Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhongyang Luo | -- | 1997 | 2023-01-06 09:52:58 | | | |
| 2 | Zhongyang Luo | + 364 word(s) | 2361 | 2023-01-06 11:14:09 | | | | |
| 3 | Catherine Yang | Meta information modification | 2361 | 2023-01-10 02:44:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Luo, Z.; Qian, Q.; Sun, H.; Wei, Q.; Zhou, J.; Wang, K. Lignin-First Biorefinery. Encyclopedia. Available online: https://encyclopedia.pub/entry/39830 (accessed on 07 February 2026).
Luo Z, Qian Q, Sun H, Wei Q, Zhou J, Wang K. Lignin-First Biorefinery. Encyclopedia. Available at: https://encyclopedia.pub/entry/39830. Accessed February 07, 2026.
Luo, Zhongyang, Qian Qian, Haoran Sun, Qi Wei, Jinsong Zhou, Kaige Wang. "Lignin-First Biorefinery" Encyclopedia, https://encyclopedia.pub/entry/39830 (accessed February 07, 2026).
Luo, Z., Qian, Q., Sun, H., Wei, Q., Zhou, J., & Wang, K. (2023, January 06). Lignin-First Biorefinery. In Encyclopedia. https://encyclopedia.pub/entry/39830
Luo, Zhongyang, et al. "Lignin-First Biorefinery." Encyclopedia. Web. 06 January, 2023.
Copy Citation
Conventional lignocellulosic-biomass-utilization strategies have difficulty in avoiding the undesirable condensation of reactive intermediates during biomass deconstruction, which poses fundamental challenges for commercial applications. Lignin-first biorefinery inhibits the condensation of reactive intermediates either by selectively catalyzing the conversion of these intermediates to stable derivatives or by avoiding their formation by functionalizing natural structures or intermediates. This strategy has attracted wide attention from researchers since it was proposed.
lignocellulosic biomass
lignin-first biorefinery
lignin and its derivatives
1. Reductive Catalytic Fractionation (RCF)
One of the most effective strategies is the direct hydrogenolysis of native lignin in lignocellulosic biomass; that is, a stable, low-Mw lignin oil (phenolic monomers, dimers, and small oligomers) can be obtained through tandem lignin depolymerization and stabilization [1]. This methodology is now termed reductive catalytic fractionation (RCF) [2], also known as catalytic upstream biorefining (CUB) or early-stage catalytic conversion of lignin (ECCL) via H-transfer reactions for the process using 2-PrOH as an H-donor [3][4]. In Table 1, selected reaction systems that achieve high-monomer yields are generalized.
Table 1. Reductive catalytic fractionation of biomass feedstock.
| Feedstock | Catalyst | Solvent | Monomer Yield | Sugar Retention | Year Ref |
|---|---|---|---|---|---|
| Miscanthus | Ni/C | Methanol | 68 wt% | 86 wt% | 2016 [5] |
| Corn Stover | Ni/C | Methanol | 24.5 wt% | 76 wt% | 2016 [6] |
| Flax Shive | Ru/C | Ethanol | 9.5 wt% | Glucan 67.2 wt% | 2020 [7] |
| Spruce | Ru/C | Ethanol | 30 wt% | Glucan 84.4 wt% | 2022 [8] |
| Bamboo | Pd/C | Methanol | 32.2 wt% | Glucan 73.4 wt% Xylan 57.4 wt% |
2019 [9] |
| Eucalyptus | Pd/C | Methanol | 49.8 wt% | Glucan 82.5 wt% Xylan 67.8 wt% |
2020 [10] |
| Poplar | Pd/C | Methanol/ H2O (7:3) |
43.5 wt% | 66.7 wt% | 2016 [11] |
| Zn/Pd/C | Methanol | 54 wt% | 79 wt% | 2015 [12] | |
| Birch | Ru/C | Methanol | 51.5% (C-Yield) | 81%(C-Yield) | 2015 [2] |
| Pd/C | Methanol | 49.3% (C-Yield) | 89% (C-Yield) | 2015 [13] | |
| Pd/C | Water | 43.8 wt% | 55 wt% | 2016 [14] | |
| Pd/C | Ethanol/ H2O (1:1) |
36% (C-Yield) | 84.4 wt% | 2016 [15] | |
| Ni/Al2O3 a | Methanol | 36 wt% | 84.9 wt% | 2017 [16] | |
| Pd/C+H3PO4 b | Methanol/ H2O (7:3) |
37 wt% | 56 wt% | 2017 [17] |
a Ni/Al2O3 pellets in catalyst cage. b Reaction operated in a flow-through reactor.
1.1. Role of the Catalyst Used
A general understanding of the RCF processes has been established through mechanistic studies, which can be summarized in three basic steps: lignin extraction, which entirely depends on the solvent; solvolytic depolymerization and catalytic hydrogenolysis; and stabilization, which is controlled by a heterogeneous, redox-active catalyst [18]. Since the hydrogenolysis of C–O bonds is metal-dependent, the type and yield of products can be controlled by selecting an appropriate metal [19]. Heterogeneous metals have been shown to catalyze lignin depolymerization efficiently, including Pt, Pd, Rh, and Ru, as well as Ni, which is abundant on Earth [5][13][20][21].
Sels and colleagues presented the RCF of birch with a Ru/C catalyst, in which the lignin fraction was degraded to a propyl-substituted phenol compound with a monomer yield of 52%. Cellulose retention reached 95%, while hemicellulose retention was only 47% among the carbohydrates, which were converted into C2–C6 sugar polyol products in the subsequent hydrolysis reaction [2]. Furthermore, Pd/C and Ru/C catalysts were compared under identical conditions. As expected, the lignin product yields were similar for the two catalysts. However, the chemical structures of the products were quite different, and the Pd/C catalyst had a higher selectivity for lignin monomers rich in hydroxyl groups and a higher retention of carbohydrate residues [13].
Luo et al. have shown that Pd/Zn synergistic catalysis is relevant to lignin conversion in terms of the cleavage of β-O-4 linkages and the follow-up hydrodeoxygenation [22]. Furthermore, when different types of biomass feedstocks were treated with Zn/Pd/C, the native lignin was converted into two main products: dihydroeugenol and 2,6-dimethoxy-4-propylphenol, with lignin monomer yields ranging from 40% to 54% [12]. Further mechanistic studies revealed a synergistic effect between Pd/C and ZnII; it was proposed that the addition of ZnII can activate and promote the removal of Cγ-OH from the β-O-4 bond [23].
From the perspective of industrial applications, the development of low-cost and highly available catalysts is imperative. Song et al. presented a selective hydrogenolysis of natural lignin fractions from birch wood to dihydroeugenol, 2,6-dimethoxy-4-propylphenol, and a small amount of propenyl-substituted phenols using a Ni/C catalyst [24]. Interestingly, the Fe-doped bimetallic catalyst showed stronger hydroxyl removal when compared to the Ni/C catalyst, and the monomer product distribution changed from PG-OH and PS-OH to PG and PS [25]. Li et al. developed a new Ni-W2C/AC bimetallic catalyst and found that there was a synergistic effect between the Ni and W2C, which could significantly promote the formation of lignin-derived monomers. Carbohydrates were further converted into ethylene glycol and other diol products. This catalyst can be widely used in birch, poplar, pine, beech, and other raw materials [26].
1.2. Influence of Solvents
In the process of the direct catalytic treatment of lignocellulosic biomass, solvent decomposition can cut the lignin–carbohydrate complex (LCC) between lignin and hemicellulose, realizing lignin stripping from the biomass substrates. Subsequently, the β-O-4 linkage bond in the lignin structure is broken under solvent decomposition. Soluble lignin fragments are then generated, which make further contact with the catalyst surface and complete the subsequent activation of the β-O-4 linkage bond into a single-molecule compound. Solvents play an important role in the delignification of biomass and lignin depolymerization, affecting the yield of aromatic monomers as well as the retention of carbohydrate pulps [27][28].
Sels et al. investigated the effects of different solvents on the RCF of birch wood. It was found that the higher the polarity of the solvent, the higher the degree of delignification. This was because highly polar solvents can better complete the dissolution of the wood fiber structure and make the solvents more accessible to lignin; among them, methanol and ethylene glycol showed the highest efficiencies for delignification. From the distribution of lignin degradation products in a Pd/C catalytic system, with the increase of solvent polarity, the monomers and dimers of degradation products increased, while the oligomer products significantly decreased, indicating that highly polar solvents can also accelerate the degradation of the lignin oligomer into monomers and dimers [14]. A techno–economic analysis of the RCF process using different solvents was carried out by Beckham et al., who replaced the solvent in the methanol-case with ethylene glycol. Due to the lower vapor pressure of ethylene glycol, the overall reactor pressure was reduced substantially. Generally, lower pressure during RCF results in lower capital costs. On the other hand, ethylene glycol has a higher cost and higher energy consumption for solvent recovery than methanol. Overall, on the basis of supporting the sale of bioethanol at USD 2.50 per gallon of gasoline equivalent, the methanol case has a higher MSP–monomer fraction at USD 3.63 per kg, while the ethylene glycol case has a lower MSP–monomer fraction at USD 3.07 per kg [29].
Sels and colleagues further investigated the effects of different alcohol/water-mixing solvent systems on the RCF, and their results showed that the addition of moderate amounts of water significantly enhanced the extraction efficiency of lignin. However, too much water resulted in a lower degree of delignification [11]. Chen et al. also confirmed the positive effect of adding water on the yield of lignin monomers [30]. It should be noted that, if pure water is used as the medium while the lignin fraction is efficiently separated and degraded, the carbohydrate fraction also undergoes hydrolysis reactions and almost all of the hemicellulose and about 20% of the cellulose are removed [14]. Similar solvent-polarity effects can also be observed in other catalytic systems. When water replaced methanol as the solvent in the case of the Ru/C system, not only did the yield of phenol monomer decrease from 52% to 25%, but the carbohydrate fraction was also degraded into soluble polyols [2]. A plausible explanation for this is the autoionization of water into H+ acid ions under high temperature conditions, which can catalyze the hydrolysis of carbohydrate [31]. In addition, the redeposition of dissolved lignin on the surface of lignocellulosic fibers should be considered when water is used as the solvent [32]. Above all, a pure water system may not be suitable for the current direct catalytic reduction process of biomass feedstocks.
1.3. Flow-Through Reactors
The new strategy of reductive catalytic fractionation has been proposed to depolymerize and stabilize lignin by mixing metal catalysts and biomass; however, this usually results in the catalyst not being recovered. Thus, flow-through systems for lignin-first biorefinery were developed (Figure 1). In 2017, two research teams introduced flow-through reactors for the RCF process, in which the biomass and catalyst were separated by filling into two different beds. The solvent was passed through the heated biomass bed to extract and partially depolymerize the lignin polymer. Then a liquid mixture of dissolved lignin fragments flowed through the catalyst bed for further depolymerization and stabilization of active intermediates [17][33].
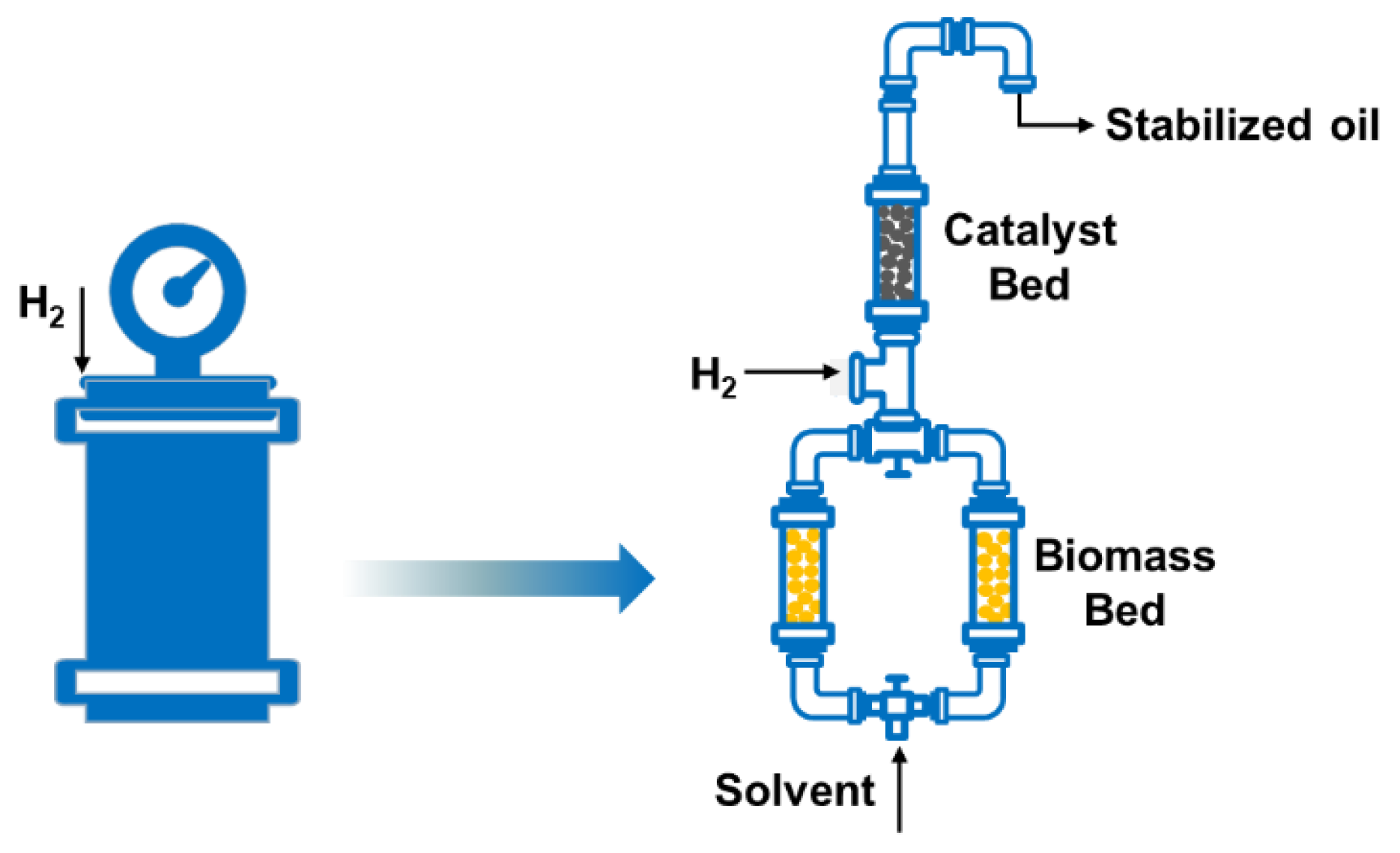
Figure 1. The evolution of reactor configurations for reductive catalytic fractionation.
However, flow-through systems also have certain limitations. For example, they require harsh reaction conditions in order to realize efficient delignification and stabilization, which significantly increases reactor costs [29]. Generally, the solvent consumption is high, because this design may increase the time taken by solvent-extracted lignin fragments to reach the catalyst bed, and partial lignin may undergo an irreversible condensation reaction, resulting in a decrease in the final phenolic-monomer yield and selectivity. Therefore, kinetic issues such as adequate mass transfer between active lignin fragments and the catalyst need to be considered [17][33][34][35].
Beckham and colleagues demonstrated that the lignin oil obtained from the flow-through system could be stored for a long time without compromising subsequent hydrogenolysis activity, but the unusually high ratio of solvent to biomass made it difficult to implement on an industrial scale [36]. In 2021, the team found that solvent usage exhibits a significant effect on the GWP; with the methanol solvent loading reducing from a 9 L/dry kg biomass to a 4 L/dry kg biomass, the GWP reduces from 0.079 kg CO2-eq/kg to a −1.078 kg CO2-eq/kg lignin fraction [29]. On this basis, a multiple flow-reduction catalytic fractionation strategy has been proposed, which successfully reduced the solvent–biomass ratio to 1.9 L/kg with no significant decline of lignin oil quality found in the case of catalyst overload. This strategy greatly reduces the energy demand and operation cost of solvent recovery, which has a good development prospect [37].
2. Stabilization Strategies
Given that the effective extraction of lignin with a high purity and less-condensed structure from lignocellulosic biomass is crucial for lignin valorization, various biomass-fractionation technologies have been developed [38]. Extraction with supercritical fluid using CO2 in a supercritical condition is generally applied, which can enhance the accessibility of biomass and reduce the pretreatment temperature [39]. Moreover, organosolv pretreatment is considered one of the most promising methods for biomass fractionation. The organic media can realize a higher lignin extraction efficiency thanks to its higher lignin solubility when compared to water [40]. In Table 2, selected extraction systems which achieve high-lignin isolated yields are generalized.
Table 2. Solvent- or co-solvent-assisted lignin extraction from biomass feedstock.
| Feedstock | Conditions | Organic Media | Isolated Lignin a | Year Ref |
|---|---|---|---|---|
| Hemp Hurds | 165 °C 20 min |
Methanol H2SO4 aqueous solution |
75 wt% | 2014 [41] |
| Switchgrass | 180 °C 60 min |
Ethanol H2SO4 aqueous solution |
60.5 wt% | 2012 [42] |
| Poplar | 160 °C 30 min |
Methanol H2SO4 aqueous solution Formaldehyde |
64 wt% | 2018 [43] |
| Walnut | 170 °C 30 min |
Methanol H2SO4 aqueous solution Formaldehyde |
50 wt% | 2021 [44] |
| 120 °C 150 min |
1-butanol H2SO4 aqueous solution |
85 wt% | 2021 [45] | |
| Birch | 85 °C 180 min |
Formaldehyde 1,4-dioxane Hydrochloric acid |
116 wt% | 2019 [46] |
| 95 °C 210 min |
Propionaldehyde 1,4-dioxane Hydrochloric acid |
89 wt% | 2019 [46] |
a Lignin isolated yields are calculated based on the theoretical amount of lignin in feedstock.
The theoretical maximum yield of lignin depolymerization to monomers is approximately the square of the cleavable interunit ether bond (β-O-4) content [47]. Therefore, the retention of the reactive β-O-4 bond is one of the means to realize lignin valorization [48]. Alcohols can act as external nucleophiles to capture benzyl carbocation intermediates and form ether at the α-position of the β-O-4 bond, which further inhibits the condensation reaction [49][50]. Lancefield et al. found that most of the β-O-4 bonds were retained in bioethanol- and biobutanol-extracted lignin [51]. Zhu et al. found that higher yields of monomers were obtained by the depolymerization of benzyl alcohol after microwave-assisted methylation, which meant that etherification improved the reactivity of the β-O-4 bond [52]. Deuss and colleagues reported the semi-continuous extraction of high β-O-4 content lignin with butanol in a flow-through system, thereby reducing the difficulty of further catalytic depolymerization [45]. However, when compared to reductive catalytic fractionation, the alcohol–etherification approach usually produces a lower yield of phenolic monomers owing to inefficient lignin extraction and incomplete intermediate capture [50].
In 2015, Barta and colleagues proposed the addition of ethylene glycol as a functional group protector to produce a stable G/S-C2-glycol acetal (1,3-dioxolane) structure through its combination with the acidolysis reaction intermediate, thus improving the yield of aromatic monomers [53][54]. On this basis, De Santi et al. used the green solvent dimethyl carbonate (DMC) to replace 1, 4-dioxane and toluene; meanwhile, sulfuric acid was used to replace the expensive iron (III) trifluoromethanesulfonate (Fe(OTf)3). The monomer yield reached 9 wt% when pine was used as raw material [55].
In 2016, Luterbacher and colleagues reported the addition of formaldehyde to organic solvent processing to avoid repolymerization during lignin extraction. This method takes advantage of the functional group protection of formaldehyde: formaldehyde reacts with α-OH and γ-OH on the side-chain of lignin to form a stable 1, 3-dioxane structure through acylation, which inhibits the formation of benzyl carbocation. At the same time, the electron-rich positions on the aromatic ring (usually the positions ortho or para to methoxyl groups) are easily replaced with protonated-formaldehyde electrophilic aromatics to form a hydroxyl methyl group, which further blocks the polycondensation reaction site [56]. Recently, the team demonstrated that the extracted lignin was able to achieve steady-state, continuous depolymerization with a Ni/C catalyst in a flow-through system, in which the yield of 45% monophenol was achieved and maintained for 125 h [57].
In 2018, Abu-Omar and colleagues used a solvent mixture of methanol and dilute sulfuric acid with a small amount of formaldehyde to extract lignin. Over 68% of the lignin in poplar was extracted and depolymerized by the Ni/C catalyst, resulting in three major phenolic monomers: isoeugenol, 4-propenyl eugenol, and guaiacol, with a total yield of 63% [43]. This extraction method is also applicable to walnut-shell biomass. Compared to ethanol, methanol—as a stronger nucleophilic reagent—is more effective in protecting carbocation intermediates [44].
In summary, the method of using alcohols or aldehydes to stabilize lignin intermediates is basically compatible with the established organic solvent pulping method. Compared to the RCF process, its biggest advantage is that it can separate the biomass fractionation from the subsequent depolymerization step so that the two steps can be optimized independently and the depolymerization is more flexible. Therefore, only the solvent and reaction conditions need to be adjusted [50].
References
- Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: The advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557.
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763.
- Ferrini, P.; Rezende, C.A.; Rinaldi, R. Catalytic Upstream Biorefining through Hydrogen Transfer Reactions: Understanding the Process from the Pulp Perspective. ChemSusChem 2016, 9, 3171–3180.
- Rinaldi, R.; Woodward, R.; Ferrini, P.; Rivera, H. Lignin-First Biorefining of Lignocellulose: The Impact of Process Severity on the Uniformity of Lignin Oil Composition. J. Braz. Chem. Soc. 2018, 30, 479–491.
- Luo, H.; Klein, I.M.; Jiang, Y.; Zhu, H.; Liu, B.; Kenttämaa, H.I.; Abu-Omar, M.M. Total Utilization of Miscanthus Biomass, Lignin and Carbohydrates, Using Earth Abundant Nickel Catalyst. ACS Sustain. Chem. Eng. 2016, 4, 2316–2322.
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive Catalytic Fractionation of Corn Stover Lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950.
- Kazachenko, A.S.; Tarabanko, V.E.; Miroshnikova, A.V.; Sychev, V.V.; Skripnikov, A.M.; Malyar, Y.N.; Mikhlin, Y.L.; Baryshnikov, S.V.; Taran, O.P. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts 2020, 11, 42.
- Taran, O.P.; Miroshnikova, A.V.; Baryshnikov, S.V.; Kazachenko, A.S.; Skripnikov, A.M.; Sychev, V.V.; Malyar, Y.N.; Kuznetsov, B.N. Reductive Catalytic Fractionation of Spruce Wood over Ru/C Bifunctional Catalyst in the Medium of Ethanol and Molecular Hydrogen. Catalysts 2022, 12, 1384.
- Zhang, K.; Li, H.; Xiao, L.P.; Wang, B.; Sun, R.C.; Song, G. Sequential utilization of bamboo biomass through reductive catalytic fractionation of lignin. Bioresour. Technol. 2019, 285, 121335.
- Chen, X.; Zhang, K.; Xiao, L.P.; Sun, R.C.; Song, G. Total utilization of lignin and carbohydrates in Eucalyptus grandis: An integrated biorefinery strategy towards phenolics, levulinic acid, and furfural. Biotechnol. Biofuels 2020, 13, 2.
- Renders, T.; Van den Bosch, S.; Vangeel, T.; Ennaert, T.; Koelewijn, S.-F.; Van den Bossche, G.; Courtin, C.M.; Schutyser, W.; Sels, B.F. Synergetic Effects of Alcohol/Water Mixing on the Catalytic Reductive Fractionation of Poplar Wood. ACS Sustain. Chem. Eng. 2016, 4, 6894–6904.
- Parsell, T.; Yohe, S.; Degenstein, J.; Jarrell, T.; Klein, I.; Gencer, E.; Hewetson, B.; Hurt, M.; Kim, J.I.; Choudhari, H.; et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 2015, 17, 1492–1499.
- Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Renders, T.; Courtin, C.M.; Sels, B.F. Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem. Commun. (Camb) 2015, 51, 13158–13161.
- Schutyser, W.; Van den Bosch, S.; Renders, T.; De Boe, T.; Koelewijn, S.F.; Dewaele, A.; Ennaert, T.; Verkinderen, O.; Goderis, B.; Courtin, C.M.; et al. Influence of bio-based solvents on the catalytic reductive fractionation of birch wood. Green Chem. 2015, 17, 5035–5045.
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.; Samec, J.S. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287.
- Van den Bosch, S.; Renders, T.; Kennis, S.; Koelewijn, S.F.; Van den Bossche, G.; Vangeel, T.; Deneyer, A.; Depuydt, D.; Courtin, C.M.; Thevelein, J.M.; et al. Integrating lignin valorization and bio-ethanol production: On the role of Ni-Al2O3catalyst pellets during lignin-first fractionation. Green Chem. 2017, 19, 3313–3326.
- Kumaniaev, I.; Subbotina, E.; Sävmarker, J.; Larhed, M.; Galkin, M.V.; Samec, J.S.M. Lignin depolymerization to monophenolic compounds in a flow-through system. Green Chem. 2017, 19, 5767–5771.
- Renders, T.; Van den Bossche, G.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201.
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678.
- Kenny, J.K.; Brandner, D.G.; Neefe, S.R.; Michener, W.E.; Román-Leshkov, Y.; Beckham, G.T.; Medlin, J.W. Catalyst choice impacts aromatic monomer yields and selectivity in hydrogen-free reductive catalytic fractionation. React. Chem. Eng. 2022, 7, 2527–2533.
- Renders, T.; Cooreman, E.; Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Vangeel, T.; Deneyer, A.; Van den Bossche, G.; Courtin, C.M.; Sels, B.F. Catalytic lignocellulose biorefining in n-butanol/water: A one-pot approach toward phenolics, polyols, and cellulose. Green Chem. 2018, 20, 4607–4619.
- Parsell, T.H.; Owen, B.C.; Klein, I.; Jarrell, T.M.; Marcum, C.L.; Haupert, L.J.; Amundson, L.M.; Kenttämaa, H.I.; Ribeiro, F.; Miller, J.T. Cleavage and hydrodeoxygenation (HDO) of C–O bonds relevant to lignin conversion using Pd/Zn synergistic catalysis. Chem. Sci. 2013, 4, 806–813.
- Klein, I.; Marcum, C.; Kenttämaa, H.; Abu-Omar, M.M. Mechanistic investigation of the Zn/Pd/C catalyzed cleavage and hydrodeoxygenation of lignin. Green Chem. 2016, 18, 2399–2405.
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007.
- Zhai, Y.; Li, C.; Xu, G.; Ma, Y.; Liu, X.; Zhang, Y. Depolymerization of lignin via a non-precious Ni–Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903.
- Li, C.; Zheng, M.; Wang, A.; Zhang, T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: Simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci. 2012, 5, 6383–6390.
- Shuai, L.; Luterbacher, J. Organic Solvent Effects in Biomass Conversion Reactions. ChemSusChem 2016, 9, 133–155.
- Zhu, S.; Guo, J.; Wang, X.; Wang, J.; Fan, W. Alcoholysis: A Promising Technology for Conversion of Lignocellulose and Platform Chemicals. ChemSusChem 2017, 10, 2547–2559.
- Bartling, A.; Stone, M.L.; Hanes, R.J.; Bhatt, A.; Zhang, Y.; Biddy, M.J.; Davis, R.; Kruger, J.S.; Thornburg, N.E.; Luterbacher, J.; et al. Techno-economic analysis and life cycle assessment of a biorefinery utilizing reductive catalytic fractionation. Energy Environ. Sci. 2021, 14, 4147–4168.
- Chen, J.; Lu, F.; Si, X.; Nie, X.; Chen, J.; Lu, R.; Xu, J. High Yield Production of Natural Phenolic Alcohols from Woody Biomass Using a Nickel-Based Catalyst. ChemSusChem 2016, 9, 3353–3360.
- Sun, J.; Li, H.; Xiao, L.-P.; Guo, X.; Fang, Y.; Sun, R.-C.; Song, G. Fragmentation of Woody Lignocellulose into Primary Monolignols and Their Derivatives. ACS Sustain. Chem. Eng. 2019, 7, 4666–4674.
- Chen, H.; Fu, Y.; Wang, Z.; Qin, M. Degradation and redeposition of the chemical components of aspen wood during hot water extraction. BioResources 2015, 10, 3005–3016.
- Anderson, E.M.; Stone, M.L.; Katahira, R.; Reed, M.; Beckham, G.T.; Román-Leshkov, Y. Flowthrough Reductive Catalytic Fractionation of Biomass. Joule 2017, 1, 613–622.
- Anderson, E.M.; Stone, M.L.; Hülsey, M.J.; Beckham, G.T.; Román-Leshkov, Y. Kinetic Studies of Lignin Solvolysis and Reduction by Reductive Catalytic Fractionation Decoupled in Flow-Through Reactors. ACS Sustain. Chem. Eng. 2018, 6, 7951–7959.
- Cooreman, E.; Vangeel, T.; Van Aelst, K.; Van Aelst, J.; Lauwaert, J.; Thybaut, J.W.; Van den Bosch, S.; Sels, B.F. Perspective on Overcoming Scale-Up Hurdles for the Reductive Catalytic Fractionation of Lignocellulose Biomass. Ind. Eng. Chem. Res. 2020, 59, 17035–17045.
- Brandner, D.; Kruger, J.S.; Thornburg, N.E.; Facas, G.G.; Kenny, J.K.; Dreiling, R.J.; Morais, A.R.C.; Renders, T.; Cleveland, N.S.; Happs, R.M.; et al. Flow-through solvolysis enables production of native-like lignin from biomass. Green Chem. 2021, 23, 5437–5441.
- Jang, J.H.; Brandner, D.G.; Dreiling, R.J.; Ringsby, A.J.; Bussard, J.R.; Stanley, L.M.; Happs, R.M.; Kovvali, A.S.; Cutler, J.I.; Renders, T.; et al. Multi-pass flow-through reductive catalytic fractionation. Joule 2022, 6, 1859–1875.
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products-strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461.
- Wang, X.; Guo, Y.; Zhou, J.; Sun, G. Structural changes of poplar wood lignin after supercritical pretreatment using carbon dioxide and ethanol–water as co-solvents. RSC Adv. 2017, 7, 8314–8322.
- Yadav, P.; Athanassiadis, D.; Antonopoulou, I.; Rova, U.; Christakopoulos, P.; Tysklind, M.; Matsakas, L. Environmental impact and cost assessment of a novel lignin production method. J. Clean. Prod. 2021, 279, 123515.
- Gandolfi, S.; Ottolina, G.; Consonni, R.; Riva, S.; Patel, I. Fractionation of hemp hurds by organosolv pretreatment and its effect on production of lignin and sugars. ChemSusChem 2014, 7, 1991–1999.
- Hu, G.; Cateto, C.; Pu, Y.; Samuel, R.; Ragauskas, A.J. Structural characterization of switchgrass lignin after ethanol organosolv pretreatment. Energy Fuels 2012, 26, 740–745.
- Luo, H.; Abu-Omar, M.M. Lignin extraction and catalytic upgrading from genetically modified poplar. Green Chem. 2018, 20, 745–753.
- Nishide, R.N.; Truong, J.H.; Abu-Omar, M.M. Organosolv Fractionation of Walnut Shell Biomass to Isolate Lignocellulosic Components for Chemical Upgrading of Lignin to Aromatics. ACS Omega 2021, 6, 8142–8150.
- Zijlstra, D.S.; de Korte, J.; de Vries, E.P.C.; Hameleers, L.; Wilbers, E.; Jurak, E.; Deuss, P.J. Highly Efficient Semi-Continuous Extraction and In-Line Purification of High beta-O-4 Butanosolv Lignin. Front. Chem. 2021, 9, 655983.
- Talebi Amiri, M.; Dick, G.R.; Questell-Santiago, Y.M.; Luterbacher, J.S. Fractionation of lignocellulosic biomass to produce uncondensed aldehyde-stabilized lignin. Nat. Protoc. 2019, 14, 921–954.
- Galkin, M.V.; Samec, J.S. Lignin Valorization through Catalytic Lignocellulose Fractionation: A Fundamental Platform for the Future Biorefinery. ChemSusChem 2016, 9, 1544–1558.
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908.
- Kaiho, A.; Kogo, M.; Sakai, R.; Saito, K.; Watanabe, T. In situ trapping of enol intermediates with alcohol during acid-catalysed de-polymerisation of lignin in a nonpolar solvent. Green Chem. 2015, 17, 2780–2783.
- Questell-Santiago, Y.M.; Galkin, M.V.; Barta, K.; Luterbacher, J.S. Stabilization strategies in biomass depolymerization using chemical functionalization. Nat. Rev. Chem. 2020, 4, 311–330.
- Lancefield, C.S.; Panovic, I.; Deuss, P.J.; Barta, K.; Westwood, N.J. Pre-treatment of lignocellulosic feedstocks using biorenewable alcohols: Towards complete biomass valorisation. Green Chem. 2017, 19, 202–214.
- Zhu, G.; Qiu, X.; Zhao, Y.; Qian, Y.; Pang, Y.; Ouyang, X. Depolymerization of lignin by microwave-assisted methylation of benzylic alcohols. Bioresour. Technol. 2016, 218, 718–722.
- Deuss, P.J.; Scott, M.; Tran, F.; Westwood, N.J.; de Vries, J.G.; Barta, K. Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J. Am. Chem. Soc. 2015, 137, 7456–7467.
- Deuss, P.J.; Lancefield, C.S.; Narani, A.; de Vries, J.G.; Westwood, N.J.; Barta, K. Phenolic acetals from lignins of varying compositions via iron(iii) triflate catalysed depolymerisation. Green Chem. 2017, 19, 2774–2782.
- De Santi, A.; Galkin, M.V.; Lahive, C.W.; Deuss, P.J.; Barta, K. Lignin-First Fractionation of Softwood Lignocellulose Using a Mild Dimethyl Carbonate and Ethylene Glycol Organosolv Process. ChemSusChem 2020, 13, 4468–4477.
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333.
- Lan, W.; Du, Y.P.; Sun, S.; Behaghel de Bueren, J.; Héroguel, F.; Luterbacher, J.S. Continuous hydrogenolysis of acetal-stabilized lignin in flow. Green Chem. 2021, 23, 320–327.
More
Information
Subjects:
Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
10 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




