Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicholas Dunne | -- | 1494 | 2023-01-04 11:59:10 | | | |
| 2 | Conner Chen | + 1 word(s) | 1495 | 2023-01-05 01:43:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sargioti, N.; Levingstone, T.J.; O’cearbhaill, E.D.; Mccarthy, H.O.; Dunne, N.J. Types of Microneedle Arrays. Encyclopedia. Available online: https://encyclopedia.pub/entry/39727 (accessed on 08 March 2026).
Sargioti N, Levingstone TJ, O’cearbhaill ED, Mccarthy HO, Dunne NJ. Types of Microneedle Arrays. Encyclopedia. Available at: https://encyclopedia.pub/entry/39727. Accessed March 08, 2026.
Sargioti, Nikoletta, Tanya J. Levingstone, Eoin D. O’cearbhaill, Helen O. Mccarthy, Nicholas J. Dunne. "Types of Microneedle Arrays" Encyclopedia, https://encyclopedia.pub/entry/39727 (accessed March 08, 2026).
Sargioti, N., Levingstone, T.J., O’cearbhaill, E.D., Mccarthy, H.O., & Dunne, N.J. (2023, January 04). Types of Microneedle Arrays. In Encyclopedia. https://encyclopedia.pub/entry/39727
Sargioti, Nikoletta, et al. "Types of Microneedle Arrays." Encyclopedia. Web. 04 January, 2023.
Copy Citation
Microneedle (MN) arrays are minimally-invasive devices that can penetrate the stratum corneum, one of the most important barriers for topically-applied drugs, thus creating a pathway for drug permeation to the dermal tissue below. MN arrays can be characterized as: (1) solid, (2) coated, (3) hollow and (4) dissolvable. They can be further categorized based on their mode of drug delivery, and the materials used for their manufacture.
micro-sized needles
solid microneedles
metallic microneedles
1. Introduction
There are many approaches currently used for the delivery of drugs and therapeutic agents including oral administration, conventional hypodermic needles, topical creams and transdermal patches [1]. Oral drug delivery is considered one of the most desired routes of administration when compared to other routes due to high patient compliance, cost-effectiveness, less sterility constraints, flexibility in dosage delivery and the ease of production. However, it results in the poor bioavailability of drugs due to factors relating to dissolution, permeability, and solubility [2]. Conventional hypodermic needles can cause pain to the patient as they penetrate deep into the dermis where pain receptors are present. Their use is particularly challenging for needle phobic patients. The traditional use of subcutaneous injection for the delivery of macromolecules also has safety concerns for healthcare workers as needle stick injuries are a common occurrence. In some cases, subcutaneous injections are expensive as there may be a need for multiple or chronic administration by trained medical professionals [3]. The topical application and administration of drugs, using a topical cream, gel or ointment or a non-invasive transdermal patch, allows for the penetration of the drug into the skin without pain [4]. These topical methods have a limited ability to administer drugs with large particles (e.g., nucleic acids, large drug molecules) as the stratum corneum layer of the skin acts as a natural barrier [5]. The ability of a drug to penetrate the skin is influenced by the skin physiology and permeability, and various other factors including the physiochemical properties of the drug (i.e., size, molecular weight, concentration, partition coefficient and solubility) and formulation characteristics (i.e., release rate, ingredients and the presence of permeation enhancer) [6]. Additionally, the administration of ionic drugs, drugs of high concentrations or with very low/high partition coefficient can create problems such as skin irritation, non-systematic circulation and poor permeability [7]. Overall, while these topical methods have the advantage of being painless, they lack bioavailability and can lead to skin irritations, allergic reactions or non-controlled drug release [8].
As a result of the disadvantages of existing techniques, there is an increasing imperative for innovative methods for the delivery of therapeutic agents. For this reason, many studies have focused on the investigation of microneedle (MN) arrays as transdermal drug delivery (TDD) systems. MN arrays are minimally-invasive devices that can penetrate the stratum corneum, one of the most important barriers for topically-applied drugs, thus creating a pathway for drug permeation to the dermal tissue below [9]. MN arrays can enhance skin permeability compared to non-invasive patches enabling a faster onset of action and good bioavailability. The use of minimally invasive MN-based transdermal patches for TDD offers several important advantages over traditional drug delivery methods. These advantages include: (1) easy and controlled drug delivery; (2) the enhancement of therapeutic efficiency with fewer side effects; (3) less pain than with traditional hypodermic needles; and (4) the maintenance of a steady plasma level of the drug [10][11]. To date, MN arrays have been used in several biomedical applications including diabetes treatment [12], cancer diagnosis and therapy [13], for infections, inflammation and chronic pain treatment and the treatment and control of obesity [14], and also for other applications including the sampling of blood and interstitial fluids [15]. However, current MN-based TDD systems have associated limitations including incomplete insertion, particularly for polymeric MNs, which results in limited drug delivery efficiency and the wastage of valuable medication [16].
Metallic MN offer potential to overcome the challenges associated with polymeric MNs systems. However, several existing challenges limiting the translation of metallic MN arrays as a successful TDD systems remain, including: (1) current methods for metallic MN array fabrication involve a multi–step process that is not cost-effective; (2) the lack of clinical data relating to cytotoxicity of the metals used for MN fabrication; (3) limited drug loading; and (4) challenges in maintaining mechanical properties and piercing capacity.
2. Types of Microneedle Arrays
MN arrays can be characterized as: (1) solid, (2) coated, (3) hollow and (4) dissolvable (Figure 1) [17][18][19]. They can be further categorized based on their mode of drug delivery, and the materials used for their manufacture [20]. In general, there are four different modes of drug delivery: (1) ‘poke–detach–diffuse’ for solid MNs (Figure 2a); (2) ‘coat and poke’ for solid coated MNs (Figure 2b); (3) ‘poke and flow’ for hollow MNs (Figure 2c); and (4) ‘poke and release’ using dissolvable MNs (Figure 2d) [21]. The ‘poke-detach-diffuse’ method involves the use of solid MNs to create micro-channels through the epidermis into the dermis [14]. After the removal of the MN system, the drug formulation is applied to the skin surface by applying topical creams or transdermal patches and the drug is delivered through the created micro-channels [17]. Coated MN systems are solid MNs coated with a particular drug formulation. They deliver the drug during the insertion of the needles into the skin, termed the ‘coat and poke’ method [14]. The coating of the MNs can be achieved by dipping or spraying the surface of the solid MNs with the solubilized drug [22]. For coated MNs, following the penetration of the MN into the skin, the delivery of the drug is achieved by the dissolution of the coating which allows diffusion of the drug and the MNs are subsequently removed [22]. Solid MNs can be fabricated from metals (e.g. stainless steel or titanium), ceramics (silicon) and polymers (poly D, L-lactic-co-glycolic acid (PLGA) and poly-ethylene glycol (PEG)) using different fabrication methods [14][21][23]. The length and the shape of the channels formed depend on the needle geometry and design. Martiano et al. used triangular shape stainless steel MNs (height = 1 mm, width = 0.2 mm) for the TDD of insulin based on the ‘poke–detach–diffuse’ method [24]. Their study demonstrated the potential for effective TDD of macromolecular drugs using solid MNs. The “coat and poke” method has also been used with titanium solid MNs of 330 µm height in an area of 1 cm2 coated with protein antigen for vaccine delivery [24]. Their study demonstrated rapid and reproducible intracutaneous administration of dry-coated antigen [24].
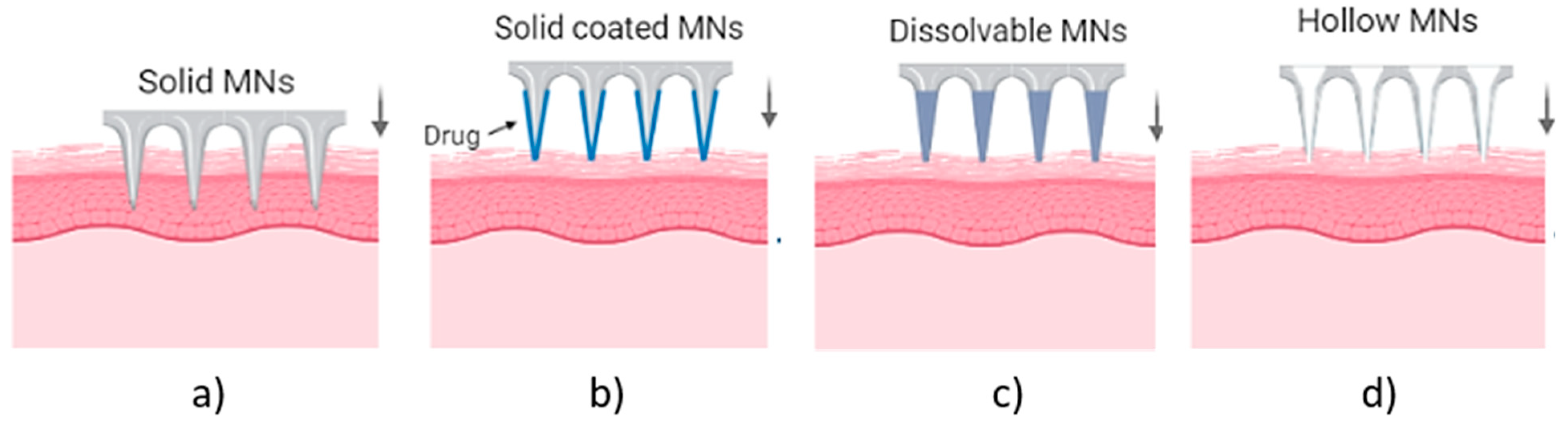
Figure 1. Types of MN array. (a) solid MNs, that require a transdermal patch for continuous drug administration after their insertion/removal, (b) coated, where the drug is coated around the needles, (c) hollow MNs for the creation of a path to administer the drug using conventional needles and (d) dissolvable that are filled with the drug and fully dissolved following skin insertion [14].
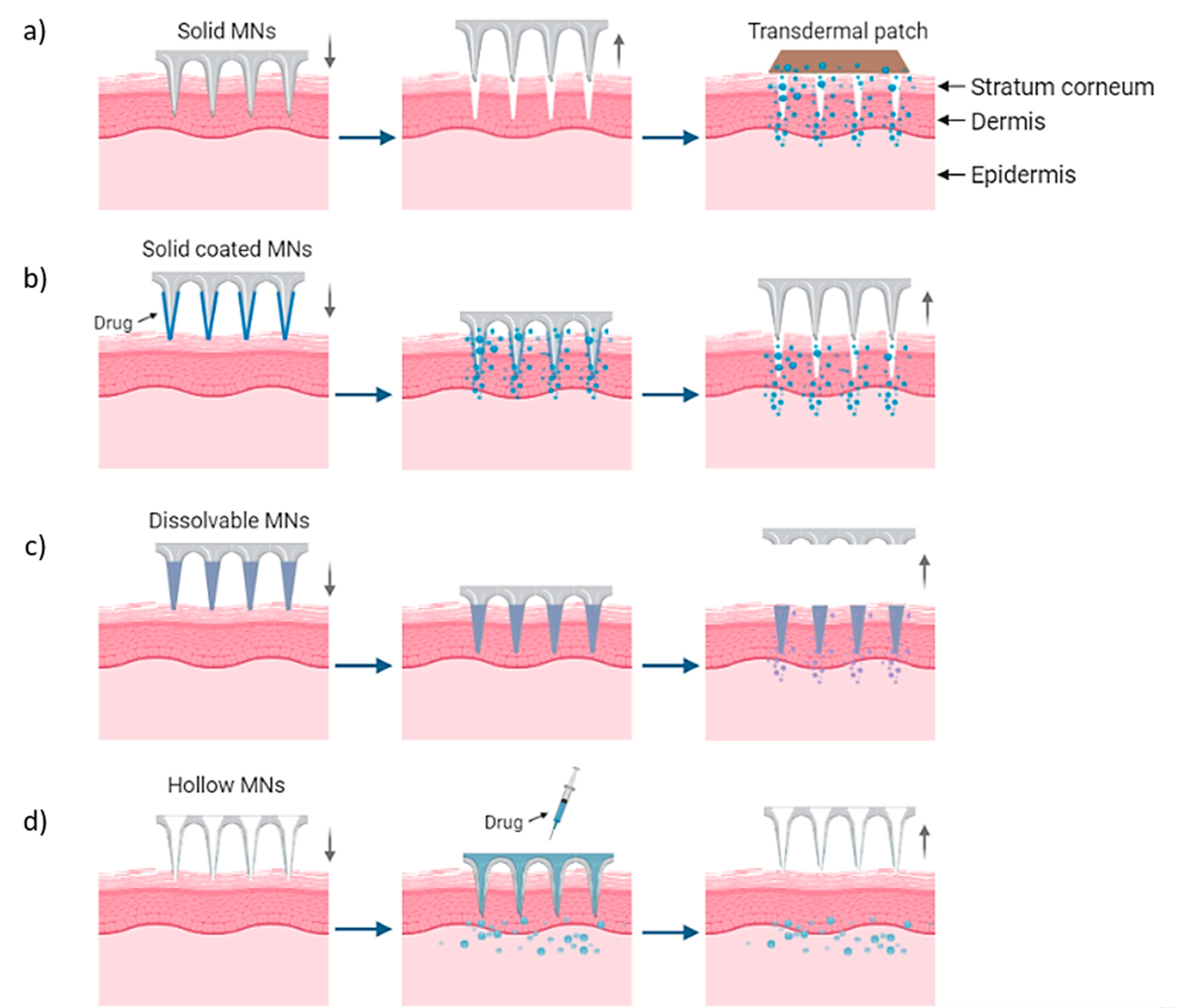
Figure 2. Delivery procedures of solid MNs through the (a) ‘poke-detach-diffuse’ method, in which the solid MNs are used only for the creation of holes and the drug administration is provided by a transdermal patch and (b) ‘coat and poke’ method where the drug is coated on the needles and the drug administration is provided from the MNs. (c) “Poke and flow” method in which the drug is inserted through hollow MNs and delivered in the body and (d) “poke and release” method for dissolvable MNs which are inserted and dissolved in the skin [14].
More recently, porosity have been introduced within the structure of solid MNs, made by metals, polymers or ceramics, to enhance their ability to delivery drugs and therapeutic agents [25]. These porous MNs have different percentages of porosity, ranging between ~30% to 40% with average pore diameter 1.3 μm to 2.22 μm within their structure offering the unique ability to absorb drugs within their pores and release them upon insertion into the skin [26][27][28]. While these MNs show potential for enabling improved TDD, the volume of voids within the structure can result in MN tip collapse due to the porous structure [26]. In particular, the porosity reduced the strength to only 2 N compressive force for titanium MNs and 0.6 N for polymeric MNs. In addition to the decrease of mechanical properties, decrease of tip sharpness was also observed with increased fragility during the fabrication process [27][29]. Thus, further optimization of the selected particle size of the powder material and the pore diameter of the final part, is required to achieve porous MNs that meet the clinical requirements [26].
Hollow MN systems contain an empty cavity within the MN and a bore at the tip. Drug delivery is achieved through the ‘coat and flow’ method whereby micro-volumes of a drug can be delivered directly into the skin. They can deliver relatively large amounts of drugs with higher accuracy in dose, directly to the skin [14][17]. Hollow MNs are typically fabricated from metals and ceramics with similar fabrication techniques as used for solid MN arrays [20]. The final type of MN systems are dissolvable MNs, which are biodegradable and can be manufactured from water-soluble materials or degradable polymers [19]. The matrix of the MNs contains the drug and has sufficient mechanical stability to enable insertion into the skin, therein the matrix dissolves and the drug is released as a consequence, thus achieving drug delivery via the ‘poke and release’ method [20].
References
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258.
- Viswanathan, P.; Muralidaran, Y.; Ragavan, G. Challenges in oral drug delivery: A nano-based strategy to overcome. In Nanostructures for Oral Medicine; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323477215.
- Cross, S.; Roberts, M. Physical Enhancement of Transdermal Drug Application: Is Delivery Technology Keeping up with Pharmaceutical Development? Curr. Drug Deliv. 2005, 1, 81–92.
- Kaur, L.P.; Guleri, T.K. Topical Gel: A Recent Approach for Novel Drug Delivery. Asian J. Biomed. Pharm. Sci. 2013, 3, 1–5.
- Patil, P.; Datir, S.; Saudagar, R. A Review on Topical Gels as Drug Delivery System. J. Drug Deliv. Ther. 2019, 9, 661–668.
- Chander Jhawat, V.; Saini, V.; Kamboj, S.; Maggon, N. Transdermal drug delivery systems: Approaches and advancements in drug absorption through skin. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 47–56.
- Chhatrani, B.M.; Shah, D.P.; Lalbhai, N. Naranjibhai A Review on Microemulsion Based Gel: A Novel Approach for Enhancing Topical Delivery of Hydrophobic Drug. Int. J. Pharm. Pharm. Res. 2017, 8, 19–35.
- Patel, D.; Chaudhary, S.A.; Parmar, B.; Bhura, N. Transdermal Drug Delivery System: A Review. Pharma Innov. 2012, 1, 66.
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv. Funct. Mater. 2012, 22, 4879–4890.
- Arunachalam, A.; Karthikeyan, M.; Kumar, D. Review Article Current Pharma Research Transdermal Drug Delivery System: A Review. J. Curr. Pharma Res. 2010, 1, 70–82.
- Shingade, G.M. Review on: Recent Trend on Transdermal Drug Delivery System. J. Drug Deliv. Ther. 2012, 2, 66–75.
- Khanna, P.; Strom, J.A.; Malone, J.I.; Bhansali, S. Microneedle-based automated therapy for diabetes mellitus. J. Diabetes Sci. Technol. 2008, 2, 1122–1129.
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Microneedle-based delivery devices for cancer therapy: A review. Pharmacol. Res. 2019, 148, 104438.
- Hao, Y.; Li, W.; Zhou, X.L.; Yang, F.; Qian, Z.Y. Microneedles-based transdermal drug delivery systems: A review. J. Biomed. Nanotechnol. 2017, 13, 1581–1597.
- Makvandi, P.; Kirkby, M.; Hutton, A.R.J.; Shabani, M.; Yiu, C.K.Y.; Baghbantaraghdari, Z.; Jamaledin, R.; Carlotti, M.; Mazzolai, B.; Mattoli, V.; et al. Engineering Microneedle Patches for Improved Penetration: Analysis, Skin Models and Factors Affecting Needle Insertion; Springer: Singapore, 2021; Volume 13, ISBN 0123456789.
- Chen, M.C.; Ling, M.H.; Kusuma, S.J. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015, 24, 106–116.
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.J.; Woolfson, A.D. Microneedles: Design, Microfabrication and Optimization. In Microneedle-Mediated Transdermal and Intradermal Drug Delivery; Wiley-Blackwell: New York, NY, USA, 2012; pp. 20–56.
- Donnelly, R.F.; Raj Singh, T.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207.
- Tuan-Mahmood, T.M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637.
- Dharadhar, S.; Majumdar, A.; Dhoble, S. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201.
- He, X.; Sun, J.; Zhuang, J.; Xu, H.; Liu, Y.; Wu, D. Microneedle System for Transdermal Drug and Vaccine Delivery: Devices, Safety, and Prospects. Dose-Response 2019, 17, 1559325819878585.
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483.
- Zhou, C.P.; Liu, Y.L.; Wang, H.L.; Zhang, P.X.; Zhang, J.L. Transdermal delivery of insulin using microneedle rollers in vivo. Int. J. Pharm. 2010, 392, 127–133.
- Matriano, J.A.; Cormier, M.; Johnson, J.; Young, W.A.; Buttery, M.; Nyam, K.; Daddona, P.E. Macroflux® microprojection array patch technology: A new and efficient approach for intracutaneous immunization. Pharm. Res. 2002, 19, 63–70.
- Bao, L.; Park, J.; Bonfante, G.; Kim, B. Recent advances in porous microneedles: Materials, fabrication, and transdermal applications. Drug Deliv. Transl. Res. 2022, 12, 395–414.
- Cahill, E.M.; Keaveney, S.; Stuettgen, V.; Eberts, P.; Ramos-Luna, P.; Zhang, N.; Dangol, M.; O’Cearbhaill, E.D. Metallic microneedles with interconnected porosity: A scalable platform for biosensing and drug delivery. Acta Biomater. 2018, 80, 401–411.
- Li, J.; Liu, B.; Zhou, Y.; Chen, Z.; Jiang, L.; Yuan, W.; Liang, L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS ONE 2017, 12, e0172043.
- van der Maaden, K.; Luttge, R.; Vos, P.J.; Bouwstra, J.; Kersten, G.; Ploemen, I. Microneedle-based drug and vaccine delivery via nanoporous microneedle arrays. Drug Deliv. Transl. Res. 2015, 5, 397–406.
- Aldawood, F.K.; Andar, A.; Desai, S. A comprehensive review of microneedles: Types, materials, processes, characterizations and applications. Polymers 2021, 13, 2815.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Revisions:
2 times
(View History)
Update Date:
05 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





