
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mahmoud Khedr | -- | 4189 | 2023-01-06 07:24:01 | | | |
| 2 | Amina Yu | -84 word(s) | 4105 | 2023-01-06 09:27:00 | | |
Video Upload Options
Welding is a joining process that permanently connects solid parts and forms components that cannot be divided without causing damage. Furthermore, welding is the most efficient and economical way to join similar or dissimilar materials with or without using filler material, heat, or external pressure. Welding can be processed in a variety of environments, including outdoors, inside, underwater, and even in outer space. The two main categories of welding methods, solid-state welding (SSW) and fusion welding, are processes to join metals. Fusion welding can be defined as the melting process of parent materials on facing surfaces with a filler material to form a weld bead. The fusion welding process comprises gas welding, arc welding, and intense-energy beam welding.
1. Solid-State Welding (SSW)
2. Diffusion Bonding (DB)
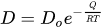
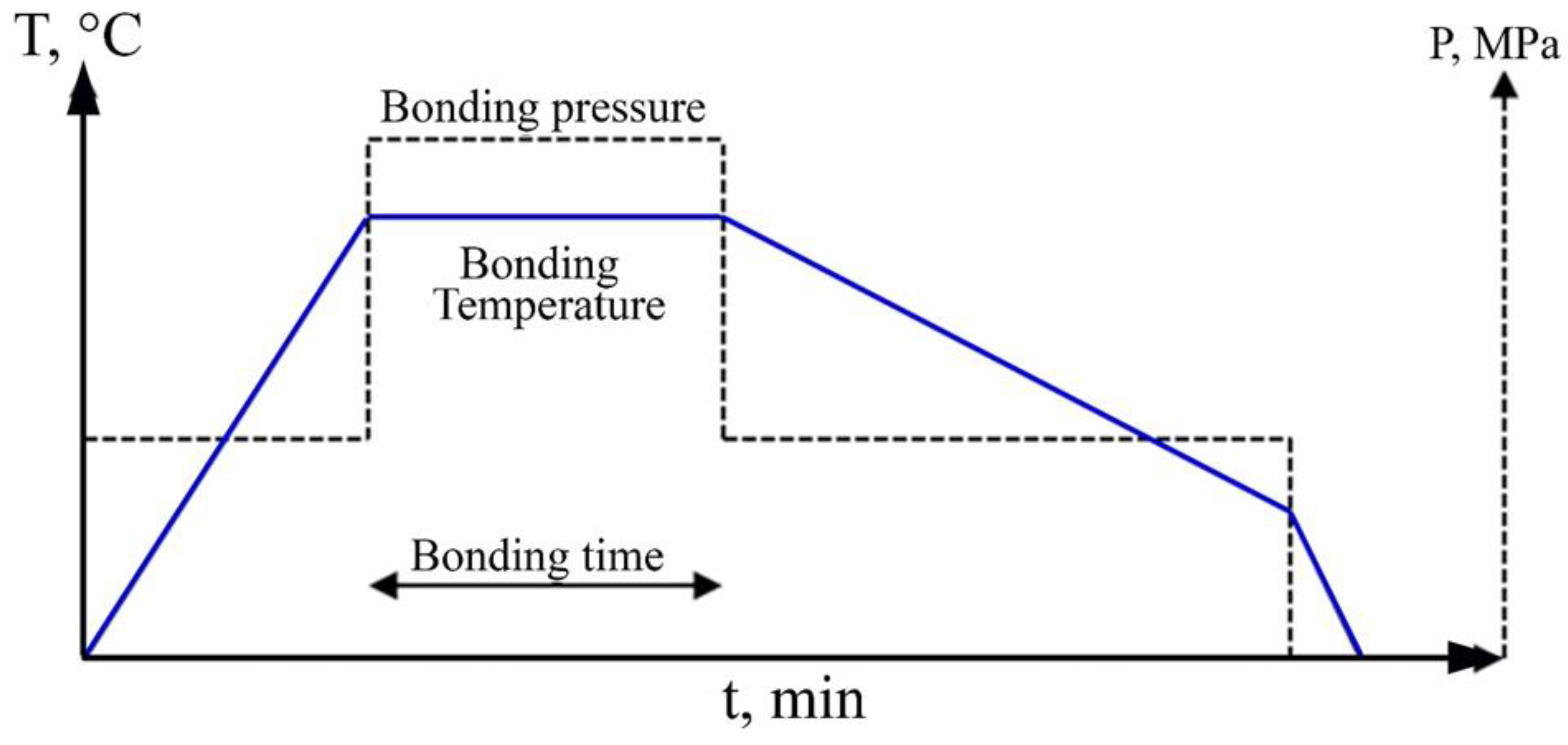

2.1. DB of Steels
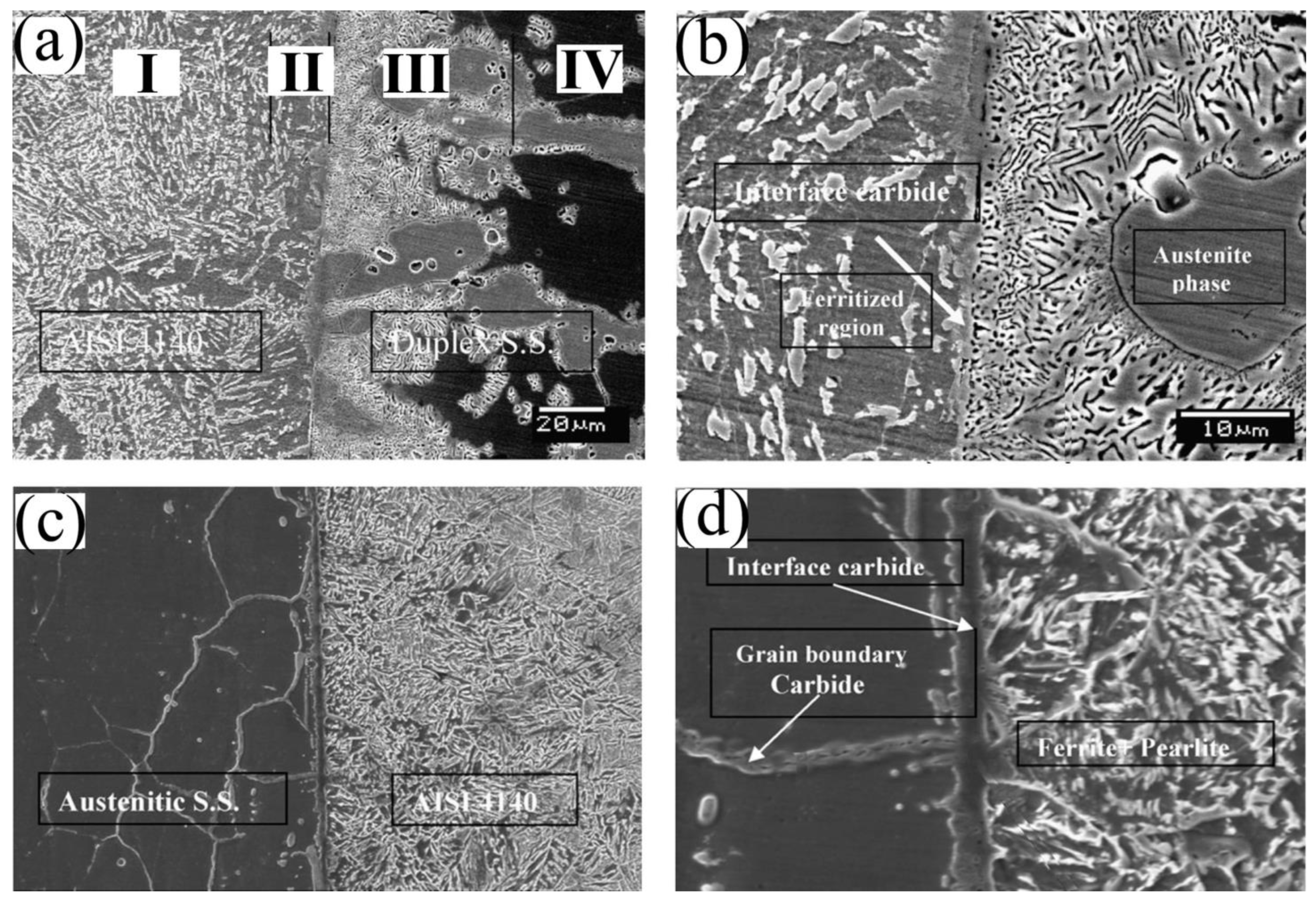
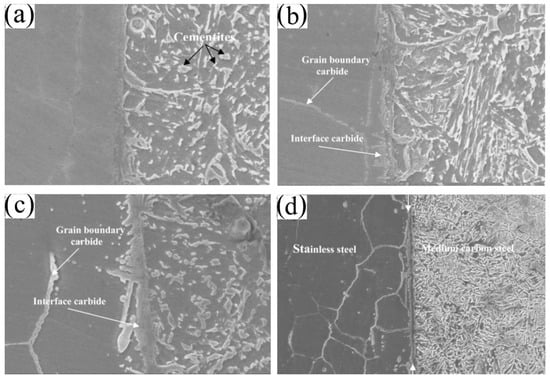
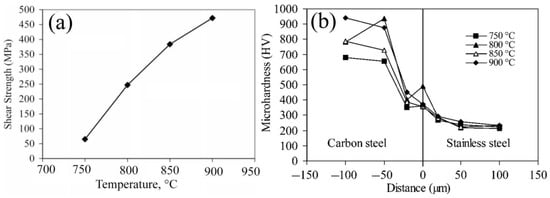
2.2. Defects in Diffusion Bonding
Two-Dimensional Defects
Three-Dimensional Defects (Precipitation)
3. Friction Stir Welding (FSW) of Steels
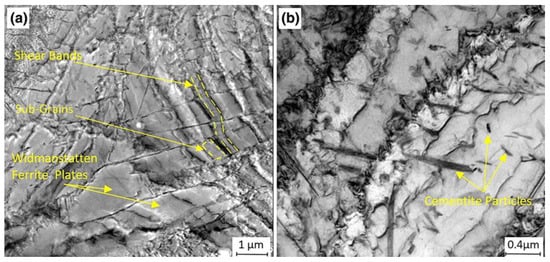
References
- Akca, E.; Gürsel, A. Solid state welding and application in aeronautical industry. Period. Eng. Nat. Sci. PEN 2016, 4, 1–8.
- Coronado, J.; Cerón, C. Fracture mechanisms of CTOD samples of submerged and flux cored arc welding. Theor. Appl. Fract. Mech. 2010, 53, 145–151.
- Brothers, D.G. The Origin of Acicular Ferrite in Gas Metal Arc and Submerged Arc Welds. Master’s Thesis, Naval Postgraduate School, Monterey, CA, USA, 1994.
- Kreye, H. Melting phenomena in solid state welding processes. Weld. J. 1977, 56, 154–158.
- Yildirim, S.; Kelestemur, M.H. A study on the solid-state welding of boron-doped Ni3Al–AISI 304 stainless steel couple. Mater. Lett. 2005, 59, 1134–1137.
- Al-Sabur, R.K.; Jassim, A.K. Friction stir spot welding applied to weld dissimilar metals of AA1100 Al-alloy and C11000 Copper. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Philadelphia, PA, USA, 2018.
- Reddy, M.G.; Rao, S.A.; Mohandas, T. Role of electroplated interlayer in continuous drive friction welding of AA6061 to AISI 304 dissimilar metals. Sci. Technol. Weld. Join. 2008, 13, 619–628.
- Miyashita, Y.; Mutoh, Y.; Akahori, M.; Okumura, H.; Nakagawa, I.; Jin-Quan, X. Laser welding of dissimilar metals aided by unsteady thermal conduction boundary element method analysis. Weld. Int. 2005, 19, 687–696.
- El Mahallawy, N.; Fathy, A.; Abdelaziem, W.; Hassan, M. Microstructure evolution and mechanical properties of Al/Al–12% Si multilayer processed by accumulative roll bonding (ARB). Mater. Sci. Eng. A 2015, 647, 127–135.
- Mahallawy, N.E.; Fathy, A.; Hassan, M.; Abdelaziem, W. Evaluation of mechanical properties and microstructure of Al/Al–12% Si multilayer via warm accumulative roll bonding process. J. Compos. Mater. 2017, 0021998317692141.
- Abdelaziem, W.; ElMahalawy, N.; Hassan, M.; Fathy, A. Effect of wire brushing on cold roll bonding of Al 1050 sheets. Egyptian J. Eng. Sci. Technol. 2015, 18, 3–4.
- Kurt, B. The interface morphology of diffusion bonded dissimilar stainless steel and medium carbon steel couples. J. Mater. Process. Technol. 2007, 190, 138–141.
- Yılmaz, O. Effect of welding parameters on diffusion bonding of type 304 stainless steel–copper bimetal. Mater. Sci. Technol. 2001, 17, 989–994.
- Akhter, J.; Ahmad, M.; Iqbal, M.; Akhtar, M.; Shaikh, M. Formation of dendritic structure in the diffusion zone of the bonded Zircaloy-4 and stainless steel 316L in the presence of Ti interlayer. J. Alloy. Compd. 2005, 399, 96–100.
- AlHazaa, A.; Haneklaus, N.; Almutairi, Z. Impulse pressure-assisted diffusion bonding (IPADB): Review and outlook. Metals 2021, 11, 323.
- Azizi, A.; Alimardan, H. Effect of welding temperature and duration on properties of 7075 Al to AZ31B Mg diffusion bonded joint. Trans. Nonferrous Met. Soc. China 2016, 26, 85–92.
- Li, P.; Wang, L.; Yan, S.; Meng, M.; Xue, K. Temperature effect on the diffusion welding process and mechanism of B2–O interface in the Ti2AlNb-based alloy: A molecular dynamics simulation. Vacuum 2020, 173, 109118.
- Akca, E.; Gursel, A. The effect of diffusion welding parameters on the mechanical properties of titanium alloy and aluminum couples. Metals 2017, 7, 22.
- Cooke, K.O.; Atieh, A.M. Current trends in dissimilar diffusion bonding of titanium alloys to stainless steels, aluminium and magnesium. J. Manuf. Mater. Process. 2020, 4, 39.
- Wang, J.; Li, Y.; Huang, W. Interface microstructure and diffusion kinetics in diffusion bonded Mg/Al joint. React. Kinet. Catal. Lett. 2008, 95, 71–79.
- Song, T.; Jiang, X.; Shao, Z.; Fang, Y.; Mo, D.; Zhu, D.; Zhu, M. Microstructure and mechanical properties of vacuum diffusion bonded joints between Ti-6Al-4V titanium alloy and AISI316L stainless steel using Cu/Nb multi-interlayer. Vacuum 2017, 145, 68–76.
- Fang, Y.; Jiang, X.; Mo, D.; Song, T.; Luo, Z. Microstructure and mechanical properties of the vacuum diffusion bonding joints of 4J29 kovar alloy and 316L stainless steel using pure cobalt interlayer. Vacuum 2019, 168, 108847.
- AlHazaa, A.; Haneklaus, N. Diffusion bonding and transient liquid phase (TLP) bonding of type 304 and 316 austenitic stainless steel—A review of similar and dissimilar material joints. Metals 2020, 10, 613.
- Islam, M.; Ridlev, N. Isostatic diffusion bonding of a microduplex stainless steel. Scr. Mater. 1998, 38, 1187–1193.
- Lee, H.-S. Diffusion bonding of metal alloys in aerospace and other applications. In Welding and Joining of Aerospace Materials, 2nd ed.; Chaturvedi, M., Ed.; Woodhead Publishing Series Elsevier Ltd.: Cambridge, UK, 2021.
- Kim, S.-J.; Gil Lee, C.; Lee, T.-H.; Oh, C.-S. Effect of Cu, Cr and Ni on mechanical properties of 0.15 wt.% C TRIP-aided cold rolled steels. Scr. Mater. 2003, 48, 539–544.
- Zhang, C.; Li, H.; Li, M. Formation mechanisms of high quality diffusion bonded martensitic stainless steel joints. Sci. Technol. Weld. Join. 2015, 20, 115–122.
- Hua, Y.; Chen, J.; Yu, L.; Si, Y.; Liu, C.; Li, H.; Liu, Y. Microstructure evolution and mechanical properties of dissimilar material diffusion-bonded joint for high Cr ferrite heat resistant steel and austenitic heat resistant steel. Acta Metall. Sin. 2021, 58, 141–154.
- Noh, S.; Kasada, R.; Kimura, A. Solid-state diffusion bonding of high-Cr ODS ferritic steel. Acta Mater. 2011, 59, 3196–3204.
- Shi, Y.; Liu, J.; Yan, Y.; Xia, Z.; Lei, Y.; Guo, F.; Li, X. Creep properties of composite solders reinforced with nano-and microsized particles. J. Electron. Mater. 2008, 37, 507–514.
- Hull, D.; Rimmer, D. The growth of grain-boundary voids under stress. Philos. Mag. 1959, 4, 673–687.
- Wang, D.; Xiu, S. Effect of bonding temperature on the interfacial micro-structure and performance of mild steel/austenite stainless steel diffusion-bonded joint. Acta Metall. Sin. 2017, 53, 567–574.
- Kurt, B.; Orhan, N.; Ozel, S. Interface microstructure of diffusion bonded austenitic stainless steel and medium carbon steel couple. Sci. Technol. Weld. Join. 2007, 12, 197–201.
- Raynor, G.V.; Rivlin, V.G. Phase Equilibria in Iron Ternar Alloys—A Critical Assessment of the Experimental Literature; The Institute of Metals: London, UK, 1988.
- Frost, H.J.; Ashby, M.F. Deformation Mechanism Maps: The Plasticity and Creep of Metals and Ceramics; Pergamon press: Elmsford, NY, USA, 1982.
- Trapp, J.; Semenov, A.; Nöthe, M.; Wallmersperger, T.; Kieback, B. Fundamental principles of spark plasma sintering of metals: Part III–densification by plasticity and creep deformation. Powder Metall. 2020, 63, 329–337.
- Sopoušek, J.; Foret, R.; Jan, V. Simulation of dissimilar weld joints of steel P91. Sci. Technol. Weld. Join. 2004, 9, 59–64.
- Race, J.; Bhadeshia, H. Carbon migration across dissimilar steel welds. In International Trends in Welding Science and Technology; ASM International: Almere, The Netherlands, 1993; pp. 1–5.
- Gomez, X.; Echeberria, J. Microstructure and mechanical properties of low alloy steel T11–austenitic stainless steel 347H bimetallic tubes. Mater. Sci. Technol. 2000, 16, 187–193.
- Silva, C.C.; Miranda, H.C.; de Sant’Ana, H.B.; Farias, J.P. Austenitic and ferritic stainless steel dissimilar weld metal evaluation for the applications as-coating in the petroleum processing equipment. Mater. Des. 2013, 47, 1–8.
- Mas, F.; Tassin, C.; Valle, N.; Robaut, F.; Charlot, F.; Yescas, M.; Roch, F.; Todeschini, P.; Bréchet, Y. Metallurgical characterization of coupled carbon diffusion and precipitation in dissimilar steel welds. J. Mater. Sci. 2016, 51, 4864–4879.
- Lippold, J.C.; Kotecki, D.J. Welding Metallurgy and Weldability of Stainless Steels; Wiley-Interscience: New York, NY, USA, 2005.
- Thomas, W.M.; Nicholas, E.D.; Needham, J.C.; Murch, M.G.; Temple-Smith, P.; Dawes, C.J. International Patent Application PCT/GB92/02203 and GB Patent Application 9125978.8; UK Patent Office: London, UK, 1991; p. 6.
- Wang, Y.; Tsutsumi, S.; Kawakubo, T.; Fujii, H. Microstructure and mechanical properties of weathering mild steel joined by friction stir welding. Mater. Sci. Eng. A 2021, 823, 141715.
- Ibrahim, I.R.; Khedr, M.; Mahmoud, T.S.; Abdel-Aleem, H.A.; Hamada, A. Study on the mechanical performance of dissimilar butt joints between low Ni medium-Mn and Ni-Cr austenitic stainless steels processed by gas tungsten arc welding. Metals 2021, 11, 1439.
- Suman, S.; Biswas, P. Comparative study on SAW welding induced distortion and residual stresses of CSEF steel considering solid state phase transformation and preheating. J. Manuf. Process. 2020, 51, 19–30.
- Meng, X.; Huang, Y.; Cao, J.; Shen, J.; dos Santos, J.F. Recent progress on control strategies for inherent issues in friction stir welding. Prog. Mater. Sci. 2021, 115, 100706.
- Meng, X.; Xie, Y.; Ma, X.; Liang, M.; Peng, X.; Han, S.; Kan, L.; Wang, X.; Chen, S.; Huang, Y. Towards friction stir remanufacturing of high-strength aluminum components. Acta Metall. Sin. Engl. Lett. 2022, 1–12.
- Sunilkumar, D.; Muthukumaran, S.; Vasudevan, M.; Reddy, G.M. Microstructure and mechanical properties relationship of friction stir-and A-GTA-welded 9Cr-1Mo to 2.25 Cr-1Mo steel. J. Mater. Eng. Perform. 2021, 30, 1221–1233.
- Sunilkumar, D.; Muthukumaran, S.; Vasudevan, M.; Reddy, M.G. Effect of friction stir and activated-GTA welding processes on the 9Cr–1Mo steel to 316LN stainless steel dissimilar weld joints. Sci. Technol. Weld. Join. 2020, 25, 311–319.
- Abd-Elaziem, W.; Khedr, M.; Newishy, M.; Abdel-Aleem, H. Metallurgical characterization of a failed A106 Gr-B carbon steel welded condensate pipeline in a petroleum refinery. Int. J. Press. Vessel. Pip. 2022, 200, 104843.
- Wang, Z.W.; Xie, G.M.; Wang, D.; Zhang, H.; Ni, D.R.; Xue, P.; Xiao, B.L.; Ma, Z.Y. Microstructural evolution and mechanical behavior of friction-stir-welded DP1180 advanced ultrahigh strength steel. Acta Metall. Sin. Engl. Lett. 2020, 33, 58–66.
- Sekban, S.M.; Xue, P.; Ma, Z.Y.; Purcek, G. Shipbuilding. Mater. Sci. Eng. A 2016, 672, 40–48.
- Venkatakrishna, A.; Lakshminarayanan, A.; Vasantharaja, P.; Vasudevan, M. Decisive impact of Filler-free joining processes on the Microstructural evolution, tensile and impact properties of 9Cr-1Mo-V-Nb to 316 L (N) dissimilar joints. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2022, 236, 2408–2427.
- Sunilkumar, D.; Muthukumaran, S.; Vasudevan, M.; Reddy, M. Tool rotational speed variant response on the evolution of microstructure and its significance on mechanical properties of friction stir welded 9Cr-1Mo steel. J. Mater. Process. Technol. 2020, 278, 116536.
- Wang, H.; Wang, K.; Wang, W.; Huang, L.; Peng, P.; Yu, H. Microstructure and mechanical properties of dissimilar friction stir welded type 304 austenitic stainless steel to Q235 low carbon steel. Mater. Charact. 2019, 155, 109803.
- Rai, R.; De, A.; Bhadeshia, H.K.D.H.; Debroy, T. Review: Friction stir welding tools. Sci. Technol. Weld. Join. 2011, 16, 325–342.
- Ghosh, M.; Kumar, K.; Mishra, R. Friction stir lap welded advanced high strength steels: Microstructure and mechanical properties. Mater. Sci. Eng. A 2011, 528, 8111–8119.
- Sunilkumar, D.; Muthukumaran, S.; Vasudevan, M.; Selvi, S.P.; Reddy, M.G. Effect of tool rotation speed on microstructure and hardness of friction-stir-welded 9Cr-1Mo steel. Trans. Indian Inst. Met. 2019, 72, 1537–1540.
- Wang, X.; Gao, Y.; McDonnell, M.; Feng, Z. Determination of the friction stir welding window from the solid-state-bonding mechanics under severe thermomechanical conditions. Materialia 2022, 21, 101350.
- Wang, X.; Gao, Y.; Liu, X.; McDonnell, M.; Feng, Z. Tool-workpiece stick-slip conditions and their effects on torque and heat generation rate in the friction stir welding. Acta Mater. 2021, 213, 116969.
- Sekban, D.; Aktarer, S.; Purcek, G. Friction stir welding of low-carbon shipbuilding steel plates: Microstructure, mechanical properties, and corrosion behavior. Metall. Mater. Trans. A 2019, 50, 4127–4140.
- Ramesh, R.; Dinaharan, I.; Kumar, R.; Akinlabi, E. Microstructure and mechanical characterization of friction stir welded high strength low alloy steels. Mater. Sci. Eng. A 2017, 687, 39–46.




