
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Maria Vettraino | -- | 4745 | 2023-01-06 17:09:25 | | | |
| 2 | Chiara Antonelli | -2131 word(s) | 2707 | 2023-01-10 01:30:20 | | | | |
| 3 | Sirius Huang | -137 word(s) | 2570 | 2023-01-10 03:05:47 | | |
Video Upload Options
Plant trade coupled with climate change has led to the increased spread of well-known and new Phytophthora species, a group of fungus-like organisms placed in the Kingdom Chromista. Their presence in plant nurseries is of particular concern because they are responsible for many plant diseases, with high environmental, economic and social impacts. This text offers a brief overview of the current status of Phytophthora species in European plant nurseries. Focus was placed on Italian sites. Despite the increasing awareness of the risk of Phytophthora spread and the management strategies applied for controlling it, the complexity of the Phytophthora community in the horticulture industry is increasing over time. Since the survey carried out by Jung et al., new Phytophthora taxa and Phytophthora-host associations were identified. Phytophthora hydropathica, P. crassamura, P. pseudocryptogea and P. meadii were reported for the first time in European plant nurseries, while P. pistaciae, P. mediterranea and P. heterospora were isolated from Italian ornamental nurseries. Knowledge of Phytophthora diversity in plant nurseries and the potential damage caused by them will help to contribute to the development of early detection methods and sustainable management strategies to control Phytophthora spread in the future.
1. Introduction
Phytophthora species are Oomycetes, classified within the Stramenopile lineage; they consist of soilborne and airborne species and require water to complete their life cycles. They produce infectious propagules, including zoospores, chlamydospores and oospores, that can be spread short or long distances. These structures enable long-term survival (oospores) and short-term survival (chlamydospores) facilitating the adaptation of Phytophthora taxa to different environments [1][2][3][4]. Some Phytophthora species are aggressive pathogens that can cause damping-off, root and collar rot, wilting and blight on over 1000 plant species. While some taxa, such as P. infestans Mont. de Bary, have a limited range of hosts, species such as P. cinnamomi Rands, can infect more than 109 plant species [1].
2. Phytophthora Species Diversity in Plant Nurseries
Numerous Phytophthora species have been documented in commercial plant nurseries worldwide, causing significant economic losses [1][5][6][7][8]. For instance, a total of 28 and 15 Phytophthora taxa, have been found in Oregon and California ornamental nurseries, respectively [9][10]. Some of the Phytophthora taxa found in plant nurseries are of regulatory concern. Among those, P. ramorum Werres, de Cock and Man in ’t Veld has received the most notoriety, but also other regulated taxa can be present. The first European detection of P. lateralis Tucker and Milbrath (EPPO List) is dated 1999 in French nurseries [11]. Of over 36 Phytophthora species identified in Pennsylvania nurseries and greenhouses, three, P. parvispora Scanu and Denman; P. chrysanthemi Naher, Watanabe, Chikuo and Kageyama; and P. sojae Kaufm. and Gerd., were listed in the U.S.- regulated Plant Pest Risk [12][13].
2.1. The Case of Phytophthora Species in Italian Plant Nurseries
The survey conducted by Jung et al. [14] described a total of 36 different Phytophthora taxa in Italian plant nurseries, forest and landscape plantings. Those taxa were not exclusive to Italy, confirming nurseries as a potential basin of plant pathogens [14][15]. Phytophthora cinnamomi, P. cambivora Petri Buisman and P. cryptogea Pethybr. and Laff. occurred mainly in oak stands, while P. palmivora E.J. Butler was isolated from all nurseries stands of Olea europaea L. Over more than 100 Phytophthora-host associations, 33 new hosts were reported exclusively in Italy, including Agave attenuata Salm-Dyck., Coronilla valentina L. and Solanum melongena L. Phytophthora ramorum, P. fragrariae Hickman and P. lateralis were the only three Phytophthora species recorded in Italian plant nurseries and plantings also included on the EPPO quarantine list. Interestingly, P. ramorum was first isolated in Italy in 2002, on R. yakushimanum Ken Janeck in a Piedmont nursery [16]. Later, in 2013 the pathogen was detected by pyrosequencing analysis in chestnut stands and by culture-based methods on Viburnum tinus L. in Pistoia, where currently it is considered eradicated [17][18]. Nevertheless, its record in Italian plant nurseries confirmed that it was not halted despite strict quarantine regulations.
A literature survey was carried out using databases available for academic research, such as Scopus [19] and Web of Science [20], using “Phytophthora and Italy” as keywords. The dataset was then restricted to nurseries. Results highlighted the presence of 43 Phytophthora species associated with horticultural, forest and ornamental plant species. The richness of Phytophthora taxa in Italy could be associated with the geographic characteristics of the country and its extensive trading traditions. Italy is a long peninsula with mostly mountainous hinterland and is surrounded on every side except the north by the Mediterranean Sea. Thus, its climate is highly diverse. The lack of information of the exact geographic locations of the nurseries reported in the dataset analyzed has prevented us from highlighting a possible link between Phytophthora diversity and climate conditions. Nevertheless, the diversity of the Phytophthora community decreased from Southern to Northern Italy (Figure 1). Sardinia, Tuscany and Piedmont were the regions with the highest Phytophthora spp. richness in the three Italian zones (Southern, Central and Northern Italy, respectively) (Figure 1). Phytophthora ramorum, P. cinnamomi, P. nicotianae Breda de Haan and P. niederhauserii Abad were present throughout the peninsula. However, the structures of Phytophthora communities varied in the three zones also in accordance with the distribution of hosts and efforts to characterize occurrence, with most of the survey conducted after 2000. The rising outbreaks of Phytophthora species in forests and natural ecosystems in Europe in the late 1990s probably stimulated the scientific community to investigate with systematic surveys the presence of pathogens in nurseries.
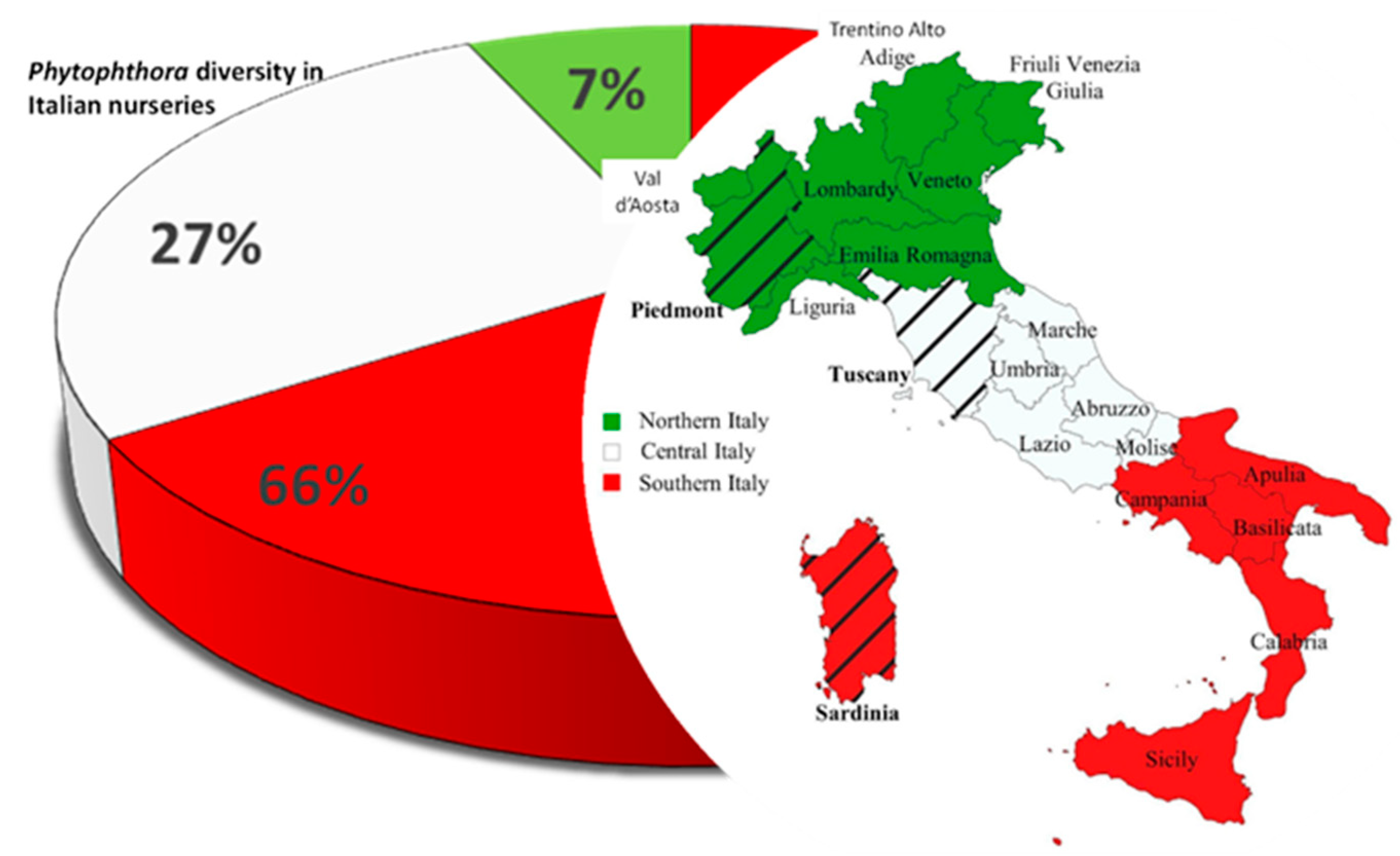
Figure 1. Distribution map of Phytophthora spp. in Italian plant nurseries. Different colors represent the three Italian zones: red = Southern Italy; white = Central Italy; green = Northern Italy. The regions within each zone with the highest Phytophthora diversity are indicated by stripes.
The previous survey by Jung et al. [14] focused on data collected from 1992 to 2013. Since then, three new Phytophthora species, P. pistaciae Mirabolfathy; P. mediterranea Bregant, Mulas and Linaldeddu; and P. heterospora Scanu, Cacciola, Linald. and T. Jung, were described in Italy. These were the first reports in Europe (Figure 2).
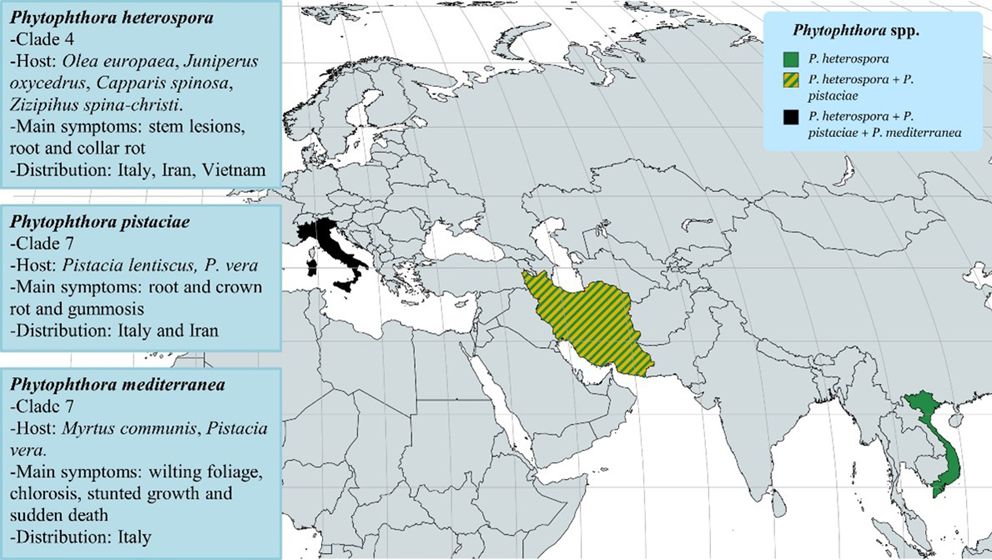
Figure 2. Worldwide geographical distribution of P. pistaciae, P. mediterranea and P. heterospora.
Phytophthora mediterranea was isolated from declining potted myrtle seedlings (Myrtus communis L.) in Italy [21]. It was previously observed on pistachio (Pistacia vera L.) in California [22]. Although phylogenetically, P. mediterranea is closely related to P. cinnamomi, the two species can be easily distinguished on the basis of some morphological differences, such as size of the sporangia, colony growth pattern and cardinal temperature values. Several Mediterranean maquis species are highly susceptible to this newly recognized pathogen [21]. Phytophthora pistaciae causes leaf reddening, wilted shoots, root and collar rot on nursery plants of P. lentiscus L. in Italy [23]. It is considered the most aggressive pathogen of P. vera in Iran [24][25]. Phytophthora heterospora has been isolated from stem lesions and root and collar rot of Olea europaea (2010, Italy), Ziziphus spina-christi L. Desf (2011, Fars Province, Iran), Juniperus oxycedrus L., Capparis spinosa L. (2013–2014, Italy) and Durio zibethinus L. (2013, Mekong River delta, Vietnam) [26]. Phytophthora heterospora and P. palmivora have many similar morphological characteristics in terms of colony morphology, sporangia, chlamydospores, and gametangia shape and size. However, Phytophthora heterospora produces pseudoconidia, a unique asexual dissemination structure of Phytophthora species. This feature was previously described on isolates obtained from Theobroma cacao L. in the Ivory Coast and named P. palmivora var. heterocystica Babacauh [27]. Unfortunately, it is unclear whether P. heterospora and P. palmivora var. heterocystica represent the same taxon. Pathways of P. heterospora and P. pistaceae introduction are unknown. It is worth noting that Italy has a long history of trade in goods with Iran, where both P. heterospora and P. pistaceae are present. It is the second-largest importer of shelled pistachios in Europe (after Germany), with a value of USD 193 million in 2020 [28]. Since 2013, the list of Phytophthora-host combinations was reviewed with novel associations including P. psychrophila Jung and Hansen/Ilex aquifolium, P. pseudosyringae/I. aquifolium, P. pseudocryptogea Safaiefarahani, Mostowfizadeh, Hardy and Burgess/Laurus nobilis L., P. megasperma Dreschsler/L. nobilis, P. citrophthora R.E. Smith and E.H. Smith Leonian /L. nobilis, P. bilorbang Aghighi and Burgess/Phyllirea latifolia L. and P. palmivora/P. latifolia [21]. Phytophthora x pelgrandis Gerlach, Nirenberg and Gräfenhan, previously observed in potted plants in the Netherlands, Hungary and Germany, and Phytophthora hydropathica Hong and Gallegly, previously never detected in European nurseries, were also reported on Lavandula spp., Buxus sempervirens L., C. lawsoniana and Viburnum tinus L. in Italy [29][30][31]. During 2012–2014, P. pseudosyringae was first detected using ITS DNA metabarcoding [32][33] and only in 2021, was isolated from potted plants of Ilex aquifolium L. in Sardinia (Italy) [21]. In all probability, this is only the beginning of the story; the final picture of the occurrence of Phytophthora species in plant nurseries will probably never be complete, as additional species are being discovered every year. Researchers believe that the diversity of Phytophthora species presently is well underestimated. Although a total number of 200 Phytophthora taxa have been described [26][34], another 200–400 species may remain to be discovered in environments not yet surveyed [35] or not yet formally identified [32][33].
3. How to Tackle the Spread of Phytophthora Species
Before the COVID-19 pandemic, the value of horticulture production in Italy exceeded EUR 2.7 billion. Regarding plant nurseries, the figures also included cut flowers and flowering plants. During the periods of lockdown, all seasonal products were irremediably lost due to the impossibility of watering, for a short period, and the demand for ceremonies and anniversaries. Fortunately, matters are gradually improving for this sector in both the domestic and foreign markets. The return to gardening practices has led to an appreciable increase in sales. Export, however, is the driving force for the sector, with a value of about USD 28,765,318.00 [36], with demand coming mainly from Northern European countries (Holland, Germany and France). Italian imports of plants and live plant materials (import values USD 86,437,699.00; data 2020) comes mainly from The Netherlands (71%), Germany, Spain and Poland [36]. In globalized trade, plants and plant products are continuously on the move. Marketing has switched from conventional to web-commerce sites, exacerbating potential phytosanitary risks as delivery often bypasses traditional screening by NPPOs [37][38][39]. Not least, the distribution of pests is clearly altered by climate change. The presence of P. cinnamomi in alpine areas is emblematic. Its quick spreading in new geographic areas was reported in forests [40][41][42] as well as in German nurseries, where generally it is rare due to its sensitivity to frost [43]. In this scenario, the future of Phytophthora spp. occurrence is dangerously uncertain. Addressing the risks of Phytophthora spread is a highly complex task. Despite good intentions to control pest introduction and spread, we must be aware of the weakness and the lack of harmonization of phytosanitary regulations and processes [39][44][45][46][47][48][49][50]. The recently adopted new Plant Health Regulation (EU) 2016/2031, enhancing more effective measures for the protection of the Union territory and its plants, ensures safer trade, as well as proposing mitigation measures for the impacts of climate change on the health of crops and forests. The application of the new law cannot tackle the issue alone. It is essential to develop pest risk assessments that underpin policy and decision-making to assess the risks of introduction, spread and the environmental impact posed by invasive alien species (IAS). However, during the introduction steps, pathogens could be particularly hard to identify. They can express a pathogenic lifestyle only following introduction into new areas and in association with new hosts. Several guidelines and protocols for risk assessments have already been drawn up, but an effort to harmonize them and enhance communication and information exchanges with other countries is suggested [51][52][53]. The development of new rapid, reliable, accurate and cost-effective detection methods is also widely desirable to prevent spread of Phytophthora spp. Apart from molecular approaches, such as environmental DNA metabarcoding, aerobiology or the use of sentinel plants, represent a challenging but helpful research line for bio-surveillance of IAS [54][55][56]. Once in a nursery, the spread of Phytophthora is difficult to stop. Several guidelines were published to help to maintain a nursery system that excludes Phytophthora pathogens [57][58]. The application of those protocols, however, could be hampered by practical issues. They could require technical practices, such as testing irrigation water for the presence of pathogens, which represent additional costs for professional growers. In this context, it is important to inform professionals in the sector of the risk and consequences of plant diseases that are often hidden by chemical treatments. It is increasingly recognized that surveillance activities should be developed for early detection both in the areas of interest and in the exporting regions outside the EU. Thus, field workers and inspectors at borders should continuously update their knowledge or skills to recognize symptoms of plant diseases. In recent years, several molecular methods have been developed for early detection of Phytophthora; however, they often require expertise not generally present in plant nurseries, meaning that growers need to pay for external services. It is a matter of fact that plant nurseries are generally small-sized enterprises, about 1.3 ha/nursery in Italy, that could hardly bear the costs of biosecurity strategies, despite the necessity. External financial aid, for example from EU plant health organizations, could support bio-surveillance practices. Among the strategies suggested for Phytophthora disease management, the biological protection approach results in one of the most eco-sustainable control methods by inhibiting plant pathogens, improving plant immunity and/or stimulating microorganisms beneficial to the plants. Gaining a better understanding of the interaction of biological control agents with the environment and the development of new eco-friendly products, such as nanoparticles as carriers of plant extracts or other chemicals [59][60][61][62], will be important for the improvement of environmentally sustainable management protocols. Given the global nature of Phytophthora disease problems, bio-surveillance should be introduced encompassing global cooperation in monitoring, detection, studying and managing the pathogen. Encouragement of better collaborations among research centers, growers and national and international organizations will optimize efforts for protecting plants. Moreover, a reciprocal exchange dialogue is required with the public and industry to work in synergy in order to fully share common control strategies, increase awareness of the risks in plant trade and the importance of protecting and maintaining local biodiversity.
4. Conclusions
The plant nursery industry is a reservoir for Phytophthora species, whose spread will be exacerbated by the effects of the ever-increasing global plant trade, climate change, the introduction of highly susceptible or asymptomatic hosts and the emergence of new threats, or a combination of these issues. These factors will have a decisive influence on the geographic distribution of pathogens, their virulence and host range into the future. It is, therefore, not surprising that in the future, new combinations of host-pathogens or new Phytophthora hybrids will occur. The growing number of publications and citations for Phytophthora species could be interpreted as an increasing awareness of their environmental, economic and social impacts. Nonetheless, there remains a lack of information about the occurrence of Phytophthora spp. in nurseries, illustrating the need to develop simple, efficient early detection methods and management strategies. More efforts should be addressed to highlight the risk posed by new introductions of Phytophthora species as a matter of urgency by government agencies, international health organizations, managers, plant nurseries and citizens. In this scenario, nurseries will play a crucial role. By enforcing appropriate biosecurity practices and early detection, they can reduce their economic losses and limit pest spread into forests and urban areas. The study highlighting the rapid increase in the number of Phytophthora species in European plant nurseries will contribute to raising awareness of managers and scientists on the importance of implementing appropriate biosecurity measures to minimize the ecological and economic threat posed to the forest and food chains as well as natural ecosystems and urban areas.
References
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press—American Phytopathological Society: St. Paul, MN, USA, 1996; 562p.
- Jung, T.; Colquhoun, I.J.; Hardy, G.E.St.J. New Insights into the Survival Strategy of the Invasive Soilborne Pathogen Phytophthora Cinnamomi in Different Natural Ecosystems in Western Australia. For. Path. 2013, 43, 266–288, doi:10.1111/efp.12025.
- Vannini, A.; Breccia, M.; Bruni, N.; Tomassini, A.; Vettraino, A.M. Behaviour and Survival of Phytophthora Cambivora Inoculum in Soil-like Substrate under Different Water Regimes: Behaviour and Survival of Phytophthora Cambivora. For. Path. 2012, 42, 362–370, doi:10.1111/j.1439-0329.2012.00768.x.
- Vettraino, A.M.; Tomassini, A.; Vannini, A. Use of mRNA as an indicator of the viability of Phytophthora cambivora. Acta Hortic. 2010, 431–434, doi:10.17660/ActaHortic.2010.866.57.
- Knaus, B.J.; Fieland, V.J.; Graham, K.A.; Grünwald, N.J. Diversity of Foliar Phytophthora Species on Rhododendron in Oregon Nurseries. Plant Disease 2015, 99, 1326–1332, doi:10.1094/PDIS-09-14-0964-RE.
- Burgess, T.I.; Edwards, J.; Drenth, A.; Massenbauer, T.; Cunnington, J.; ostowfizadeh-Ghalamfarsa, R.; Dinh, Q.; Liew, E.C.Y.; White, D.; Scott, P.; et al. Current Status of Phytophthora in Australia. persoonia 2021, 47, 151–177, doi:10.3767/persoonia.2021.47.05.
- Langenhoven, S.; Halleen, F.; Spies, C.F.J.; Stempien, E.; Mostert, L. Detection and Quantification of Black Foot and Crown and Root Rot Pathogens in Grapevine Nursery Soils in the Western Cape of South Africa. Phytopathologia Mediterranea 2018, 57, doi:10.14601/Phytopathol_Mediterr-23921.
- Simamora, A.V.; Stukely, M.J.C.; Hardy, G.E.StJ.; Burgess, T.I. Phytophthora Boodjera Sp. Nov., a Damping-off Pathogen in Production Nurseries and from Urban and Natural Landscapes, with an Update on the Status of P. Alticola. IMA Fungus 2015, 6, 319–335, doi:10.5598/imafungus.2015.06.02.04.
- Parke, J.L.; Knaus, B.J.; Fieland, V.J.; Lewis, C.; Grünwald, N.J. Phytophthora Community Structure Analyses in Oregon Nurseries Inform Systems Approaches to Disease Management. Phytopathology® 2014, 104, 1052–1062, doi:10.1094/PHYTO-01-14-0014-R.
- Rooney-Latham, S.; Blomquist, C.L.; Kosta, K.L.; Gou, Y.Y.; Woods, P.W. Phytophthora Species Are Common on Nursery Stock Grown for Restoration and Revegetation Purposes in California. Plant Disease 2019, 103, 448–455, doi:10.1094/PDIS-01-18-0167-RE.
- Hansen, E.M.; Streito, J.-C.; Delatour, C. First Confirmation of Phytophthora Lateralis in Europe. Plant Disease 1999, 83, 587–587, doi:10.1094/PDIS.1999.83.6.587B.
- Molnar, C.; Nikolaeva, E.; Kim, S.; Olson, T.; Bily, D.; Kim, J.-E.; Kang, S. Phytophthora Diversity in Pennsylvania Nurseries and Greenhouses Inferred from Clinical Samples Collected over Four Decades. Microorganisms 2020, 8, 1056, doi:10.3390/microorganisms8071056.
- USDA APHIS Home Landing Page. Available online: https://www.aphis.usda.gov/aphis/home/ (accessed on 1 September 2022)
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora Infestations in European Nurseries Put Forest, Semi-Natural and Horticultural Ecosystems at High Risk of Phytophthora Diseases. For. Path. 2016, 46, 134–163, doi:10.1111/efp.12239.
- Henricot, B.; Pérez Sierra, A.; Jung, T. Phytophthora Pachypleura Sp. Nov., a New Species Causing Root Rot of Aucuba Japonica and Other Ornamentals in the United Kingdom. Plant Pathol 2014, 63, 1095–1109, doi:10.1111/ppa.12194.
- Gullino, C.; Garofalo, M.C.; Moretti, F.; Gianetti, G.; Mainenti, E. Rinvenimento su rododendro di Phytophthora ramorum. L’Inf. Agrar. 2003, 19, 87–89.
- Ginetti, B.; Carmignani, S.; Ragazzi, A.; Werres, S.; Moricca, S. Foliar Blight and Shoot Dieback Caused by Phytophthora Ramorum on Viburnum Tinus in the Pistoia Area, Tuscany, Central Italy. Plant Disease 2014, 98, 423–423, doi:10.1094/PDIS-07-13-0767-PDN.
- Vannini, A.; Bruni, N.; Tomassini, A.; Franceschini, S.; Vettraino, A.M. Pyrosequencing of Environmental Soil Samples Reveals Biodiversity of the Phytophthora Resident Community in Chestnut Forests. FEMS Microbiol Ecol 2013, 85, 433–442, doi:10.1111/1574-6941.12132.
- Scopus Welcome to Scopus. Available online: https://www.scopus.com/home.uri (accessed on 1 October 2022).
- Web of Science—Clarivate. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 1 October 2022).
- Bregant, C.; Mulas, A.A.; Rossetto, G.; Deidda, A.; Maddau, L.; Piras, G.; Linaldeddu, B.T. Phytophthora Mediterranea Sp. Nov., a New Species Closely Related to Phytophthora Cinnamomi from Nursery Plants of Myrtus Communis in Italy. Forests 2021, 12, 682, doi:10.3390/f12060682.
- Trouillas, F.P.; Nouri, M.T.; Bourret, T.B. Identification and Characterization of Phytophthora Species Associated with Crown and Root Rot of Pistachio Trees in California. Plant Disease 2022, 106, 197–206, doi:10.1094/PDIS-05-21-1064-RE.
- Linaldeddu, B.T.; Mulas, A.A.; Bregant, C.; Piras, G.; Montecchio, L. First Report of Phytophthora Pistaciae Causing Root and Collar Rot on Nursery Plants of Pistacia Lentiscus in Italy. Plant Disease 2020, 104, 1564–1564, doi:10.1094/PDIS-12-19-2567-PDN.
- Mirsoleimani, Z.; Mostowfizadeh-Ghalamfarsa, R. Characterization of Phytophthora pistaciae, the causal agent of pistachio gummosis, based on host range, morphology, and ribosomal genome. Phytopathologia Mediterranea 2013, 52, doi:10.14601/Phytopathol_Mediterr-11549.
- Mirabolfathy, M.; Cooke, D.E.L.; Duncan, J.M.; Williams, N.A.; Ershad, D.; Alizadeh, A. Phytophthora Pistaciae Sp. Nov. and P. Melonis: The Principal Causes of Pistachio Gummosis in Iran. Mycological Research 2001, 105, 1166–1175, doi:10.1016/S0953-7562(08)61987-5.
- Scanu, B.; Jung, T.; Masigol, H.; Linaldeddu, B.T.; Jung, M.H.; Brandano, A.; Mostowfizadeh-Ghalamfarsa, R.; Janoušek, J.; Riolo, M.; Cacciola, S.O. Phytophthora Heterospora Sp. Nov., a New Pseudoconidia-Producing Sister Species of P. Palmivora. Journal of Fungi 2021, 7, 870, doi:10.3390/jof7100870.
- Babacauh, K.D. Structure des populations de Phytophthora palmivora (Butl.) Butl. emend. Bras. et Griff. parasite du Cacaoyer (Theobroma cacao L.). Bulletin de la Société Botanique de France. Lettres Botaniques 1983, 130, 15–25, doi:10.1080/01811797.1983.10824567.
- Statista-The Statistics Portal for Market Data, Market Research and Market Studies. Available online: https://www.utu.fi/en/news/news/statista-the-statistics-portal-for-market-data-market-research-and-market-studies (accessed on 26 October 2022).
- Vitale, S.; Luongo, L.; Galli, M.; Belisario, A. First Report of Phytophthora Hydropathica Causing Wilting and Shoot Dieback on Viburnum in Italy. Plant Disease 2014, 98, 1582–1582, doi:10.1094/PDIS-03-14-0308-PDN.
- Faedda, R.; Cacciola, S.O.; Pane, A.; Szigethy, A.; Bakonyi, J.; Veld, W.A.M. in’t; Martini, P.; Schena, L.; di San Lio, G.M. Phytophthora × Pelgrandis Causes Root and Collar Rot of Lavandula Stoechas in Italy. Plant Disease 2013, 97, 1091–1096, doi:10.1094/PDIS-11-12-1035-RE.
- Szigethy, A.; Nagy, Z.Á.; Vettraino, A.M.; Józsa, A.; Cacciola, S.O.; Faedda, R.; Bakonyi, J. First Report of Phytophthora × Pelgrandis Causing Root Rot and Lower Stem Necrosis of Common Box, Lavender and Port-Orford-Cedar in Hungary. Plant Disease 2013, 97, 152–152, doi:10.1094/PDIS-07-12-0662-PDN.
- Prigigallo, M.I.; Mosca, S.; Cacciola, S.O.; Cooke, D.E.L.; Schena, L. Molecular Analysis of Phytophthora Diversity in Nursery-Grown Ornamental and Fruit Plants. Plant Pathol 2015, 64, 1308–1319, doi:10.1111/ppa.12362.
- Prigigallo, M.I.; Abdelfattah, A.; Cacciola, S.O.; Faedda, R.; Sanzani, S.M.; Cooke, D.E.L.; Schena, L. Metabarcoding Analysis of Phytophthora Diversity Using Genus-Specific Primers and 454 Pyrosequencing. Phytopathology® 2016, 106, 305–313, doi:10.1094/PHYTO-07-15-0167-R.
- Brasier, C.M.; Cooke, D.E.L.; Duncan, J.M.; Hansen, E.M. Multiple New Phenotypic Taxa from Trees and Riparian Ecosystems in Phytophthora Gonapodyides-P. Megasperma ITS Clade 6, Which Tend to Be High-Temperature Tolerant and Either Inbreeding or Sterile. Mycological Research 2003, 107, 277–290, doi:10.1017/S095375620300738X.
- Brasier, C.M. Phytophthora biodiversity: How many Phytophthora species are there? In Proceedings of the Phytophthoras in Forests and Natural Ecosystems: Fourth Meeting of the International Union of Forest Research Organizations (IUFRO)Working Party S07.02.09, Monterey, CA, USA, 26–31 August 2007.
- TrendEconomy Open Data Portal. Available online: https://trendeconomy.com/data (accessed on 21 October 2022).
- Franić, I.; Prospero, S.; Hartmann, M.; Allan, E.; Auger‐Rozenberg, M.; Grünwald, N.J.; Kenis, M.; Roques, A.; Schneider, S.; Sniezko, R.; et al. Are Traded Forest Tree Seeds a Potential Source of Nonnative Pests? Ecol Appl 2019, 29, doi:10.1002/eap.1971.
- Cleary, M.; Oskay, F.; Doğmuş, H.T.; Lehtijärvi, A.; Woodward, S.; Vettraino, A.M. Cryptic Risks to Forest Biosecurity Associated with the Global Movement of Commercial Seed. Forests 2019, 10, 459, doi:10.3390/f10050459.
- Hulme, P.E. Unwelcome Exchange: International Trade as a Direct and Indirect Driver of Biological Invasions Worldwide. One Earth 2021, 4, 666–679, doi:10.1016/j.oneear.2021.04.015.
- Vettraino, A.M.; Barzanti, G.P.; Bianco, M.C.; Ragazzi, A.; Capretti, P.; Paoletti, E.; Luisi, N.; Anselmi, N.; Vannini, A. Occurrence of Phytophthora Species in Oak Stands in Italy and Their Association with Declining Oak Trees. Forest Pathol 2002, 32, 19–28, doi:10.1046/j.1439-0329.2002.00264.x.
- Garibaldi, A.; Bertetti, D.; Poli, A.; Bizioli, L.; Gullino, M.L. First Report of Root Rot Caused by Phytophthora Cinnamomi on Mountain Laurel (Kalmia Latifolia) in Italy. Plant Disease 2012, 96, 1381–1381, doi:10.1094/PDIS-04-12-0402-PDN.
- Scanu, B.; Linaldeddu, B.T.; Franceschini, A.; Anselmi, N.; Vannini, A.; Vettraino, A.M. Occurrence of Phytophthora Cinnamomi in Cork Oak Forests in Italy. For. Path. 2013, 43, 340–343, doi:10.1111/efp.12039.
- Nechwatal, J.; Jung, T. A Study of Phytophthora Spp. in Declining Highbush Blueberry in Germany Reveals That P. Cinnamomi Can Thrive under Central European Outdoor Conditions. J Plant Dis Prot 2021, 128, 769–774, doi:10.1007/s41348-021-00445-y.
- Vettraino, A.M.; Santini, A. Forest Health in Italy: Learning From the Xylella Incursion. Front. For. Glob. Change 2021, 4, 699393, doi:10.3389/ffgc.2021.699393.
- Eschen, R.; Rigaux, L.; Sukovata, L.; Vettraino, A.M.; Marzano, M.; Grégoire, J.-C. Phytosanitary Inspection of Woody Plants for Planting at European Union Entry Points: A Practical Enquiry. Biol Invasions 2015, 17, 2403–2413, doi:10.1007/s10530-015-0883-6.
- Vettraino, A.; Potting, R.; Raposo, R. EU Legislation on Forest Plant Health: An Overview with a Focus on Fusarium Circinatum. Forests 2018, 9, 568, doi:10.3390/f9090568.
- Engler, A.; Nahuelhual, L.; Cofré, G.; Barrena, J. How Far from Harmonization Are Sanitary, Phytosanitary and Quality-Related Standards? An Exporter’s Perception Approach. Food Policy 2012, 37, 162–170, doi:10.1016/j.foodpol.2011.12.003.
- Brasier, C.M. Preventing invasive pathogens: Deficiencies in the system. Plantsman 2005, 4, 54–57.
- Day, R.K. More trade, safer trade: Strengthening developing countries’ sanitary and phytosanitary (SPS) capacity. CABI Work. Paper 2013, 4, 33.
- Kumar, P.L.; Cuervo, M.; Kreuze, J.F.; Muller, G.; Kulkarni, G.; Kumari, S.G.; Massart, S.; Mezzalama, M.; Alakonya, A.; Muchugi, A.; et al. Phytosanitary Interventions for Safe Global Germplasm Exchange and the Prevention of Transboundary Pest Spread: The Role of CGIAR Germplasm Health Units. Plants 2021, 10, 328, doi:10.3390/plants10020328.
- Roy, H.E.; Rabitsch, W.; Scalera, R.; Stewart, A.; Gallardo, B.; Genovesi, P.; Essl, F.; Adriaens, T.; Bacher, S.; Booy, O.; et al. Developing a Framework of Minimum Standards for the Risk Assessment of Alien Species. J Appl Ecol 2018, 55, 526–538, doi:10.1111/1365-2664.13025.
- EFSA Panel on Plant Health. Guidance on the environmental risk assessment of plant pests. EFSA J. 2011, 9, 2460. doi: https://doi.org/10.2903/j.efsa.2011.2460.
- Kenis, M.; Bacher, S.; Baker, R.H.A.; Branquart, E.; Brunel, S.; Holt, J.; Hulme, P.E.; MacLeod, A.; Pergl, J.; Petter, F.; et al. New Protocols to Assess the Environmental Impact of Pests in the EPPO Decision-Support Scheme for Pest Risk Analysis*: Environmental Impact Assessment in PRA. EPPO Bulletin 2012, 42, 21–27, doi:10.1111/j.1365-2338.2012.02527.x.
- Vettraino, A.; Roques, A.; Yart, A.; Fan, J.; Sun, J.; Vannini, A. Sentinel Trees as a Tool to Forecast Invasions of Alien Plant Pathogens. PLoS ONE 2015, 10, e0120571, doi:10.1371/journal.pone.0120571.
- Casarin, N.; Hasbroucq, S.; López-Mercadal, J.; Ángel Miranda, M.; Bragard, C.; Grégoire, J.-C. Measuring the threat from a distance: Insight into the complexity and perspectives for implementing sentinel plantation to test host range of Xylella fastidiosa. bioRiv 2022. bioRiv:07.22.500186. doi: https://doi.org/10.1101/2022.07.22.500186.
- Vettraino, A.M.; Santini, A.; Nikolov, C.; Grégoire, J.-C.; Tomov, R.; Orlinski, A.; Maaten, T.; Sverrisson, H.; Økland, B.; Eschen, R. A Worldwide Perspective of the Legislation and Regulations Governing Sentinel Plants. Biol Invasions 2020, 22, 353–362, doi:10.1007/s10530-019-02098-3.
- Parke, J.L.; Grünwald, N.J. A Systems Approach for Management of Pests and Pathogens of Nursery Crops. Plant Disease 2012, 96, 1236–1244, doi:10.1094/PDIS-11-11-0986-FE.
- Weiland, J.E. The Challenges of Managing Phytophthora Root Rot in the Nursery Industry. Plant Health Progress 2021, 22, 332–341, doi:10.1094/PHP-02-21-0036-FI.
- Kutawa, A.B.; Ahmad, K.; Ali, A.; Hussein, M.Z.; Abdul Wahab, M.A.; Adamu, A.; Ismaila, A.A.; Gunasena, M.T.; Rahman, M.Z.; Hossain, M.I. Trends in Nanotechnology and Its Potentialities to Control Plant Pathogenic Fungi: A Review. Biology 2021, 10, 881, doi:10.3390/biology10090881.
- Vettraino, A.M.; Zikeli, F.; Scarascia Mugnozza, G.; Vinciguerra, V.; Tabet, D.; Romagnoli, M. Lignin Nanoparticles Containing Essential Oils for Controlling Phytophthora Cactorum Diseases. Forest Pathology 2022, 52, doi:10.1111/efp.12739.
- Abd El Aty, A.A.; Zohair, M.M. Green-Synthesis and Optimization of an Eco-Friendly Nanobiofungicide from Bacillus Amyloliquefaciens MH046937 with Antimicrobial Potential against Phytopathogens. Environmental Nanotechnology, Monitoring & Management 2020, 14, 100309, doi:10.1016/j.enmm.2020.100309.
- Ahmad, H.; Venugopal, K.; Bhat, A.H.; Kavitha, K.; Ramanan, A.; Rajagopal, K.; Srinivasan, R.; Manikandan, E. Enhanced Biosynthesis Synthesis of Copper Oxide Nanoparticles (CuO-NPs) for Their Antifungal Activity Toxicity against Major Phyto-Pathogens of Apple Orchards. Pharm Res 2020, 37, 246, doi:10.1007/s11095-020-02966-x.




