Wales [27]. The subsequent investigation suggested that the most likely ignition source was sparking at a battery-powered signal bell system [28]. Since this was the third mining disaster in two years attributed to sparking in low-voltage signaling systems [29], the UK government responded with a research program by Wheeler et al.[11][12][30], who developed the first version of a ‘break-flash’ testing apparatus. The intention was that the circuits which are qualified via that test apparatus will not be able to ignite flammable gas atmospheres. This work became the origin of what today is known as intrinsic safety.
NFPA 70, the National Electrical Code (NEC) [31] defines intrinsic safety as: “A circuit in which any spark or thermal effect is incapable of causing ignition of a mixture of flammable or combustible material in air under prescribed test conditions.” It then refers to UL 913 [32] for test requirements. Zborovszky [33] provides a more detailed explanation: “A circuit is considered intrinsically safe when any spark or thermal effect produced normally (that is, by operating the equipment in its correct operational manner to fulfill its purpose) or accidentally (caused by short circuit, earth fault, defective components, breaking the wiring, etc.) is incapable under prescribed test conditions of causing ignition of a prescribed gas mixture. The test conditions and gas mixture should be at least as hazardous as the true parameters of the environment where the circuit will be operated.” This exposition makes it clear that this is not a concept derived from some fundamental theory; instead, it is to be determined experimentally in the context of some agreed-upon test apparatus and test conditions. Magison [34] provides a detailed explanation of intrinsic safety and the means of achieving it. Such circuits normally exhibit the following characteristics:
- Very low power consumption.
- No electrical components which could store (and later discharge) a significant amount of energy. In practice, this means that values of capacitance and inductance must be kept very low. If higher values are needed, sometimes it is possible to arrange diode shunts which do not interfere with normal function, but which limit the amount of energy that could be made available in case of fault.
- Protection circuits to limit the current that can flow into the device. Zener diodes placed across power supplies are a common way of meeting this requirement.
Devices with minimal capability of storing or generating energy can automatically qualify as intrinsically safe equipment. A device which cannot generate or store more than 1.2 V, 0.1 A, 20 mJ, nor 25 mW under normal or failure conditions qualifies without further testing [34]. Such a device is exemplified by a thermocouple, which generally produces less than 0.1 V. The limits are very tough to meet and devices other than transducers will rarely qualify. A device within these limits is sometimes referred to as a simple apparatus. The basis for the establishment of these minimum values is not known, and presumably represents committee action rather than laboratory research.
8.2. Power-Limited Circuits
A milder form of safety concept is involved in the NEC concept of Class 1, Class 2, or Class 3 power-limited circuits (Sec. 725). The requirements for Class 1 circuits are so broad that there clearly is no basis for assuming any fire-safety effectiveness. Class 3 devices are only rarely encountered. Class 2 power-limited circuits or power supplies are common, however. The basic requirements for Class 2 and Class 3 power supplies are given in Tables 11(A) and 11(B) of the NEC, while additional details are provided in UL standards, there being at least 8 different UL standards for this purposeiv. Perhaps the most general of these is UL 1310 [35], for Class 2 power supplies. The NEC typically limits Class 2 power supplies to an output of 100 volt-amps, or less. Current output is limited to 8 A for voltage ratings of 30 V or lower, while power supplies providing 60 – 150 V, have the output is limited to 5 mA; however, there is not widespread usage of 60 – 150 V Class 2 power supplies.
Small and Vicars [36] studied some details of these power supplies and pointed out that designers using these supplies are sometimes unaware of the 8 A output potential and assume that for a 24 V, 40 V-A power supply only the steady-state output of 1.67 A will be present, thus not providing for safe handling of currents up to 8 A. Durham et al. [37] noted that Class 2 power supplies are sometimes assumed to be incapable of igniting fires, but neither the NEC nor the UL make such claims. They then conducted a series of tests with commercial Class 2 power supplies, and found that one in six trials led to a flaming fire. The test in question involved a Class 2 power supply rated for a 12 VDC, 900 mA output. The ignition mode was by hooking up grossly under-rated resistors to the output of the device. The power delivered was measured at 21.6 W. The authors also demonstrated that they could ignite paper with an 11.6 W output (not from a Class 2 power supply).
9. Conclusions
No electrical ignition will occur under conditions of zero voltage, current, power, or energy. Conversely, ignitions can occur when substantial amounts of voltage, current, power, or energy are available. This leads one to consider that there may be some lower limits for ignition to be possible. This is consistent with the general observation that for ignition to occur of substances which are not self-heating or exothermically reacting, some finite amount of external energy must be provided. Due to the mass per volume of substance, it is much more difficult to ignite solids, compared to gases. But for either state of matter, the minimum conditions needed will depend on the geometry and operating details of the experimental rig.
Minimum ignition energy (MIE) values for flammable gas mixtures have been studied for decades. Under ideal (worst-case) conditions, values of around 0.2 to 2.0 mJ are found for many common fuel gases in air [5]. For solids, the corresponding energy criterion would be an energy fluence criterion. This is valid for certain explosives, but it has been shown not to be valid for common combustibles.
Despite over a century’s worth of research, guidance on the minimum voltage or the minimum current needed to create an arc is limited and conflicted. No published research has been uncovered to justify the requirements for a ‘simple apparatus,’ nor for Class 2 or Class 3 power supplies.
A case history demonstrates that, under some circumstances, a 1.2 V voltage source can ignite a solid material. This should be viewed in the context that it is much more difficult to ignite solids, rather than gases.
Many more fires occurs where electrical failures ignite solids materials, as opposed to gaseous atmospheres. But existing research is inverted with regards to this need—most research has been on gaseous atmospheres. Significantly more research is needed to be able to give useful guidance on minimum circuit conditions needed for electrical ignitions of solid materials.
References
- Campbell, R. C., Home Electrical Fires, National Fire Protection Assn., Quincy MA (2019).
- Fire in the United States, 2008-2017, 20th ed., U.S. Fire Administration, Emmitsburg MD (2019).
- Icove, D. J., and Hargrove, T. K., Project Arson: Uncovering the True Arson Rate in the United States, pp. 283-292 in Proc. Intl. Symp. on Fire Investigation (ISFI 2014), NAFI, Sarasota FL (2014).
- Babrauskas, V., Electrical Fires and Explosions, Fire Science Publishers, New York (2021).
- Babrauskas, V., Ignition Handbook, Fire Science Publishers/Society of Fire Protection Engineers, Issaquah WA (2003).
- Standard Test Method for Determining Ignition Temperature of Plastics (ASTM D1929), ASTM Intl., West Conshohocken PA.
- Standard Test Method for Autoignition Temperature of Liquid Chemicals (ASTM E659), ASTM International.
- Babrauskas, V., Electrical Fires and Explosions, Fire Science Publishers, New York (2021).Blanc, M. V., Guest, P. G., von Elbe, G., and Lewis, B., Ignition of Explosive Gas Mixtures by Electric Sparks. III. Minimum Ignition Energies and Quenching Distances of Mixtures of Hydrocarbons and Ether with Oxygen and Inert Gases, pp. 363-367 in 3rd Symp. on Combustion and Flame and Explosion Phenomena, Williams & Wilkins, Philadelphia (1949).
- Atalla, M. M., Arcing of Electrical Contacts in Telephone Switching Circuits. Part IV—Mechanisms of the Initiation of the Short Arc, Bell System Tech. J. 34, 203-220 (1955).
- Nottingham, W. B., A New Equation for the Static Characteristic of the Normal Electric Arc, J. AIEE 42, 12-19 (1923).
- Wheeler, R. V., Dr. Wheeler’s Report on Experiments with Signalling Apparatus, pp. 36-40 in Redmayne, et al., op cit.
- Wheeler, R. V., Report of Battery-Bell Signalling Systems as Regards the Danger of Ignition of Firedamp-Air Mixtures by the Break-Flash at the Signal-Wires, HMSO, London (1915).
- Zborovszky, Z., and Cotugno, L. A., Evaluation of the Cadmium Disc Breakflash in Testing Electrical Circuits—Safety in Explosive Atmospheres—A Comprehensive Study of Intrinsic Safety Criteria (BuMines OFR 68-76), Bureau of Mines, Pittsburgh (1974).
- Pugh, S. N., Forensic Evidence of Electric Arcs (M.S. thesis), Univ. California, Davis (2006).
- Stanback, H. I. jr., Predicting Damage from 277 V Single Phase to Ground Arcing Faults, IEEE Trans. Ind. Appl. IA-13, 308-314 (1977); and IA-14, 93-95 (1978).
- Babrauskas, V., Ignition of Gases, Vapors, and Liquids by Hot Surfaces, Fire Technology 58, 281–310 (2022).
- Babrauskas, V., Unexposed-Face Temperature Criteria in Fire Resistance Tests: A Reappraisal, Fire Safety J. 44, 813-818 (2009).
- Babrauskas, V., Smoldering Fires, Fire Science Publishers, New York (2021).
- Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products using an Oxygen Consumption Calorimeter (E1354), ASTM International, West Conshohocken PA.
- Tokyo Fire Department, Fire Report: Fires of Small Battery, J. Japan Assn. of Fire Science and Engineering 44:5, 57-60 (Oct. 1994).
- Verbeek, R., and Bouma, R. H. B., Evaluation of the Energy Fluence in the Small Gap Test, Propellants, Explosives, Pyrotechnics 36, 16-21 (2011).
- Wypych, G., PVC Formulary, 2nd ed., ChemTec Publishing, Toronto (2015).
- Babrauskas, V., Mechanisms and Modes for Ignition of Low-voltage, PVC-insulated Electrotechnical Products, Fire & Materials 30, 150-174 (2006).
- Babrauskas, V., Ignition of Wood: A Review of the State of the Art, J. Fire Protection Engineering 12, 163-189 (2002).
- Babrauskas, V., Gray, B. F., and Janssens, M. L., Prudent Practices for the Design and Installation of Heat-Producing Devices near Wood Materials, Fire & Materials 31, 125-135 (2007).
- Guide for Fire and Explosion Investigations (NFPA 921), National Fire Protection Assn., Quincy MA (2021).
- Redmayne, R. A. S., Williams, E., and Smillie, R., Causes and Circumstances Attending the Explosion Which Occurred at the Senghenydd Colliery on Tuesday, 14th October, 1913, Home Office, HMSO, London (1914).
- McMillan, A., Electrical Installations in Hazardous Areas, Butterworth-Heinemann, Oxford, UK (1998).
- Lloyd, H., and Guénault, E. M., The Use of Break-Flash Apparatus No. 3 for Intrinsic Safety Testing (Research Report 33), Safety in Mines Research Establishment, Sheffield, England (1951).
- Wheeler, R. V., and Thornton, W. M., Report on Electric Signalling with Bare Wires so far as Regards the Danger of Ignition of Inflammable Gaseous Mixtures by the Break-flash at the Signal Wires, HMSO, London (1916).
- National Electrical Code (NFPA 70), National Fire Protection Assn., Quincy MA.
- Standard for Safety—Intrinsically Safe Apparatus and Associated Apparatus for Use in Class I, II, and III, Division 1, Hazardous (Classified) Locations (ANSI/UL 913), UL.
- Zborovszky, Z., and Cotugno, L. A., A Comprehensive Study of Intrinsic Safety Criteria (Report DRI 2597; Bureau of Mines OFR 23-73), University of Denver Research Institute, Denver (1972).
- Magison, E. C., Electrical Instruments in Hazardous Locations, 4th ed., ISA, Research Triangle Park NC (1998).
- Class 2 Power Units (UL 1310), UL.
- Small, J. E., and Vicars, R. J., Class 2 Transformers and Plastic Enclosed Printed Circuit Boards: A Potentially Perilous Combination, IEEE Symp. on Product Compliance Engineering, IEEE (2010).
- Durham, M. O., Durham, R. A., Ozment, C. I., and Coffin, J., Unraveling the Myths of Low Energy Electrical Ignition, Paper X, Frontiers in Power Conf. 2009, Oklahoma State Univ., Stillwater (2009).




