Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Nikolova | -- | 10404 | 2023-01-04 09:01:13 | | | |
| 2 | Camila Xu | Meta information modification | 10404 | 2023-01-04 09:28:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nikolova, M.P.; Apostolova, M.D. Advances in Multifunctional Bioactive Coatings. Encyclopedia. Available online: https://encyclopedia.pub/entry/39713 (accessed on 07 February 2026).
Nikolova MP, Apostolova MD. Advances in Multifunctional Bioactive Coatings. Encyclopedia. Available at: https://encyclopedia.pub/entry/39713. Accessed February 07, 2026.
Nikolova, Maria P., Margarita D. Apostolova. "Advances in Multifunctional Bioactive Coatings" Encyclopedia, https://encyclopedia.pub/entry/39713 (accessed February 07, 2026).
Nikolova, M.P., & Apostolova, M.D. (2023, January 04). Advances in Multifunctional Bioactive Coatings. In Encyclopedia. https://encyclopedia.pub/entry/39713
Nikolova, Maria P. and Margarita D. Apostolova. "Advances in Multifunctional Bioactive Coatings." Encyclopedia. Web. 04 January, 2023.
Copy Citation
This entry outlines the main materials used for metal implant manufacturing and some general coating techniques. It focuses on recent trends in the design and performance of biomedical coatings for metallic implants used for orthopedic and dental applications. The ways of improving the bioactive coating performance by incorporating bioactive moieties such as growth factors, osteogenic factors, immunomodulatory factors, antibiotics, or other drugs that are locally released in a controlled manner have also been addressed. The influence of the bioactive films on in vitro behavior of cells cultured on coated implants and in vitro/in vivo performance of the implant systems is underlined.
metallic implants
bioactive coatings
surface modifications
1. Bioactive Coatings
Surface modifications of metallic implants have been extensively explored to hinder a range of adverse effects such as long-term stability, low biomechanical properties, lack of biocompatibility, restriction of implant surface corrosion, post-surgery infections, etc. Therefore, the development and design of biometallics rely on surface modification since, by applying appropriate coatings, the surface properties can be tailored and improved. In connection with this, two approaches for surface modification have been applied: (a) direct coating deposition on the unmodified metal substrate; (b) initial substrate modification by grinding, sand-blasting, etching, or other treatment and deposition of overlaying coating. In the second case, improved surface roughness parameters for synergy of both textural properties and mechanical interlocking of coating are obtained.
The main requirements for a selection of coating material include (a) adequate mechanical reliability, adhesion strength and fracture toughness to withstand the applied forces; (b) corrosion resistance in the body fluid environment; (c) biocompatibility and lack of toxicity, allergic or other undesirable effects or inflammation [1]. Depending on their performance in the organism, the biomedical coatings can be subdivided into three main groups: bioinert, bioactive and bioresorbable [2]. In contrast to bioinert (such as Al2O3 and ZrO2), bioactive coatings refer to biomaterials that can stimulate the surrounding tissue and cells to regenerate around the exogenous graft and to release bioactive molecules for elimination the post-operative complications [3]. Absorbable (bioresorbable) coatings are designed to degrade in the human body via an electrochemical mechanism of dissolution and then metabolized by cells and tissue [4].
2. Inorganic Coatings
Various research groups focus on the development of inorganic coatings for biomedical applications because of their stability in the body environment, good mechanical properties, corrosion and wear resistance. To be called “bioactive”, these coatings should have surface-located functional groups that in an aqueous solution create conditions favoring heterogeneous mineral nucleation and growth on the surface. However, some of these inorganic coatings have shown disadvantages, including the release of toxic ions, cytotoxicity, lack of biodegradability, low bonding strength, etc. Some recent studies on the wide range of such inorganic coating materials are reviewed in the next sections.
2.1. Nitrides
Various transitional metal nitride coatings such as TiN, ZrN, NbN, TaN, etc. have been studied as protective films against wear and corrosion of medical metal surfaces of the prosthesis and surgical implants [5]. Nitride films have a high melting point, chemical resistance to oxidation and acceptable adhesion [6]. Titanium nitride (TiN) films are often used in industry because of their high surface hardness and chemical properties. For biomedical applications, TiN was found to be well tolerated by tissue due to its inertness [7]. On orthopedic implants, nitride coatings protect the surface against wear and act as a diffusion barrier, preventing ion release from the metal to the body fluids [8]. Compared to other nitrides, TiN shows better biological properties for orthopedic applications [9]. However, because of dissimilarities in the hardness of TiN coating and substrate, plastic deformation at the coating/substrate interface occurs resulting in the formation of flakes, defects in the coating and fracture [10]. Compared with CVD-deposited TiN coatings, PVD utilizes a higher deposition rate and delivers improved bonding strength [11]. To improve the adhesion strength and wear performance “hard” nano-TiN and “soft” Ti4N3−x transitional phase with variable composition was prepared by DC magnetron sputtering on Ti6Al4V alloy [12]. The coating showed excellent bonding and wear resistance because of the match of the mechanical properties at the substrate/coating interface together with good biocompatibility. Except for higher resistance to plastic deformation and improved wear behavior than the bare Ti20Nb13Zr (TNZ) alloy, TiN coating deposited by the cathodic arc PVD process indicated better corrosion resistance in SBF than TNZ [13]. Compared to bare Ti substrate, TiN coating showed approximately eight times more corrosion resistance and 4 times more wear resistance [14]. For that reason, sputtered TiN was used as an interior layer between HAp films and pure Ti rendering the controllability of thin film structural properties because of the improvement in bonding, strong fatigue resistance and better mechanical performance of the HAp layer [15].
Because the release of metal ions such as nickel, cobalt and chromium may cause serious problems in joint replacement due to metal hypersensitivity, considerable attention was also paid to PVD deposited ternary nitrides such as TiNbN that can act as a surface coat to hide the metal beneath affording an immuno-privileged state [16]. Copper-doped TiN (TiCuN) deposited by axial magnetic field enhanced arc ion plating has proved to own excellent corrosion resistance, wear resistance and good antibacterial properties and displayed no cytotoxic effect on human umbilical vein endothelial cells [17]. Compared with TiN coating, TiCuN promoted mRNA expression of endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF), enhancing cell migration and angiogenesis ability. Similarly, ZrN/Cu coating deposited on SS and Ti substrate enhanced their wear- and corrosion resistance in SBF and their antibacterial activity by using ion release and a contact killing mechanism [18].
Similar to other transitional metals, Ta has a high affinity to nitrogen and forms inert TaN. TaN is also a commonly used material for the production of hard thin coatings because of its chemical inertness, corrosion resistance [19] and biocompatibility [20]. Mendizabal et al. discovered that the highest corrosion resistance was observed for Ta and low nitrogen content TaNx films (lower than at30%) when deposited on AISI 316L steel by modulated pulse power magnetron sputtering [21]. The excessive amount of nitrogen on the film worsened its corrosion protection. Deposited on Ti substrate, the magnetron sputtered TaN film exhibited stronger bonding properties than TiN, and optimal compressive performance [22]. Deposited on Mg alloy by reactive magnetron sputtering, TaN exhibited a 95-fold decrease in corrosion current density in SBF solution compared to uncoated Mg-Y-Re alloy [23]. Additionally, the incorporation of Ag and Cu in TaN nanocomposite films gave the condensates anti-bacterial and anti-wear properties [24].
In contrast to crystalline TiN and TaN, amorphous SiN was also shown to be biocompatible and slow dissolving in a water-based solution [25][26]. It also has high hardness (up to 26 GPa) and Young modulus (up to 212 GPa), low wear rates, acts as a barrier for ion release from the metal and generates biocompatible wear particles [27]. However, some challenges such as inadequate adhesion and high dissolution rate that can reduce scratch resistance and cause premature coating failure are still faced by these coatings [28]. By alloying SiN with Fe and C in the Si-Fe-C-N system [29] or with Nb and Cr in the Si-Nb-Cr-N system [30], better adhesion, optimized dissolution rate and ion release can be obtained without compromising the adherence and morphology of MC3T3 or L929 cells. Overall, the composite coatings have low surface roughness, high hardness and elastic modulus and no evident cytotoxicity as opposed to SiN controls and CoCrMo alloy. With the increase of Cr between 4 to 6 at% the release of Si, Cr and Nb ions and the dissolution rate of the coating reduced, while the cell viability was reduced.
2.2. Oxides
TiO2 is an important material in biomedical applications, since it has good mechanical properties, antibacterial and catalytic activity and long-term stability under photo- and chemical corrosion [31]. TiO2 can promote the formation of bone-like apatite or calcium phosphate on its surface when soaked in SBF solution which makes it suitable for bone reconstruction and replacement [32]. Moreover, it was found that the formation of TiO2 coating by anodization on the surface of Ti substrates was an effective method to reduce the temperature rise of the implant during microwave diathermy treatment that would provide a potential rehabilitation solution to internal fixation of bone fracture [33]. Similar to TiO2, tantalum oxide (Ta2O5) could facilitate the formation of bone-like apatite and stimulate the rapid attachment of bone and soft tissue [20]. Tantalum oxide produced by reactive magnetron sputtering was able to enhance both early-stage corrosion resistance and in vitro biocompatibility of Mg alloy [34]. Ta2O5 coating with 12.5 at% Ag deposited by a twin-gun magnetron sputtering system exhibited both improved antibacterial effects against S. aureus and good skin fibroblast cell cellular biocompatibility [35].
Grafting metal ions and compounds is also a common method to improve the osteogenic ability of oxide coatings, since various metal ions (Ca2+, Sr2+, Mg2+, etc.) have been demonstrated to possess the property of enhancing osseointegration [36]. An illustration of the influence of different metallic ions on various processes involved in bone regeneration is presented in Figure 1. Zhao and co-authors used MAO to produce Mn-TiO2 coatings on the Ti surface that showed good biocompatibility and osteogenic properties while the Mn2+ release from the coating promoted surface biomineralization [37]. TiO2 nanotubes produced by anodizing and loaded with Sr combined with icariin (ICA) showed a better effect on cell adhesion, proliferation and higher mineralization activity than pure Ti and TiO2 coatings. Furthermore, in osteoporotic rats, more bone was formed around the implants loaded with Sr and ICA [38]. Similarly, Y-doped TiO2 coatings on Ti6Al4V produced by PEO demonstrated good biocompatibility on osteoblastic precursor cells and fibroblast cells with increasing doping concentration of yttrium and excellent antibacterial activity against E. coli and S. aureus [39]. MAO processed Fe2O3/TiO2 composite coating on Ti implants with sensitivity to the micro-magnetic field because of super-para-magnetism, which was able to enhance fibroblast response including proliferation, phenotype and extracellular collagen secretion by increasing the amount of Fe2O3 NPs [40]. Compared to pure TiO2 coatings, Fe2O3 (4.41 wt% Fe)/TiO2 composite coatings reduced bacterial growth by 60% and efficiently prevented recession and inflammatory reaction of soft tissue. By immobilizing anionic polypeptides on the surface of TiO2 nanospike coating by coordination, Gao et al. demonstrated that the obtained film was able to kill pathogenic bacteria, inhibit biofilm formation for up to 2 weeks and promote the formation of HA on the surface [41].
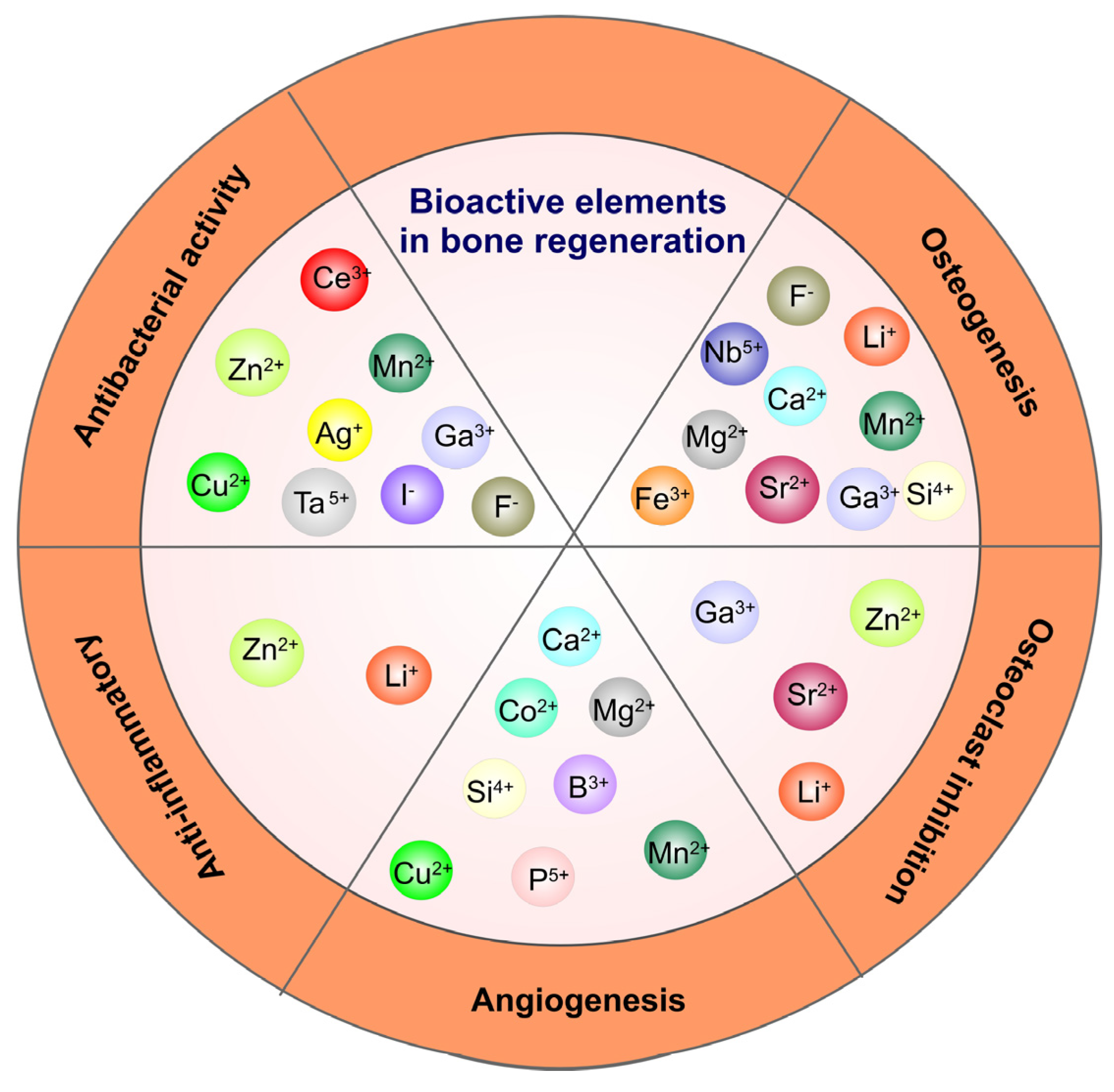
Figure 1. The therapeutic impact of some ions with angiogenic, osteogenic, anti-inflammatory and anti-bacterial activity.
TiO2 can not only be used to promote osseointegration but, also as a photosensitizer. As a stable photocatalyst, TiO2 produces ROS to kill bacteria under UV radiation but UV light is harmful to the body. For that reason, Nagay et al. prepared N- and Bi-codoped TiO2 coating on Ti by PEO that produced ROS to kill microorganisms under visible light [42]. By embedding silver (Ag) and zinc (Zn) nanoparticles into a 3D printed porous titanium oxide layer, the surface promoted the release of Ag+ and Zn2+ which favored antibacterial effect and osteogenesis, respectively [43]. Moreover, this synergetic effect was able to reduce the toxicity of Ag to the host cell. In contrast to bare Ti substrate and undoped TiO2, Ag-doped TiO2 coatings produced by sol-gel technology enhanced the corrosion resistance of Ti in SBF solution [44]. Nanoclusters of Ag incorporated in silica coatings obtained by RF co-sputtering technique displayed both good adhesion on steel substrate and antibacterial activity against S. aureus [45].
Aiming at modifying the surface of TiO2 to generate reactive oxygen species (ROS) to eradicate bacteria under near-infrared (NIR) light, different photosensitizers can be used. Chai et al. synthesized hydrothermally produced MoSe2 nanosheets on the surface of porous MAO-prepared TiO2 coatings and covered them with chitosan by electrostatic bonding to improve biocompatibility [46]. Under NIR irradiation because of the synergistic effect of hyperthermia and ROS generation, the coatings demonstrated excellent in vivo and in vitro antibacterial properties against S. mutans, whereas chitosan improved hydrophilicity and biocompatibility of the hybrid coating, promoting osseointegration even in the presence of infection under NIR light. Han and co-authors chose MoS2 with a broad spectral response to modify the surface of composite collagen/polydopamine/TiO2 coatings on Ti implants produced by MAO and hydrothermal treatment [47]. Under the combined action of photothermal and photodynamic therapy, the biofilm of S. aureus was quickly eradicated while the collagen promoted the adhesion and proliferation of osteoblasts. TiO2 nano-shovel/quercetin/L-arginine coatings doped with ytterbium (Yb) and erbium (Er) exhibited the production of ROS under near-infrared II light irradiation that could kill bacteria. At the same time, ROS catalyzed the release of nitrogen oxide (NO) free radicals from L-arginine which promoted angiogenesis and osseointegration [48]. The electrons and hole complexes generated by TiO2 reduced the photocatalytic properties, whereas the nano-shovel structure and quercetin that was coupled to the surface by organo-silanes, promoted the differentiation of bone marrow stem cells (BMSCs). Li et al. produced thermosensitive chitosan-glycerin-hydroxypropyl methylcellulose hydrogel (CGHH) to layer the top of simvastatin-loaded TiO2 nanotubes [49]. At 37 °C, the CGHH was found to be in a sol state which facilitated the controlled release of simvastatin to enhance MC3T3-E1 cell differentiation. The results of subcutaneous infection animal models indicated that CGHH had almost no antibacterial activity but, at high temperatures caused by infection, GCHH transitioned into a gel state and released a large amount of glycerin that induced acute inflammatory reaction and antibacterial activity against S. aureus and E. coli.
TiO2 can be used as a sound sensitizer to produce ROS by ultrasound-triggered electron-hole separation. Applying photoacoustic therapy sulfur-doped titanium oxide (S-TiO2-x) to titanium implants endowed the implant with good sonodynamic and photothermal properties [50]. Under NIR irradiation and ultrasound, the killing rate of S. aureus was equal to 99.995% after 15 min of exposure while the coating displayed good stability after soaking in water for 6 months.
The principle weakness of bio-ceramics originates from the low mechanical strength that makes them inappropriate for load-bearing application. When combined with metallic implants and bio-ceramic films, their mechanical properties are preserved while the integration with the bone is improved. However, the metal–ceramic interface accumulates residual stresses causing delamination at the interface.
2.3. Oxynitrides
To combine superior mechanical properties such as hardness and adhesion to the substrate and enhanced corrosion resistance, oxynitrides of transitional metals have been developed. Moreover, transitional metal nitride-oxide coatings are interesting materials because of their low degree of dissolution, corrosion resistance and inertness in body fluids [51]. The oxygen addition in cathodic arc deposited TiN decreased the grain size and enhanced the formation of a passive layer resulting in superior corrosion resistance in aggressive H2O2 augmented saline solution [52]. Sputtered TiNxOy coatings with chemical composition ranging from TiN to TiO2 deposited on microroughened titanium plates showed a significantly high level of bioactivity as compared to bare Ti substrates (1.2 up to 1.4 fold increase in cell proliferation) that made them biocompatible over a broad range of compositions [53]. Similar results were reported for TiNxOy coatings deposited on roughened SS [54] and CoCr alloy during the first two weeks of healing [55]. Therefore, in addition to the enhanced wear resistance of TiNxOy coatings, they can “isolate” the substrate metal from the bone and accelerate the effect on the growth of bone cells. For that reason, the research group synthesized gradient TiN/TiO2 coatings by a cathodic arc (for TiN) and glow discharge deposition (for TiO2) on an initial surface treated by electron beam Ti6Al4V alloy [56]. The initial electron beam treatment (EBT) of the alloy not only roughened the surface of the alloy forming regular grooves and heights but also enhanced the hardness of the substrate, thus generating a smooth gradient in stiffness from the substrate to the coating. Because of the decreased grain sizes and increased number of defects in the substrate, the EBT lowers the heat conductivity (λ) of the surface. Therefore, due to trapping heat near the surface, this initial substrate treatment not only improved the adhesion of TiN/TiO2 coating to the Ti6Al4V alloy but also triggered reorientation in the micro-volumes of the nitride and rutile to anatase ratio of the oxide (Figure 2), thus decreasing its surface hardness and bringing it closer to that of trabecular bone and human teeth and decreasing the elastic modulus mismatch between the bone and implant.
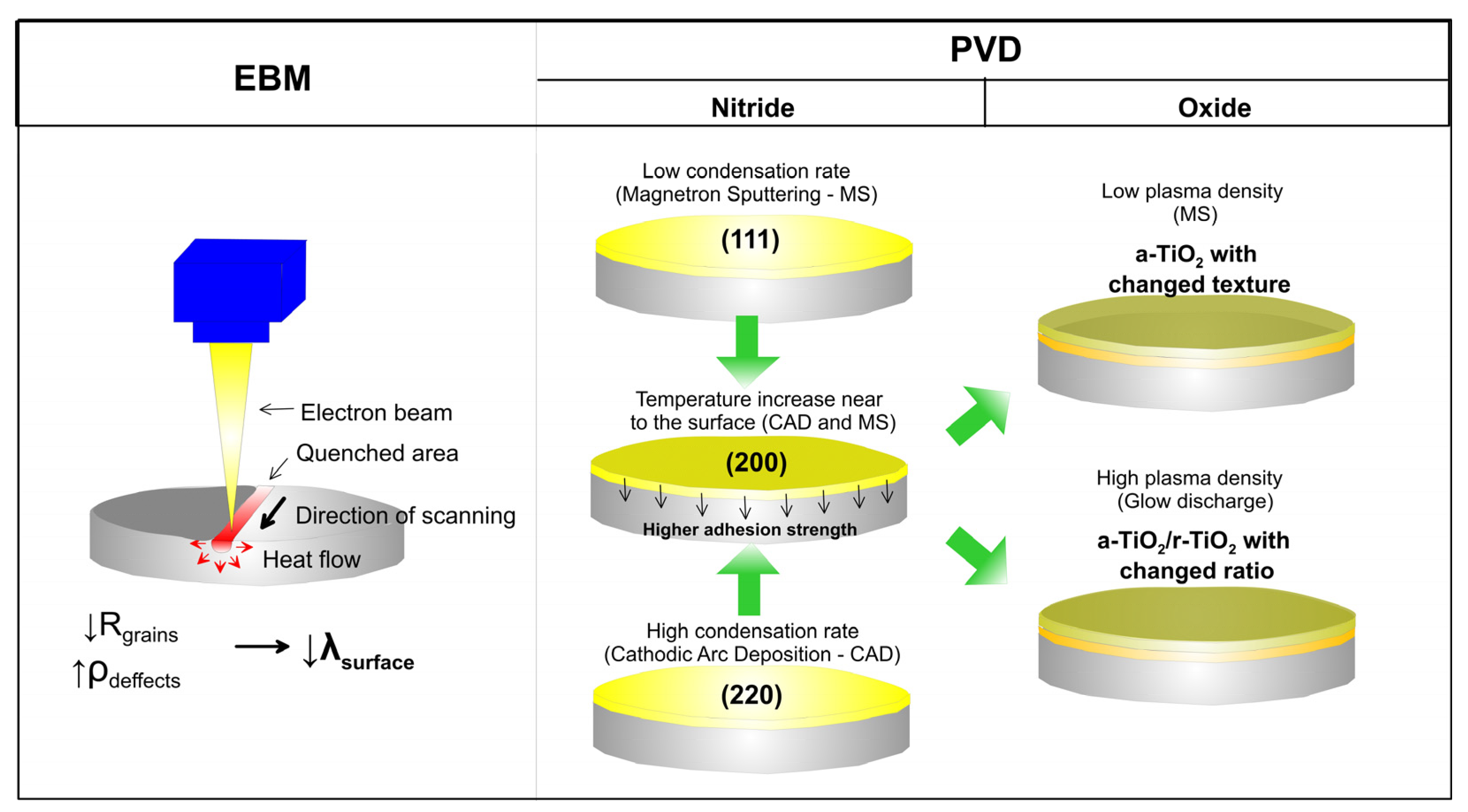
Figure 2. Schematic representation of the changes occurring after EBT of the metal surface and during deposition of the nitride/oxide coatings deposited by different methods.
Researchers observed similar reorientation of the micro volumes of the nitride and oxide and a decrease in microhardness after EBT for the magnetron sputtered TiN/TiO2 coating deposited on both Ti5Al4V alloy [57] and Co-Cr alloy [58]. The TiN/TiO2 coatings displayed adhesion bonding between the nitride and oxide layers [59] with substantially improved tribological performance and corrosion resistance as opposed to the Ti-Al-V substrate. Compared to the magnetron sputtered TiN/TiO2 coatings, those deposited by cathodic arc deposition (CAD) and glow discharge oxidation showed better human osteoblast-like cell (MG63) adhesion, viability and bone mineralization activity on both polished and EBT Ti6Al4V samples (Figure 3). This is because, in contrast to the less rough magnetron sputtered (MS) TiN/TiO2 coatings, on the surface of CAD and glow discharge oxidized coatings there are many surface elements for focal adhesion in the coating, such as droplet phase particles, disoriented crystallographic planes at the tips of oxide crystals, defects such as pores, etc., all of which support cell movement and proliferation. Simultaneously, cells cultured on the grooved surface with a smaller channel spacing (AR500) tended to have a stronger orientation along the groove axis compared to the AR850 surface with greater groove spacing. The results show that the deposited TiN/TiO2 coating on the micro-rough EBM surface stimulates and accelerates cell differentiation [56].
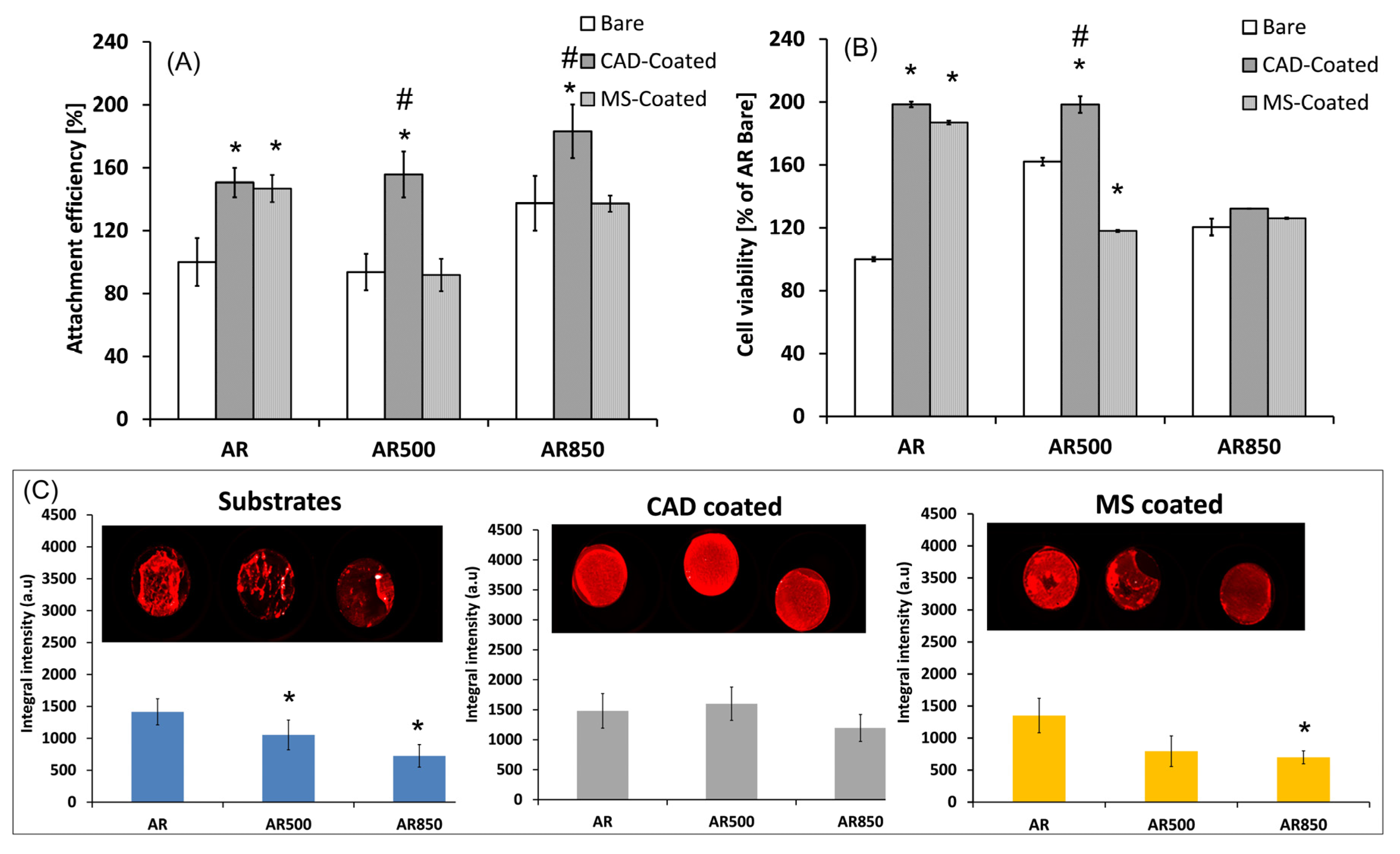
Figure 3. Cell adhesion after 6-h residence time. (A), cell viability for 24 h (B) and bone mineralization activity of MG63 osteoblasts after 31 days of incubation (C) with uncoated and TiN/TiO2 coated, polished and EBM samples of Ti64 in as-received (AR) state. Three independent studies of the cell culture samples were performed. Results are averaged and the standard deviation is indicated. * p < 0.05 compared to the polished uncoated AR sample; # p < 0.05 compared to the MS coated sample in the respective series.
Besides, magnetron-sputtered TiON and ZrON films on SS 316L substrates indicated a drastic reduction of bacterial adhesion (P. aeruginosa) as well as inhibition of biofilm formation at different time durations [60]. The bactericide activity of TiON and TiON-Ag sputtered films under visible light irradiation was reported by Rtimi et al. [61]. They stated that compared to TiON film which inactivated bacteria within 2 h, TiON-Ag coatings with Ag concentration below the cytotoxicity level showed faster and repetitive inactivation of E. coli. Similarly, ZrO2-Ag and ZrON-Ag coatings had lower bacterial retention while ZrON-Ag with porous structure and 11.8 at% Ag possessed the best antibacterial performance against S. aureus and A. actinomycetemcomitans together with excellent human gingival fibroblast (HGF) cell compatibility [62]. For magnetron-sputtered ZrON-Cu coatings, only the presence of CuO species caused bactericidal activity against S. epidermidis while Cu2+ ion release did not influence the antibacterial properties of the coating [63].
2.4. Carbon-Based Coatings
There are several types of carbon-based materials that are used for biomedical applications: (a) amorphous carbon nanostructures (diamond-like carbon (DLC), graphite-like carbon (GLC), pyrolytic carbon); (b) nanocrystalline diamond (NCD); (c) graphene and its derivates. Recent studies indicate that these carbon materials have exceptional biocompatibility, stability and mechanical properties [64][65]. Amorphous carbon with high sp3 content is referred to as diamond-like carbon (DLC) while higher sp2 content yields materials closer to graphite (graphite-like carbon). However, in contrast to graphite, the latter has higher hardness and high corrosion resistance. Pyrolytic carbon is also an amorphous carbon allotrope with dominating sp2 bonding. It is conventionally produced by CVD from gaseous hydrocarbon precursors. Depending on the process conditions, pyrolytic carbon coatings can have isotropic, granular, lamellar, columnar, etc., structures. Although mainly used for heart valve protection [66], pyrolytic coatings have been applied for the replacement of small joints such as knuckles, wrist joints and proximal interphalangeal joints [67].
DLC-based coatings are considered promising for bioimplant application because they have excellent mechanical properties, a low coefficient of friction and good wear resistance. For that reason, applied as coatings on Ti substrates by a CVD technique, the DLC film substantially improved the nano-hardness and tribological performance, decreasing the coefficient of friction by one order of magnitude [68][69]. The in vivo behavior of PVD-deposited DLC coatings on Ti substrates indicated no inflammatory reactions, confirming its good biocompatibility [70]. However, some disadvantages such as high internal stress, low toughness and high sensitivity to ambient conditions can be observed for a single layer of DLC coating that can explain the high revision rates of single-layered DLC-coated orthopedic joints [71]. Compared to TiN-coated joint prosthesis, DLC coatings demonstrated lower wear resistance [72]. To address this problem, multilayered coatings on Ti-6Al-4V alloy consisting of (a) alternating Zr and ZrN sublayers responsible for corrosion resistance and load carrying capacity, (b) overlaying Zr/DLC composite film for enhanced adhesion and reduced fatigue residual stresses and (c) top N-doped DLC to reduce friction and enhance, have been designed [73]. The resultant coatings showed a decreased coefficient of friction by more than 50% and two to three times increased hardness than that of bare Ti substrate. Except for a substantial decrease in wear, the middle layer improved the delamination strength, which is low in single DLC coatings. Additionally, fluorinated DLC coatings also exhibited good antibacterial properties against E. coli and S. aureus by decreasing their counts from 2.4 × 104 and 2.54 × 104 to less than 20, in contrast to two orders of magnitude growth of bacteria in the control groups [74]. At the same time, no substantial difference in cytotoxicity between the groups was observed confirming good biocompatibility of the coating. To improve the biocompatibility of magnetron-sputtered DLC coatings, Si-doping was also applied. Deposited on Ti6Al7Nb alloy, the addition of silicon up to 14–22 at% to the DLC coatings had a very positive effect on the proliferation and viability of endothelial cells [75]. Increasing the Si content resulted in a rise in the hydrophilic character of the coating, film hardness by up to 40% and reduced colonization by E. coli bacteria compared to the uncoated substrate [76]. Wachesk et al. deposited hybrid DLC coatings incorporating TiO2 nanoparticles by plasma-enhanced CVD on AISI 316 and implanted them in CF1 mice peritoneum [77]. The in vivo results showed that the presence of TiO2 nanoparticles enhanced healing activity and reduced the inflammatory reactions increasing DLC biocompatibility. However, a major concern with DLC coatings is their instability in an aqueous environment, which promotes delamination of the coatings [78].
Nanodiamonds possess a high surface-area-to-volume ratio together with good biocompatibility and bioactivity [79]. Additionally, diamonds were reported to have high wear resistance and low friction coefficient, which make them ideal for protective layers [80]. Nanodiamond coatings also show high surface roughness, hydrophobicity of the surface, high stability, superior electrochemical properties and biocompatibility [81][82]. On metallic substrates nanocrystalline diamond (NCD) coatings behaved as well-adhering and highly cohesive films [83]. It was found that the cell performance on NCD films depended on surface atoms or chemical groups [84]. For example, on micropatterned NCD films, human dental stem cells adhered and grew preferentially on O-terminated domains rather than on H-terminated areas [85]. The low boron (100 to 1000 ppm of B) doping of NCD films was also found to support cell proliferation and early osteogenic differentiation of MG63 cells because of the increased electroconductivity of the doped films [86]. A similar effect of enhanced attachment and spreading of MG63 cells was observed for composite apatite-nanodiamond coatings compared to pure SS and apatite coatings without nanodiamonds [87]. The authors explained the observed effect of the increased adsorption of fibronectin on the composite coatings. Simultaneously, Medina and co-authors observed that NCD coatings were able to establish a chemical bond with the cell wall or membrane of Gram-negative P. aeruginosa bacteria, thus hindering the bacterial adhesion and colonization of the surface [88].
Two-dimensional (2D) allotropes of carbon–graphene, a single atom thick layer of sp2 carbon and related graphene oxide (GO) and reduced graphene oxide (rGO) are innovative materials in the medical sector because of their unique biological properties. Graphene oxide is an oxygenated derivate of graphene with abundant functional groups on planes and edges allowing desirable dispersion behavior in aqueous media [89]. rGO consists of fewer oxygen-containing groups because of interactions with reducing agents [90]. The chemical properties of rGO resemble those of pristine graphene [91]. Many scientific works report the ability of rGO to promote osteogenic stem cell differentiation [92][93]. Graphene can be directly grown on metallic surfaces such as Ti6Al4V [94] and Mg [95] to improve bioactivity and corrosion resistance. It is also popular material with antibacterial, antifouling and hemo-compatible properties but the layered structure of graphene nanosheets limits its benefits and advantages [96]. For that reason, graphene nanoplatelets with improved biocompatibility and effectiveness for biomedical devices have been introduced [97]. In such a form, graphene is usually combined with natural or synthetic biopolymers to enhance the osteogenic potential and mechanical properties of the coating. For example, by using electrophoretic deposition, Suo et al. deposited GO/chitosan/HAp coatings on Ti that showed higher bonding strength to the substrate than HAp, GO/HAp and chitosan/HAp coatings and significantly enhanced cell–coating interactions in vitro and osseointegration in vivo [93][94][95][96][97][98]. Simultaneously, the fracture toughness of HAp rose by 200% by including only 1 wt% rGO [99]. Graphene-based materials have powerful antimicrobial properties and inhibit bacterial colonization. For example, Agarwalla and co-authors [100] tested graphene coatings on Ti against P. aerugimosa, E. faecalis, S. mutans and C. albicans and found that, when repeated twice, the film reduced biofilm formation due to the hydrophobicity of graphene. Similarly, functionalized GO nanocomposite with Ag NPs showed excellent antimicrobial activity against E. coli and S. aureus [101]. Despite these impressive properties, there is still a concern about the biodegradability of nanodiamonds and graphene in the organism. Additionally, in vitro studies with GO nanomaterials indicated the generation of ROS, DNA damage and mitochondrial disturbance [102].
2.5. Calcium Phosphates and Hydroxyapatite
Calcium phosphate ceramic coatings are extensively used to boost the biocompatibility of metal implants because of their superior adaptation to in vivo conditions. Bioactivity properties are varied according to the type of calcium phosphates. Both calcium phosphate types, HAp and tricalcium phosphate (TCP) have different crystallinity, stability, solubility, ion release and mechanical properties. The crystallinity is affected by the Ca/P ratio and a higher amount of Ca2+ or PO43- can trigger amorphous phase formation such as dicalcium phosphate dihydrate, CaHPO4.H2O and Ca3(PO4)2 [103]. In contrast to highly crystalline HAp, calcium phosphate-based coatings have high solubility in an aqueous medium that reduces coating stability and can cause implant loosening. Because of its similar properties to the inorganic composition of hard tissue such as bone and teeth [104], HAp (Ca10(PO4)6(OH)2, Ca/P = 1.67) is frequently employed as bioactive material. HAp has shown exceptional biocompatibility, osteo-inductivity, osteoconductive and bioactivity [105]. By releasing calcium and phosphate ions, HAp enhances bone regeneration and promotes mineralization [106]. By covering the metal biomaterial, HAp coating helps in maintaining stability fixed to the bone while minimizing the side effects of ion release in the bio-environment. The enhanced osteoconductive properties of HAp coatings can be attributed to the bone-like apatite chemistry of the coating (Na+, Mg2+, CO32−, Ca2+ and PO43−) and reduced degradation rate that allows a balance between ion release by the coating and ion absorption by the tissue during the bone formation [107].
However, due to their ceramic nature, highly porous or highly crystalline HAp coatings can show low mechanical properties (very brittle, with low flexibility), poor adhesion to the metal surfaces and low corrosion resistance, which makes them inappropriate for load-bearing applications. The difference in the thermal expansion coefficient of metallic alloys and HAp results in residual thermal stresses which can promote cracking or delamination of the coatings. The corrosion resistance also depends on the deposition method. Sankar et al. compared the corrosion behavior of HAp coatings obtained by EPD and pulse laser deposition (PLD) method and found that EPD coating had lower corrosion protection than PLD films due to the formation of denser and pore-free structures [108]. Dispersion strengthening by introducing a second phase to its microstructure such as other ceramics, carbon nanotubes or other compounds is deemed to overcome the poor mechanical properties of these coatings. For example, the addition of TiO2 to HAp (20–80 wt%) coating produced by High-Velocity Oxygen Fuel (HVOF) spraying on Ti6Al4V alloy delayed the HAp dissolution and increased the coating stability in Hank’s solution [109]. Similarly, by introducing TiO2 to fiber HAp by the EPD process it was discovered that the pores of the HAp coating produced from suspension with 50 and 75 wt% fiber HAp can be efficiently infiltrated and filled with TiO2 nanoparticles which increase the corrosion resistance of the coatings in SBF solution [110]. Evcin et al. [111] produced a series of HAp/Al2O3, HAp/B2O3 coatings on Ti6Al4V alloys by the HVOF method and found that increasing the amount of Al2O3 and B2O3 in HAp increased the adhesion strength and wettability.
The addition of specific trace elements to HAp like Zn, Mg, Cu, Si, Sr, Mn and F can also have a role in bone regeneration. For example, Zn was found to increase alkaline phosphatase activity and stimulate bone formation by osteoblasts [112]. For that reason, Zhou and co-authors produced Zn-doped HAp coating on ZK60 magnesium alloy by one-pot hydrothermal method with nano-whisker structure and showed that the film had a higher corrosion resistance compared to HAp coatings, promoting adhesion and differentiation of rat bone marrow mesenchymal stem cells at Zn concentration of 5% and obvious antibacterial activity [113]. Si-doped CaP was deposited on AZ31 magnesium alloy and the osteoblast cytocompatibility, evaluation showed that Si ion played a vital role in the nucleation and growth of apatite thus influencing the biological metabolism of osteoblast cells [114]. Pure Mg demonstrated an antimicrobial effect because of the increase in pH by degradation, while F is a basic element in bones. F can stimulate the differentiation of mesenchymal stem cells into osteoblasts, induce bone formation and promote the nucleation of HAp [115]. Comparing F-doped HAp, Mg-doped HAp and Mg/F-doped HAp coatings deposited on Ti substrate by pulsed laser deposition, it was found that Mg-F-HAp coating better promoted the transformation of apatite-like to HAp phase due to the synergistic effect of Mg and F. The porous 3D structure of the coatings enhanced the viability of rBMSCs, especially for Mg-F-HAp coatings, where a regulated biodegradable rate and good cellular proliferation were observed [116]. Sr ions were also found to increase bone-to-implant contact by osteoblastic cell proliferation, accelerate bone matrix synthesis and inhibit bone resorption [117]. Consequently, Sr-doped HAp coatings prepared on Mg-4Zn substrates by electrochemical deposition showed better corrosion resistance, improved protein adsorption and initial adhesion of mesenchymal stem cells, as well as improved osteogenic differentiation compared to HAp coatings [118]. Similarly, Ca-Sr-P coatings with dense crystalline structure deposited on biodegradable Mg alloy by chemical immersion method demonstrated improved corrosion resistance, higher bone formation and better osteointegration around the coating than the Mg alloy after 4 weeks of implantation in a rabbit model [119]. The biocompatible properties of HAp coatings were also enhanced by imparting antibacterial properties by incorporating silver. For example, in the presence of F in Ag-F-HAp coatings developed on Ti substrate by sol-gel method with a silver concentration of 0.3 wt%, the crystalline size and pores in the coating decreased whereas the antibacterial activity against E. coli bacteria increased with the amount of F [120].
The incorporation of polymers in HAp coating structures was also found to have a positive effect on the ceramic coating properties. For example, electro-phoretically deposited HAp-CaSiO3-chitosan composite coatings that were made porous by heat treatment at 700 °C in a controlled atmosphere indicated improved corrosion resistance and bioactivity in SBF compared to Ti substrate [121]. Similarly, biomimetically deposited Ce-doped HAp/collagen coatings on initially alkali-thermal oxidation pretreated Ti substrate showed good antibacterial activity against both E. coli and S. aureus, being more effective against E. coli [122]. By electrostatic interaction, the negatively charged surface absorbed positively charged collagen and negatively charge HAp that incorporated Ce ions in its lattice.
The general requirements for the properties of HAp coatings are listed in Table 1, although the relatively high thickness of HAp coatings, the ease of delamination of the film from the base metal and the possibility of the coating fracture occurrence can reduce the functional performance of the implant. The debris may cause inflammation in the host body.
Table 1. Requirements for HAp coatings for biomedical application [123].
| Property | Specification |
|---|---|
| Ca/P ratio | 1.67–1.76 |
| Heavy metals | <50 ppm |
| Density | 2.98 g/cm3 |
| Crystallinity | >62% |
| Thickness | 5–70 μm |
| Abrasion | Mass loss < 65 mg at 100 cycles |
| Tensile strength | >50.8 MPa |
| Shear strength | >22 MPa |
2.6. Bioactive Glasses
The bioactive ceramic materials include silica, calcium, phosphorous and sodium ions (glass composition CaO-SiO2-P2O5-Na2O) that are released when the bioactive glass interacts with cells and the bio-environment, leading to fast bone growth. The 45S5 bio-glass (45 wt% SiO2, 24.5 wt% CaO, 24.5 wt% Na2O, 6 wt% P2O5) has shown the most effective bioactive properties, namely class A bioactivity, allowing it to bond to soft and hard tissues [124]. Similar to many other ceramic materials, bioactive glasses can be produced as particles with micron and nano-size, or fibers, 3D scaffolds, mesoporous coatings, or monoliths [125]. Compared to other biomaterials, bioactive glasses can make possible better integration between the metal implant and the growing tissue because of their significant bioactive behavior [108]. For example, when comparing the in vivo efficacy of CaO-MgO-SiO2-based bioactive glass-ceramic on Ti6Al4V alloy (deposited by atmospheric plasma spraying) with HAp-coated samples implanted in New Zealand rabbits, the significant growth of new bone confirmed the superior biological activity of bio-glass coatings in treating load-bearing bone defects [126].
Although showing excellent bioactivity, because of their semicrystalline or amorphous structure, bio-glasses can exhibit poor mechanical strength, low tensile strength, fatigue resistance, elastic modulus and corrosion resistance especially as regards porous coatings. The porosity formation of coatings obtained by plasma spraying occurred due to the evaporation of volatile Na2O and P2O5 [127]. To overcome this disadvantage, composites with metal oxides such as ZrO2, TiO2, Al2O3 or graphene and its derivates were made [128]. These could improve the thermal, electrical and strength properties of bioactive glasses [129]. Additionally, except for excellent bioactivity, such bioactive glass composite coatings exhibit improved antibacterial activity, angiogenic properties and corrosion resistance [130]. For example, one-dimensional bioactive glass nanorods of 45S5 composition produced by sol-gel process and hybridized with reduced graphene oxide sheets (rGO), following different methods for developing composites such as constant stirring, sonification and simultaneous reduction in GO–bio-glass composite, showed better results in bioactivity, hemocompatibility, cell proliferation and antibacterial activity as compared to pure bioactive glass nanorods [131]. Similarly, electro-phoretically deposited bioactive glass-rGO hybrid thin films (2 μm thickness) deposited on TiO2 nanotubes with a diameter of around 100 nm were advantageous in antibacterial activity, hemocompatibility and MG-63 cell proliferation [132].
Bioglass composites with additions of metals, metal oxides and HAp also demonstrated promising bioactive properties. For example, laser process bio-glass coatings reinforced with Ti on Ti substrate with excellent coating interfacial characteristics, improved hardness, corrosion protection and in vitro wear resistance, also indicating better cell-material interaction than bare -Ti [133]. However, when comparing HAp-based HAp/Ag coatings with bio-glass-Ag bio-composite coatings on NiTi alloy, both deposited by sol-gel method, higher corrosion resistance and adhesion strength were found for HAp/Ag coatings [134]. Often, there are challenges in creating a good adhesion between the glass topcoat and the metal substrate due to low metal–amorphous ceramic interface bonding and the formation of cracks [135]. A solution to the problem can be the utilization of a polymeric matrix to create a nano-composite coating. These are characterized by low processing temperature and elimination of the sintering process if required. Among various polymers, chitosan, a natural polymer, is often used because of its biodegradability, biocompatibility, osteo=conductivity and antimicrobial properties [136]. Recently, Alaei and co-authors produced chitosan-bioactive glass nanocomposite coatings that provided significant corrosion protection to Mg alloy and controlled biological properties [137]. A new family of chitosan-based composite coatings incorporating HAp–bio-glass and different concentrations of Fe2O3 particles was electro-phoretically deposited on Ti13Nb13Zr alloy [138]. All Fe2O3-containing coating formulations showed favorable hemocompatibility, better surface properties, improved corrosion resistance and better cytocompatibility with MG63 cells as opposed to bare alloy and Hap–bio-glass coatings. Similarly, Mn-modified bio-glass/alginate nanostructure composites deposited on SS 316L by electrophoretic deposition demonstrated that the increased manganese in bio-glass had a positive effect on corrosion resistance in SBF and improved bioactivity [139].
3. Organic Coatings
Recently, the interest in applying polymer materials as coatings has increased substantially because of their easy fabrication, affordable price, low toxicity, corrosion resistance and eco-friendly nature. Polymers show low strength and elastic moduli as compared to ceramics and metals and are not used for load-bearing applications. They can be both non-biodegradable and biodegradable with complete degradation over time. However, except for low mechanical properties, another issue faced by polymers is their inadequate degradation rate and inflammatory reaction which limitations prevent them from being widely used as biomaterials for hard tissue coatings [140].
3.1. Synthetic Polymers
The most commonly used synthetic polymers for periosteum development are polylactic L-lactic-co-glycolic acid (PLGA), polyurethanes (PU), polyethylene glycol, (PEG), polycaprolactone (PCL) and poly L-lactic acid (PLLA), and polymethylmethacrylate (PMMA) [141]. Synthetic polymers are usually hydrophobic and possess no antibacterial activity. They can also deteriorate the adhesion of bone cells [142] restricting their widespread application in the medical sector. To improve the biological performance of polymer coatings, composite systems based on biocompatible polymers modified with various compounds or particles are often used [143]. For example, combining PU, which is usually used in medicine because of its favorable mechanical properties and high biocompatibility, with 0.25 wt% graphene (used as an antibacterial agent) and 2 wt% β-TCP (as a bioactive component) in dip coatings on Ti implants gave positive cell response in normal human osteoblast (NHOst) cells and effective antibacterial activity in contrast to the other examined composites with higher graphene content [142].
Similarly, PMMA is characterized by high thermal and chemical stability, biocompatibility and advanced mechanical properties [144]. Extensive studies on PMMA composites containing HAp, metal oxides and bio-glass showed that they are attractive for surface modification of biomedical implants because of their high biocompatibility, bioactivity and antimicrobial properties [145]. High molecular PMMA composite coatings with TiO2, Al2O3, HAp, bio-glass and Hap–bio-glass also provided enhanced corrosion protection compared to pure PMMA coatings [146].
Conductive polymer coatings have been also used as coatings on hard implants. Among them, poly-pyrrole (PPy) which has good biocompatibility, is frequently examined for biomedical applications [147]. However, once fabricated, pristine PPy has a rigid, brittle and insoluble nature [148]. To overcome this shortcoming, composites with various additives have been developed. For example, a composite coating of PPy with ZnO was developed by Guo et al. to protect the biodegradable Mg alloys from fast decomposition and to impart cyto-compatible and antibacterial properties [149]. Multifunctional composite coatings of PPy with pectin and 10 wt% gentamicin deposited on TiNbZr substrate demonstrated effective antibacterial performance, lower corrosion rate, controlled degradation because of the slow release of gentamicin and improved biocompatibility [150].
A synthetic polymer matrix was also used to augment HAp and bio-glass coatings and improve their mechanical strength. For example, nanostructured HAp was incorporated in polyetheretherketone (PEEK) to form PEEK–HAp composite coating which was deposited by EPD on 316 SS and heat treated at 375 °C to densify the coating and enhance the adhesion to the substrate [151]. In contrast to the as-deposited film where the HAp covered the PEEK and stimulated bioactivity, after heat treatment the HAp became encapsulated in PEEK and reduced bioactivity. Both adhesion strength and bioactivity were dependent on PEEK/HAp ratio. The increased amount of HAp caused improved bioactivity and reduced adhesion strength. Biodegradable PCL coatings on 316L SS containing 10 wt% gelatin (GE) and 3 wt% bio-glass showed drastically improved corrosion resistance and significant apatite formation as opposed to only PCL/GE coatings [152]. The bio-glass-containing composites also revealed increased MG63 cell viability compared to PCL/GE coatings while the results in an animal model (New Zealand white rabbits) demonstrated no inflammation and granulation, endothelial swelling, fibrotic tissue or other toxic effects.
More merits can be offered by biodegradable and resorbable polymers than non-degradable ones in terms of low levels of possible infections and implant rejection. However, the degradation of polyesters such as polylactic acid (PLA) and polyglycolic acid (PGA) and their co-polymers is known to create an acidic environment. The process can trigger host tissue response and foreign body reactions during degradation, as well as moderate cytotoxic reactions [153]. For that reason, cationic polymers with low toxicity such as poly(glycidyl methacrylate) or PGMA can be used. When Ti implants were functionalized with PGMA coupled with quaternized polyethyleneimine (bactericidal agent) and alendronate with high affinity to bone minerals, the obtained coating inhibited bacterial infections and promoted osseointegration in the late stages [154]. However, synthetic polymers do not have signaling sequences that are naturally present in biological polymers such as collagen, fibrinogen or fibronectin.
3.2. Polymeric Gels (Natural Polymers)
Various natural polymers such as chitosan, silk fibroin, collagen, etc., have been used in the production of bioactive coatings. Chitosan, representing the de-acetylated derivative of chitin, is considered to have osteoconductive properties. Coatings of chitosan/heparinized GO deposited by layer-by-layer technique on Mg alloy showed that substantial endothelial cell adhesion and proliferation were promoted [155]. Chitosan-Mg composite dip-coatings on Mg-Gd alloys also showed a higher amount of newly formed bone in rabbits [156]. Similarly, improved cell adhesion and proliferation of osteoblasts were observed on coated AZ31D alloy with bioactive carboxymethyl chitosan by immersion treatment [157]. As cationic macromolecule, chitosan can bind to the negatively charged cell membrane of bacteria and display, albeit weak, antibacterial properties. To impart a stronger bactericidal effect of the chitosan-containing coatings, films of chitosan and hyaluronic acid (HA) on rough Ti substrate were designed to release β-amino acid-based peptidomimetic antimicrobial peptide [158]. The layer-by-layer prepared coating showed a strong chemical cross-linking of chitosan with HA films which caused prolonged β-peptide retention that selectively prevented S. aureus biofilm formation for up to 24 days and remained its bactericidal properties after being challenged sequentially five times with S. aureus inoculum over 18 days. Simultaneously, no significant cytotoxicity on osteoblast precursor cell line derived from mouse (MC3T3-E1) compared to uncoated and film-coated controls without β-peptide was observed. Such a novel localized delivery approach that can maintain long-term antibacterial properties is promising for the development of coated medical devices prone to biofilm-associated infections. However, the adhesion and durability of chitosan coatings might raise some concerns [159].
Polydopamine, the final oxidation product of dopamine or other catecholamines, was found to form layers with an adjustable thickness (from a few to about 100 nm [160]) with good adhesion and high cell affinity [161]. This fact was confirmed by the study of Peng and co-authors who demonstrated an enhanced osteogenic differentiation on Zn-containing polydopamine films on AZ31 magnesium alloy together with improved osteogenesis and osteointegration in Sprague-Dawley rats after 8 weeks post-implantation [162]. A hybrid coating consisting of hydrothermally grown ZnO nanorods on Ti modified with polydopamine and covalently immobilized Arg-Gly-Aspartic acid-Cys (RGDC) peptide promoted cytocompatibility, new bone tissue formation and osteointegration between the implant and the new bone even in the presence of injected bacteria, or demonstrated simultaneous osseointegration and infection prevention [163]. Polymer coating produced via reversible addition-fragmentation chain transfer polymerization from glutamic acid and dopamine metha-crylamide was immobilized on Ti substrate by catechol pendants on the polymer chain [164]. Besides promoting mineral deposition, the coating was found to promote osteoblast adhesion and proliferation. Dopamine-silver loaded coating prepared at different pH values (4, 7 and 10) and different Ag+ concentrations (0.01 and 0.1 mg/mL) showed that the pH10/0.1 group displayed osteogenesis in the bacterial environment due to the great antibacterial properties and promoted mineralization activity [165]. To reduce the well-known cytotoxicity of Ag, Guo et al. prepared Poly-L-lysin (PLL)/sodium alginate/PLL self-assembled coating loaded with nano-silver on Ti that effectively inhibited the adhesion of bacteria [166]. At the same time, the PLL/SA/PLL coating induced mineralization in SBF and improved cytocompatibility and reduced cytotoxicity. Similarly, by double chelation of dopamine and chitosan, a hybrid coating consisting of HAp/dopamine/chitosan and nano-silver achieved a long-term release of silver and a continuous bacteriostatic effect [167]. This effect was accompanied by substantial osteogenic potential demonstrated in both in vitro and in vivo tests.
Silk fibroin consists of light and heavy chains and hydrophobically linked glycoprotein P25 that are all crosslinked to form a complex with antiparallel beta-sheets [168]. Because of the formation of β-sheets, silk fibroin scaffolds have better mechanical properties than collagen and chitosan but they are still insufficient compared with bone tissue [162][163][164][165][166][167][168][169]. In the form of hybrid coatings on WE43 magnesium alloy consisting of an inner layer of Mg(OH)2 produced by anodization, a middle layer of HAp formed by EDP and an outer silk fibroin layer deposited by spin coating, the surface modification was shown to improve not only corrosion resistance but also cell attachment, viability and proliferation [170]. To increase the osteogenic capacity and mechanical properties of silk fibroin, besides different organic and inorganic components, surface modifications by bioactive moieties that form hybrid films can be applied. For example, blends of silk fibroin/chitosan/rGO were fabricated by solvent casting method as films whose hydrophilicity, swelling and degradability decreased with increasing silk fibroin content, whereas the tensile strength increased [171]. The cell behavior of the G-292 cell demonstrated promoted osteogenic performance by increasing chitosan content while the increase in rGO reduced the porosity and tensile strength. The optimum result corresponded to SF:CS:rGO equal to 84:7:9 weight ratio.
3.3. ECM Proteins/Cell Coatings
Synthetic or natural multifunctional peptides can be used as coating materials on metallic grafts because introducing organic molecules that contain functional fragments can stimulate the interaction with proteins of the extracellular matrix (ECM). The organic part of ECM consists of collagen type I fibrils embedded in the amorphous substance of glycosaminoglycans and different bone proteins. Since ECM components actively participate in the regulation of cellular processes and interactions, the modification of the implant surface with components of the ECM is an attractive approach. Collagen is known to enhance tissue regeneration of bone, tendon, ligaments and vascular and connective tissue [172]. Collagen type I coating extracted from rat tail and deposited on Mg-Zr-Ca alloy implants by dip-coating showed accelerated protein bonding capacity resulting in better osteoblast activity and a tendency to form superior trabecular bone structure in male New Zealand white rabbits compared to the uncoated samples in a shorter period of implantation [173]. Another derivate of extracellular matrix proteins promoting cell adhesion as integrin ligand [174] is RGD. By using high-affinity inorganic peptides such as TiBP that contain a Ti-binding domain, RGD can be combined with antibacterial peptides to form durable and stable coatings with both bone-promoting and antibacterial properties [175]. Similarly, Zhang et al. used TiBP to connect antimicrobial sequence from human β-defebsin-3 and RGD in a coating that was found to significantly reduce the bacterial colonization onto the Ti surface and better-supported MC3T3-E1 cell growth compared with PBS-treated Ti samples [176].
Not only proteins but also vesicles and cells can be immobilized on the surface of metallic implants to form biogenic coatings. For example, using secreted extracellular vesicles (EVs) by mesenchymal stem cells, it was found that tissue repair and regeneration can be promoted since their membranes contained signaling molecules and, additionally, EVs can carry and transfer different cargos [177]. For that reason, Chen et al. immobilized adipose-derived stem cell extracellular vesicles with physisorbed fibronectin onto the Ti surface and observed enhanced osteoblast compatibility and osteo-induction activity [178]. Another approach included the immobilization of Lactobacillus casei on the surface of heat-treated Ti to form a probiotic coating [179]. The polysaccharides in the film promoted osteogenic differentiation through immunoregulation of macrophages that secreted osteogenic factors, while the surface showed 99.98% antibacterial effectiveness against S. aureus. However, the limitations of all these organic coatings are related to low mechanical strength, difficult sterilization and rapid degradation that can be overcome by designing composites with bio-ceramics or strong materials such as synthetic polymers or metals [2].
Some benefits and shortcomings of the main types of materials used for the construction of bioactive coatings are summarized in Table 2.
Table 2. Pros and cons of various bioactive coatings applied for hard tissue applications.
| Coating Material | Benefits | Shortcomings | Ref. |
|---|---|---|---|
| Metal nitrides (TiN, ZrN, TaN, SiN) and oxynitrides (TiNxOy, TiN/TiO2, TiON, ZrON) | Acceptable adhesion to metal substrates, high wear and corrosion resistance, biocompatibility | High hardness, premature coating failure, formation of flakes | [6][8][10][14][27][28] |
| Metal oxides (TiO2, Ta2O5) |
Good mechanical properties, bioactivity, antibacterial and catalytic activity, long-term stability under the photo- and chemical corrosion | Brittleness, low fracture toughness | [31][32][140] |
| Carbon-based (DLC, nanodiamonds, graphene, GO) |
Biocompatibility, stability, good mechanical properties, low coefficient of friction, superior electrochemical properties, antimicrobial properties | Single-layered DLC films suffer from high internal stress, delamination in an aqueous environment, low toughness, high sensitivity to ambient conditions; Nanodiamonds and graphene display hydrophobicity; GO nanomaterials indicated the generation of ROS, DNA damage and mitochondrial disturbance; |
[64][65][68][69][70][71][78][81][82][102] |
| Calcium phosphates and hydroxyapatite | Exceptional biocompatibility, osteo-inductivity, osteoconductive, bioactivity | Very brittle, high stiffness, low flexibility, high solubility in aqueous media | [2][103][105] |
| Bioactive glasses | Class A bioactivity, no toxic effects | Semicrystalline or amorphous structure, high brittleness, low fracture toughness, interfacial delamination, need for sintering to achieve adequate adhesion to a metallic substrate | [124][129][134][180] |
| Synthetic polymers (PMMA, PPy, PU, PCL, PGMA) |
Inexpensive, biodegradable (PCL, PGMA), chemically stable, good tensile properties and flexural rigidity | Low mechanical properties, hydrophobicity, insolubility (PMMA, PU, PPy), inflammatory reactions, inadequate degradation rate, deteriorate bone cell adhesion | [140][142][144][148][153][181] |
| Natural polymers (chitosan, silk fibroin, dopamine) | Good cell adhesion, high cell affinity | Low adhesion to metals, low durability, insufficient mechanical properties | [159][161][169] |
| ECM proteins/cell coatings | Enhance tissue regeneration of bone, tendon, ligaments and vascular and connective tissue, promoting cell adhesion, probiotic activity | Low mechanical strength, difficult sterilization, rapid degradation | [2][172][174][179] |
4. Active Moiety-Containing Coatings
Cellular active substances such as growth factors, chemokines, drugs, etc., and their application on the surface of orthopedic implants are intensively examined because these substances can effectively improve surface biocompatibility and promote osseointegration. Depending on the active moieties, these coatings can be divided into (a) drug-containing; (b) osteogenic-factor-containing; (c) immunomodulatory factors-containing and (d) antibacterial films.
4.1. Drug-Containing Coatings
Osteoinductive drugs that accelerate bone formation and enhance implant fixation are zoledronic acid and simvastatin. They can be both loaded onto the surface of the coating or within the film. For instance, zoledronate is a long-acting bisphosphonate that was found to cause cytoskeletal alterations in osteoclasts which decreased their activity and triggered apoptosis [182]. Bilayer coating of zoledronic acid associated with CaP on Mg-Sr alloy enhanced the proliferation, osteogenic differentiation and mineralization of pre-osteoblast MC3T3-E1 cells but also inhibited osteoclast differentiation and induced apoptosis which balanced the bone remodeling process [183]. Because zoledronic acid shows numerous side effects such as osteonecrosis of the jaw, gastrointestinal irritation and impairment of renal function during systemic use [184], the administration of the drug in a controlled manner by a coating seems to have potential effectiveness.
Simvastatin is a molecular analog of HMG-CoA (3-hydroxy-3-methyl-glytaryl-coenzyme A) that was found to promote mesenchymal cell differentiation into osteoblast, downregulating osteoblast apoptosis and upregulating BMP-2 [185]. Electrohoretically deposited coatings consisting of simvastatin/gelatin nanospheres/chitosan composite on WE43 magnesium alloy were found to enhance the degradation resistance of the alloy substrate and simultaneously promoted osteogenic activity [186]. However, studies with rats proved that a high dosage of simvastatin (0.5–2.2 mg per site) may induce inflammation or even impair bone healing [187][188]. Therefore, controlled delivery and drug release in an appropriate dose are of prime importance.
4.2. Coatings Containing Osteogenic Factors
Hormones, cytokines and growth factors such as bone morphogenetic proteins (BMPs), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) are known to play an essential role in bone repair and are also used to construct bioactive surfaces. BMPs are widely used cytokines to confer osteo-inductivity, but their burst release can decrease the osteogenic effect [189]. That is why porous coating are expected to be suitable for this purpose. Teng and co-authors prepared porous structured coating by 3D printing and MAO and grafted BMPs onto the surface [190]. The release of BMP-2 sustained for more than 35 days and stimulated osseointegration between the implant and bone. Kim et al. loaded BMP-2 at different concentrations in a MgO and Mg(OH)2 layer produced by micro-arc coating on AZ31B magnesium alloy and found substantial proliferation and differentiation of osteoblast cells when BMP-2 was released continuously in a concentration of 50 ng/mL after four weeks, thus stimulating stable bone growth and bone formation [191].
When incorporating BMP-9 in thermosensitive collagen and depositing it onto porous Ti, Zhu et al. found that the thermosensitive collagen degraded slowly at 37 °C thus ensuring temperature-controlled sustained release and enhanced osteogenesis around the implant [192]. Similarly, powder-processed dopamine/gelatin/rhBMP-2 coated β-TCP films on Mg-Zn alloy facilitated cell proliferation and significantly enhanced the osteogenic differentiation of Sprague-Dawley rat bone marrow-derived mesenchymal stem cells in vitro [193]. The in vivo results in New Zealand rabbits showed strong stimulation of new bone formation and matched composite degradation and bone regeneration rate. Another coating strategy accounted for the polydopamine-mediated assembly of HAp-coated alkaline treated nanoparticles and immersion of BMP-2 onto the surface of AZ31 magnesium alloy, where the coated sample showed substantial BMSCs adhesion and proliferation and stimulated osteo-inductivity and osseointegration in the New Zealand rabbit model [194]. However, some clinical and pre-clinical side effects of BMP-2 include inflammatory and wound complications, ectopic bone, osteoclast activation and osteolysis, radiculopathy and urogenital events [195]. Therefore, more research is required to understand the long-term results and bio-functionality of conjugated coatings with BMPs.
4.3. Immunomodulatory Factors Containing Coatings
Since the implant material is a foreign body, a series of immune responses can occur mainly from macrophage activation. Simultaneously, the formation of wear particles (debris) can also aggravate inflammatory reactions and dynamic imbalance between osteoblasts and osteoclasts which can trigger bone resorption and implant loosening [196]. Therefore, adapting the immunoreaction by incorporating immune factors to regulate immune response can have a beneficial effect on osseointegration. For example, Li et al. used spraying to deposit GO on Ti and loaded it with interleukin 4 (IL 4) [197]. During acute inflammation, IL-4 from the coating induced macrophage polarization to the Type 2 phenotype that is known to inhibit the development of inflammation. Besides weakened inflammatory response, the film also promoted osteogenesis.
Another strategy is based on immune regulation of the balance between osteoclasts and osteoblasts. Taking this into consideration, Lui et al. [198] conjugated osteogenic growth peptide (OGP) with N-acetylcysteine (NAC) and functionalized Ti substrates to examine bone metabolism balance in vitro. Their studies on RAW 264.7 cells demonstrated that the peptide-modified surfaces inhibited the cells from secreting inflammatory cytokines (IL-1β and TNF-α) and suppressed important transcription factors for osteo-clastogenesis. Simultaneously, the modified surfaces stimulated osteoblast spreading, proliferation and differentiation.
4.4. Antibacterial Coatings
The ideal antibacterial coating should kill pathogens during the primary contact, thus preventing biofilm formation. Since implants exist in the organism for a long time, the implant surface should also provide antibacterial properties against late infections. Except for adding different metal ions such as Ag+, Cu2+ and Zn2+ with broad antimicrobial effects as previously discussed, some non-metallic compounds and biomolecules are also prominent candidates for the production of bactericidal coatings. For instance, iodine is found to have wide antibacterial activity without developing drug resistance [199]. Kato and Shirai deposited anodic oxide film on Ti substrate and ionized iodine was electrodeposited within the pores to achieve iodine content of 0, 20, 50, 60 and 100%, where 100% corresponded to 13 μg/cm2 [200]. In vitro and in vivo experiments showed a temporal pattern of rapid initial release and subsequently slow attenuation of iodine with approximately 30% of initial iodine content remaining after 1 year. Implants with iodine contents of >20% demonstrated sufficient antibacterial activity to prevent implant-related infections even after 1 year of implantation. Similarly, chlorhexidine can be absorbed on the bacteria’s surface and destroy the membrane permeability [201]. Micro- and nano-porous Ti surface prepared by alkaline and heat treatment and covalently conjugated with amino-silane was used to graft chlorhexidine via glutaraldehyde [202]. The surface containing 1 mg/mL chlorhexidine indicated the best antibacterial results together with good osteoblast compatibility. Even in the presence of bacteria, the surface displayed great potential for osteoblast adhesion at the implant-bone interface. Another antibacterial agent—dimethyl-amino-dodecyl methacrylate (DMADDM)—introduced in HAp-modified surface via polydopamine was gradually released during the first 4 weeks after implantation and exhibited both inhibition of the pathogenic bacteria growth and osteogenic differentiation [203].
The use of natural antimicrobial peptides (AMPs) represents another strategy for imparting the bactericidal properties of coatings. For example, a hybrid antibiofilm coating of immobilized antimicrobial peptide (D-GL13K) by functional linkers—elastin-like recombinamers (ELRs—was applied on a titanium surface [204]. The presence of AMPs in the hybrid coatings provided strong antibiofilm activity against mono-species and microcosm biofilm models together with excellent cytocompatibility towards primary gingival fibroblasts. Another coating consisting of polydopamine, cationic antimicrobial peptide LL-37 and phospholipid (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine or POPC) was deposited on MAO-modified titanium substrates [205]. The multilayered coating was found to alleviate the burst release of LL-37 in the initial phase leading to antibacterial activity against S. aureus and E. coli. LL-37 killed bacteria by blocking the expression of bacterial-related genes and stimulating immune response under controlled release of POPC. However, certain dose-dependent cytotoxicity of antimicrobial agents remains a concern.
Another recent strategy is based on photothermal therapy with near-infrared (NIR) irradiation that allows deeper tissue penetration than ultraviolet (UV) light and high selectivity. Because of the local warming effect, the biofilm or bacterial integrity can be destroyed but due to low selectivity heating may cause adverse side effects to the surrounding tissue [206]. By forming a multifunctional coating on the Ti surface of Indocyanine green (ICG) and mesoporous polydopamine Yang et al. were able to convert NIR light energy into heat to kill bacteria, while simultaneously ICG produced ROS to destroy bacteria cell walls (Figure 4) [207]. Moreover, the mesoporous polydopamine was functionalized with RGD peptide to endow the coated Ti with good cytocompatibility. After biofilm eradication, the coating still displayed osteogenetic and osteointegration potential. This strategy has the potential for remotely controlled eradication in vivo avoiding invasive treatment without side effects on the surrounding tissue. Song and co-authors modified TiO2 nanorods on titanium surfaces with dopamine and ferrocene (PDA-Fc) to obtain efficient antibacterial surfaces [208]. Because of ROS generation by PDA-Fc redox reactions and local temperature increase by photothermal transformation of ferrocene, synergetic and more efficient bactericidal activity of the coating was observed.
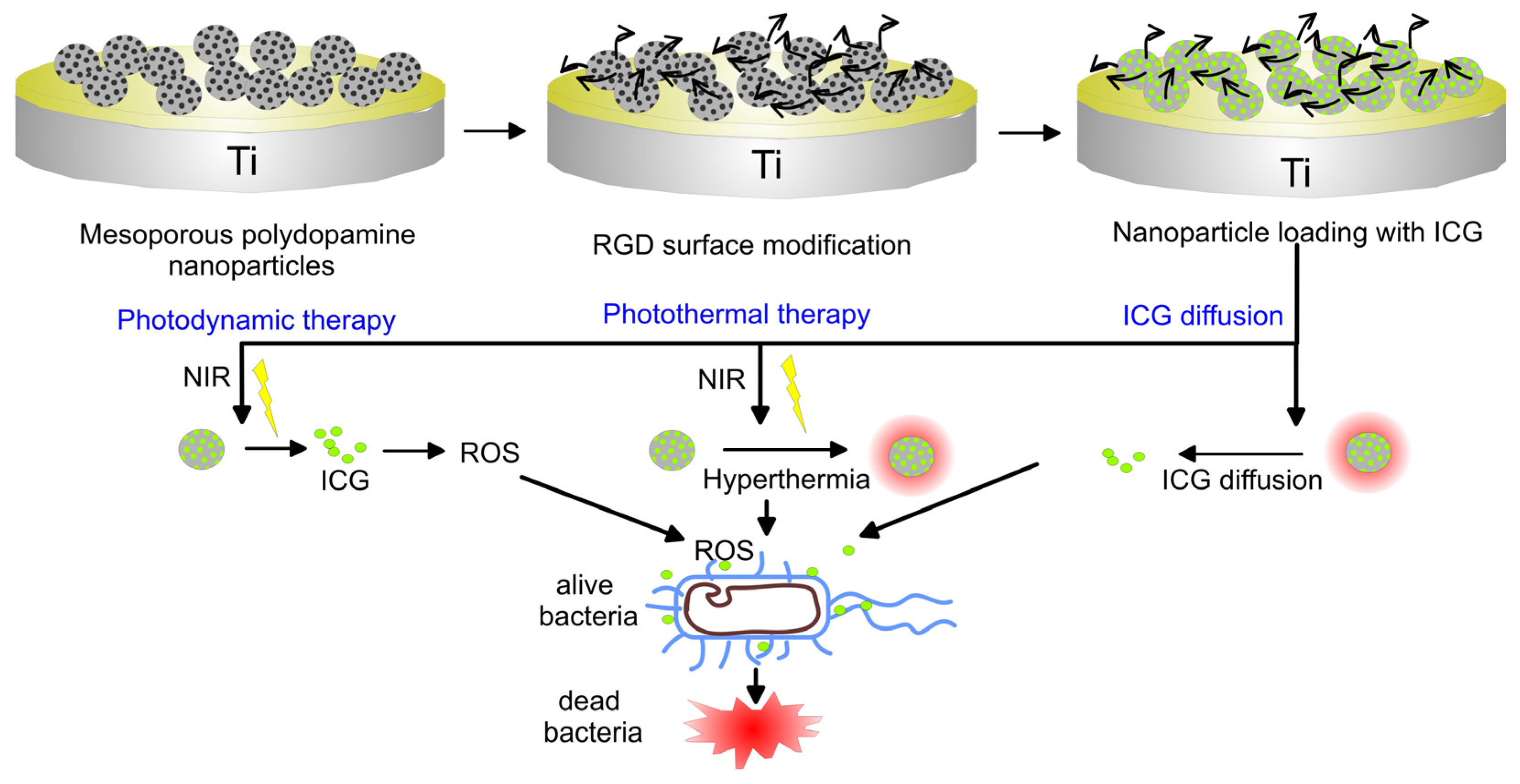
Figure 4. Schematic illustration revealing the coating construction and the elimination process of bacteria biofilm through NIR light triggering remote photodynamic and photothermal synergetic treatment.
To realize an intelligent release of antibacterial moiety when the microenvironment changes, Sang et al. coated silk protein coating with gentamicin on the Ti surface [209]. The coating exhibited a faster gentamicin release rate in an acidic environment (characteristic for the first weeks after implantation) than in alkaline media. Another mechanism relies on controlled release based on the heating effect during infection. This possibility is shown by Li et al. who produced thermosensitive chitosan-glycerin-hydroxypropyl methylcellulose hydrogel (CGHH) that layered the top of simvastatin-loaded TiO2 nanotubes [49] and was discussed in Section 2.2.
Some approaches rely on the modification of the microenvironment to achieve osseointegration and bactericidal properties. For example, zeolitic imidazolate frameworks-67 (ZIF-67) coating loaded with osteogenic growth peptide prepared on TiO2 nanotubes was found to rapidly dissolve under an acidic environment, as during inflammation [210]. The hydrolysis of ZIF-67 nanoparticles released Co ions and formed an alkaline microenvironment that effectively kills E. coli, S. aureus, S. mutans and methicillin-resistant S. aureus. The coating was able to suppress the inflammatory response and simultaneously improved the mesenchymal stromal cell (MSCs) differentiation under an inflammatory environment. In vivo results also pointed to a strong antimicrobial and anti-inflammatory properties of the coated implants at the early stages of implantation and enhancement of bone-implant osteointegration at the late stage.
Table 3 reveals the typical production techniques used for the deposition of nitride, oxide, oxynitride, carbon-based, calcium phosphates and hydroxyapatite, bioactive glass, synthetic polymer and natural polymer bioactive coating materials on metallic implants.
Table 3. Typical production techniques used for the deposition of various bioactive coating materials on metallic implants.
| Coating Material | Type | Deposition Technique | Ref. |
|---|---|---|---|
| Nitrides | TiN on Ti6Al4V | Ion implantation | [211] |
| TiN and “soft” Ti4N3−x on Ti6Al4V alloy | DC magnetron sputtering | [12] | |
| TiN coating on Ti20Nb13Zr (TNZ) alloy | Cathodic arc PVD | [13] | |
| Copper-doped TiN (TiCuN) deposited on 316L SS | Axial magnetic field enhanced arc ion plating | [17] | |
| Oxides | ZnO and ZnO/Ag on Mg-Ca alloy | Electroless deposition | [212] |
| TiO2 layers on Ti sheets and TiO2 nanotubes | Atomic layer deposition | [213] | |
| Tantalum oxide on Mg alloy | Reactive magnetron sputtering | [34] | |
| Doped-TiO2 coatings | MAO (PEO) | [37][39][40][42] | |
| TiO2 nanotubes on pure Ti | Anodization | [38] | |
| TiO2 layer embedding silver (Ag) and zinc (Zn) nanoparticles | 3D printing | [43] | |
| Ag-doped TiO2 coatings on Ti | Sol-gel | [44] | |
| Collagen/polydopamine/TiO2 coatings on Ti implants | MAO and hydrothermal treatment | [47] | |
| Oxynitrides | TiN/TiO2 coatings on Ti6Al4V | Cathodic arc deposition (CAD) and glow discharge oxidation | [56] |
| TiON and ZrON films on SS 316L | Magnetron sputtering | [60] | |
| Carbon-based | Hybrid DLC coatings incorporating TiO2 nanoparticles on AISI 316 | plasma-enhanced CVD | [77] |
| Nanocrystalline and microcrystalline diamonds on Mo substrates | Hot filament CVD | [88] | |
| Modified ultra-nanocrystalline diamond coatings on Ti | Microwaved plasma-assisted CVD and electron-beam evaporation | [84] | |
| DLC with Zr-containing interlayers | Unbalanced magnetron sputtering | [73] | |
| Mg-functionalized GO coating on Ti6Al4V | Electrophoretic deposition | [94] | |
| Graphene on pure Ti | Liquid-free technique | [100] | |
| Apatite-nanodiamond coating on SS | Electrodeposition | [87] | |
| GO loaded with interleukin 4 on Ti | Spraying | [197] | |
| Calcium phosphates and hydroxyapatite | Ultra-porous HAp on Ti alloy | Spray pyrolysis | [214] |
| HAp-graphene on Ti substrates | Cold spraying | [89] | |
| HAp on Ti substrates | Hot isostatic pressing | [215] | |
| CaP layer on Mg alloy | Hydrothermal crystallization | [216] | |
| Si-HAp coating on Mg-5Zn-0.3Ca alloy | Pulse electrodeposition | [217] | |
| HAp coatings on WE43 Mg alloy | EPD and pulse laser deposition (PLD) | [108] | |
| HAp with TiO2 on Ti6Al4V alloy | High-Velocity Oxygen Fuel (HVOF) spraying | [109] | |
| Ca-Sr-P coatings on Mg alloy | Chemical immersion method | [119] | |
| Ag-F-HAp coatings on Ti substrate | Sol-gel method | [120] | |
| Ce-doped HAp/collagen coatings on Ti | Biomimetic deposition | [122] | |
| Bioactive glasses | Zirconia-incorporated bioactive glass films on pure Ti substrates | Spray pyrolysis | [218] |
| Bioactive glass onto ultra-fine-grained Ti substrates | Laser cladding | [219] | |
| CaO-MgO-SiO2-based bioactive glass-ceramic on Ti6-Al-4V alloy | Atmospheric plasma spraying | [126] | |
| Bioactive glass nanorods of 45S5 integrated with rGO sheets | Sol-gel deposition | [131] | |
| Bioactive glass-rGO hybrid thin on TiO2 nanotubes | Electrophoretic deposition | [132] | |
| Bioglass coatings reinforced with Ti on Ti substrate | Laser Engineered Net Shaping (LENS) | [133] | |
| Synthetic polymers | Poly(sodium styrene sulfonate) on Ti | Wet-chemical method | [220] |
| PU coating with graphene and β-TCP on Ti implants | Dip coating | [142] | |
| PPy/ZnO composite coating on Mg alloy | Electrochemical synthesis | [149] | |
| PCL/gelatin coatings on 316 SS | Electrospinning | [153] | |
| PEEK-HAp composite coating on 316 SS | Electrophoretic deposition | [151] | |
| PGMA coupled with quaternized polyethyleneimine and alendronate on Ti implants | Immersion treatment | [154] | |
| Polymeric gels (natural polymers) | Methoxyl pectin and xanthan incorporating indomethacin coatings on SS | Sol-gel | [221] |
| Chitosan over porous oxide layer on Ti6Al4V | Electrodeposition | [222] | |
| Chitosan-Mg composite coating on Mg-Gd alloy | Dip coating | [156] | |
| Carboxymethyl chitosan coating on AZ31D alloy | Immersion treatment | [157] | |
| Chitosan/hyaluronic acid coating on Ti substrate | Layer-by-layer synthesis | [158] | |
| Silk fibroin layer on HAp/Mg(OH)2 coating on WE43 magnesium alloy | Spin coating | [170] | |
| Simvastatin/gelatin nanospheres/chitosan composite on WE43 magnesium alloy | Electrophoretic deposition | [186] |
References
- Ghasemi-Mobarakeh, L.; Kolahreez, D.; Ramakrishna, S.; Williams, D. Key terminology in biomaterials and biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 45–50.
- Sarian, M.N.; Iqbal, N.; Sotoudehbagha, P.; Razavi, M.; Ahmed, Q.U.; Sukotjo, C.; Hermawan, H. Potential bioactive coating system for high-performance absorbable magnesium bone implants. Bioact. Mater. 2022, 12, 42–63.
- Zhao, X.; Courtney, J.; Qian, H. 1—Introduction to bioactive materials in medicine. In Bioactive Materials in Medicine; Woodhead Publishing: Sawston, UK, 2011.
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110.
- Hübler, R.; Cozza, A.; Marcondes, T.L.; Souza, R.B.; Fiori, F.F. Wear and corrosion protection of 316-L femoral implants by deposition of thin films. Surf. Coat. Technol. 2001, 142–144, 1078–1083.
- Magnus, F.; Sveinsson, O.B.; Olafsson, S.; Gudmundsson, J.T. Current-voltage-time characteristics of the reactive Ar/N2 high power impulse magnetron sputtering discharge. J. Appl. Phys. 2011, 110, 083306.
- Kao, C.-T.; Ding, S.-J.; Chen, Y.-C.; Huang, T.-H. The anticorrosion ability of titanium nitride (TiN) plating on an orthodontic metal bracket and its biocompatibility. J. Biomed. Mater. Res. 2002, 63, 786–792.
- Gobbi, S.J.; Gobbi, V.J.; Reinke, G.; Rocha, Y. Orthopedic Implants: Coating with TiN. Biomed. J. Sci. Tech. Res. 2019, 16, 11740–11742.
- van Hove, R.P.; Sierevelt, I.N.; van Royen, B.J.; Nolte, P.A. Titanium-Nitride Coating of Orthopaedic Implants: A Review of the Literature. BioMed Res. Int. 2015, 2015, 1–9.
- Yuan, Z.; Han, Y.; Zang, S.; Chen, J.; He, G.; Chai, Y.; Yang, Z.; Fu, Q. Damage evolution behavior of TiN/Ti multilayer coatings under high-speed impact conditions. Surf. Coat. Technol. 2021, 426, 127807.
- Caiazzo, F.C.; Sisti, V.; Trasatti, S.P.; Trasatti, S. Electrochemical Characterization of Multilayer Cr/CrN-Based Coatings. Coatings 2014, 4, 508–526.
- Cui, W.; Qin, G.; Duan, J.; Wang, H. A graded nano-TiN coating on biomedical Ti alloy: Low friction coefficient, good bonding and biocompatibility. Mater. Sci. Eng. C Mater. 2017, 71, 520–528.
- Hussein, M.A.; Adesina, A.Y.; Kumar, A.M.; Sorour, A.A.; Ankah, N.; Al-Aqeeli, N. Mechanical, in-vitro corrosion, and tribological characteristics of TiN coating produced by cathodic arc physical vapor deposition on Ti20Nb13Zr alloy for biomedical applications. Thin Solid Films 2020, 709, 138183.
- Uddin, G.M.; Jawad, M.; Ghufran, M.; Saleem, M.W.; Raza, M.A.; Rehman, Z.U.; Arafat, S.M.; Irfan, M.; Waseem, B. Experimental investigation of tribo-mechanical and chemical properties of TiN PVD coating on titanium substrate for biomedical implants manufacturing. Int. J. Adv. Manuf. Technol. 2019, 102, 1391–1404.
- Carradò, A.; Schmerber, G.; Pelletier, H. Structural and mechanical investigations of magnetron sputtering TiO2/Ti/TiN multilayer films on Si(100) substrate. J. Coat. Technol. Res. 2010, 7, 821–829.
- Blunn Gw, M.R.M. Four Station Knee Simulator Wear Testing Comparing Titanium Niobium Nitride with Cobalt Chrome. J. Bioeng. Biomed. Sci. 2013, 3, 1000125.
- Liu, H.; Zhang, X.; Jin, S.; Zhao, Y.; Ren, L.; Yang, K. Effect of copper-doped titanium nitride coating on angiogenesis. Mater. Lett. 2020, 269, 127634.
- Kumar, D.D.; Kaliaraj, G.S. Multifunctional zirconium nitride/copper multilayer coatings on medical grade 316L SS and titanium substrates for biomedical applications. J. Mech. Behav. Biomed. Mater. 2018, 77, 106–115.
- Chen, Y.-I.; Lin, J.-H.; Chou, C.-C. Oxidation resistance and mechanical properties of Ta–Al–N coatings. Surf. Coat. Technol. 2016, 303, 41–47.
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681.
- Mendizabal, L.; Bayón, R.; G-Berasategui, E.; Barriga, J.; Gonzalez, J. Effect of N2 flow rate on the microstructure and electrochemical behavior of TaNx films deposited by modulated pulsed power magnetron sputtering. Thin Solid Films 2016, 610, 1–9.
- Zhang, Y.; Zheng, Y.; Li, Y.; Wang, L.; Bai, Y.; Zhao, Q.; Xiong, X.; Cheng, Y.; Tang, Z.; Deng, Y.; et al. Tantalum Nitride-Decorated Titanium with Enhanced Resistance to Microbiologically Induced Corrosion and Mechanical Property for Dental Application. PLoS ONE 2015, 10, e0130774.
- Jin, W.; Wang, G.; Peng, X.; Li, W.; Qasim, A.M.; Chu, P.K. Tantalum nitride films for corrosion protection of biomedical Mg-Y-RE alloy. J. Alloys Compd. 2018, 764, 947–958.
- Hsieh, J.H.; Chiu, C.H.; Li, C.; Wu, W.; Chang, S.Y. Development of anti-wear and anti-bacteria TaN-(Ag,Cu) thin films—A review. Surf. Coat. Technol. 2013, 233, 159–168.
- Schmidt, S.; Hänninen, T.; Wissting, J.; Hultman, L.; Goebbels, N.; Santana, A.; Tobler, M.; Högberg, H. SiNxcoatings deposited by reactive high power impulse magnetron sputtering: Process parameters influencing the residual coating stress. J. Appl. Phys. 2017, 121, 171904.
- Lal, S.; Caseley, E.A.; Hall, R.M.; Tipper, J.L. Biological Impact of Silicon Nitride for Orthopaedic Applications: Role of Particle Size, Surface Composition and Donor Variation. Sci. Rep. 2018, 8, 9109.
- Skjöldebrand, C.; Hulsart-Billström, G.; Engqvist, H.; Persson, C. Si–Fe–C–N Coatings for Biomedical Applications: A Combinatorial Approach. Materials 2020, 13, 2074.
- Correa Filho, L.; Schmidt, S.; López, A.; Cogrel, M.; Leifer, K.; Engqvist, H.; Högberg, H.; Persson, C. The Effect of Coating Density on Functional Properties of SiNx Coated Implants. Materials 2019, 12, 3370.
- Pettersson, M.; Bryant, M.; Schmidt, S.; Engqvist, H.; Hall, R.M.; Neville, A.; Persson, C. Dissolution behaviour of silicon nitride coatings for joint replacements. Mater. Sci. Eng. C Mater. 2016, 62, 497–505.
- Skjöldebrand, C.; Echeverri, E.; Hulsart-Billström, G.; Persson, C. Tailoring the dissolution rate and in vitro cell response of silicon nitride coatings through combinatorial sputtering with chromium and niobium. Biomater. Sci. 2022, 10, 3757–3769.
- Cao, Y.-Q.; Zi, T.-Q.; Zhao, X.-R.; Liu, C.; Ren, Q.; Fang, J.-B.; Li, W.-M.; Li, A.-D. Enhanced visible light photocatalytic activity of Fe2O3 modified TiO2 prepared by atomic layer deposition. Sci. Rep. 2020, 10, 1–10.
- He, X.; Zhang, G.; Zhang, H.; Hang, R.; Huang, X.; Yao, X.; Zhang, X. Cu and Si co-doped microporous TiO2 coating for osseointegration by the coordinated stimulus action. Appl. Surf. Sci. 2020, 503, 144072.
- Hua, Z.; Zhang, C.; Xu, Y.; Jia, H.; Chen, X.; Bai, Y.; Wei, B. Efficiently reduced heat rise in TiO2 coating Ti-based metallic implants using anodic oxidation method. Surf. Coat. Technol. 2019, 363, 75–79.
- Jin, W.; Wang, G.; Lin, Z.; Feng, H.; Li, W.; Peng, X.; Qasim, A.M.; Chu, P.K. Corrosion resistance and cytocompatibility of tantalum-surface-functionalized biomedical ZK60 Mg alloy. Corros. Sci. 2017, 114, 45–56.
- Huang, H.-L.; Chang, Y.-Y.; Chen, H.-J.; Chou, Y.-K.; Lai, C.-H.; Chen, M.Y.C. Antibacterial properties and cytocompatibility of tantalum oxide coatings with different silver content. J. Vac. Sci. Technol. A 2014, 32, 02B117.
- Xu, N.; Fu, J.; Zhao, L.; Chu, P.K.; Huo, K. Biofunctional Elements Incorporated Nano/Microstructured Coatings on Titanium Implants with Enhanced Osteogenic and Antibacterial Performance. Adv. Healthc. Mater. 2020, 9, 2000681.
- Zhao, Q.-M.; Sun, Y.-Y.; Wu, C.-S.; Yang, J.; Bao, G.-F.; Cui, Z.-M. Enhanced osteogenic activity and antibacterial ability of manganese–titanium dioxide microporous coating on titanium surfaces. Nanotoxicology 2020, 14, 289–309.
- Zhu, Y.; Zheng, T.; Wen, L.-M.; Li, R.; Zhang, Y.-B.; Bi, W.-J.; Feng, X.-J.; Qi, M.-C. Osteogenic capability of strontium and icariin-loaded TiO2 nanotube coatings in vitro and in osteoporotic rats. J. Biomater. Appl. 2021, 35, 1119–1131.
- Zhang, B.; Li, B.; Gao, S.; Li, Y.; Cao, R.; Cheng, J.; Li, R.; Wang, E.; Guo, Y.; Zhang, K.; et al. Y-doped TiO2 coating with superior bioactivity and antibacterial property prepared via plasma electrolytic oxidation. Mater. Des. 2020, 192, 108758.
- Li, K.; Liu, S.; Xue, Y.; Zhang, L.; Han, Y. A superparamagnetic Fe3O4–TiO2 composite coating on titanium by micro-arc oxidation for percutaneous implants. J. Mater. Chem. B 2019, 7, 5265–5276.
- Gao, Q.; Feng, T.; Huang, D.; Liu, P.; Lin, P.; Wu, Y.; Ye, Z.; Ji, J.; Li, P.; Huang, W. Antibacterial and hydroxyapatite-forming coating for biomedical implants based on polypeptide-functionalized titania nanospikes. Biomater. Sci. 2019, 8, 278–289.
- Nagay, B.E.; Dini, C.; Cordeiro, J.M.; Ricomini-Filho, A.P.; de Avila, E.D.; Rangel, E.C.; da Cruz, N.C.; Barao, V.A.R. Visible-Light-Induced Photocatalytic and Antibacterial Activity of TiO2 Codoped with Nitrogen and Bismuth: New Perspectives to Control Implant-Biofilm-Related Diseases. ACS Appl. Mater. Interfaces 2019, 11, 18186–18202.
- van Hengel, I.A.J.; Tierolf, M.W.A.M.; Valerio, V.P.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B 2020, 8, 1589–1602.
- Yetim, T. Corrosion Behavior of Ag-doped TiO2 Coatings on Commercially Pure Titanium in Simulated Body Fluid Solution. J. Bionic Eng. 2016, 13, 397–405.
- Ferraris, M.; Balagna, C.; Perero, S.; Miola, M.; Ferraris, S.; Baino, F.; Battiato, A.; Manfredotti, C.; Vittone, E.; Verné, E. Silver nanocluster/silica composite coatings obtained by sputtering for antibacterial applications. IOP Conf. Series: Mater. Sci. Eng. 2012, 40, 012037.
- Chai, M.; An, M.; Zhang, X. Construction of a TiO2/MoSe2/CHI coating on dental implants for combating Streptococcus mutans infection. Mater. Sci. Eng. C Mater. 2021, 129, 112416.
- Han, X.; Zhang, G.; Chai, M.; Zhang, X. Light-assisted therapy for biofilm infected micro-arc oxidation TiO2 coating on bone implants. Biomed. Mater. 2021, 16, 025018.
- Zhang, G.; Wu, Z.; Yang, Y.; Shi, J.; Lv, J.; Fang, Y.; Shen, Z.; Lv, Z.; Li, P.; Yao, X.; et al. A multifunctional antibacterial coating on bone implants for osteosarcoma therapy and enhanced osteointegration. Chem. Eng. J. 2022, 428, 131155.
- Li, B.; Zhang, L.; Wang, D.; Peng, F.; Zhao, X.; Liang, C.; Li, H.; Wang, H. Thermosensitive -hydrogel-coated titania nanotubes with controlled drug release and immunoregulatory characteristics for orthopedic applications. Mater. Sci. Eng. C Mater. 2021, 122, 111878.
- Su, K.; Tan, L.; Liu, X.; Cui, Z.; Zheng, Y.; Li, B.; Han, Y.; Li, Z.; Zhu, S.; Liang, Y.; et al. Rapid Photo-Sonotherapy for Clinical Treatment of Bacterial Infected Bone Implants by Creating Oxygen Deficiency Using Sulfur Doping. ACS Nano 2020, 14, 2077–2089.
- Leonova, L.A.; Boytsova, E.L.; Pustovalova, A.A. The study of titanium oxynitride coatings solubility deposited by reactive magnetron sputtering. IOP Conf. Series: Mater. Sci. Eng. 2016, 135, 012026.
- Pana, I.; Braic, V.; Dinu, M.; Mouele, E.S.M.; Parau, A.C.; Petrik, L.F.; Braic, M. In Vitro Corrosion of Titanium Nitride and Oxynitride-Based Biocompatible Coatings Deposited on Stainless Steel. Coatings 2020, 10, 710.
- Banakh, O.; Moussa, M.; Matthey, J.; Pontearso, A.; Cattani-Lorente, M.; Sanjines, R.; Fontana, P.; Wiskott, A.; Durual, S. Sputtered titanium oxynitride coatings for endosseous applications: Physical and chemical evaluation and first bioactivity assays. Appl. Surf. Sci. 2014, 317, 986–993.
- Rieder, P.; Scherrer, S.; Filieri, A.; Wiskott, H.W.A.; Durual, S. TiNOx coatings increase human primary osteoblasts proliferation independently of the substrate—A short report. Bio-Med. Mater. Eng. 2012, 22, 277–281.
- Durual, S.; Rieder, P.; Garavaglia, G.; Filieri, A.; Cattani-Lorente, M.; Scherrer, S.S.; Wiskott, H.W.A. TiNOx coatings on roughened titanium and CoCr alloy accelerate early osseointegration of dental implants in minipigs. Bone 2013, 52, 230–237.
- Nikolova, M.; Ormanova, M.; Nikolova, V.; Apostolova, M.D. Electrochemical, Tribological and Biocompatible Performance of Electron Beam Modified and Coated Ti6Al4V Alloy. Int. J. Mol. Sci. 2021, 22, 6369.
- Petrov, P.; Dechev, D.; Ivanov, N.; Hikov, T.; Valkov, S.; Nikolova, M.; Yankov, E.; Parshorov, S.; Bezdushnyi, R.; Andreeva, A. Study of the influence of electron beam treatment of Ti5Al4V substrate on the mechanical properties and surface topography of multilayer TiN/TiO2 coatings. Vacuum 2018, 154, 264–271.
- Valkov, S.; Parshorov, S.; Andreeva, A.; Bezdushnyi, R.; Nikolova, M.; Dechev, D.; Ivanov, N.; Petrov, P. Influence of Electron Beam Treatment of Co–Cr Alloy on the Growing Mechanism, Surface Topography, and Mechanical Properties of Deposited TiN/TiO2 Coatings. Coatings 2019, 9, 513.
- Nikolova, M.P.; Valkov, S.; Yankov, E.; Petrov, P. Composition, structure and adhesion of cathodic-arc and glow-discharge deposited TiN/TiO2 coatings: A case study. J. Phys. Conf. Ser. 2020, 1492, 012037.
- Kaliaraj, G.S.; Kumar, N. Oxynitrides decorated 316L SS for potential bioimplant application. Mater. Res. Express 2018, 5, 036403.
- Rtimi, S.; Baghriche, O.; Sanjines, R.; Pulgarin, C.; Bensimon, M.; Kiwi, J. TiON and TiON-Ag sputtered surfaces leading to bacterial inactivation under indoor actinic light. J. Photochem. Photobiol. A Chem. 2013, 256, 52–63.
- Chang, Y.-Y.; Huang, H.-L.; Chen, Y.-C.; Weng, J.-C.; Lai, C.-H. Characterization and antibacterial performance of ZrNO–Ag coatings. Surf. Coat. Technol. 2013, 231, 224–228.
- Castro, J.D.; Lima, M.J.; Carvalho, I.; Henriques, M.; Carvalho, S. Cu oxidation mechanism on Cu-Zr(O)N coatings: Role on functional properties. Appl. Surf. Sci. 2021, 555, 149704.
- Chen, X.; Zhang, W. Diamond nanostructures for drug delivery, bioimaging, and biosensing. Chem. Soc. Rev. 2017, 46, 734–760.
- Kulisch, W.; Popov, C. On the growth mechanisms of nanocrystalline diamond films. Phys. Status Solidi A 2006, 203, 203–219.
- Serino, G.; Gusmini, M.; Audenino, A.L.; Bergamasco, G.; Ieropoli, O.; Bignardi, C. Multiscale Characterization of Isotropic Pyrolytic Carbon Used for Mechanical Heart Valve Production. Processes 2021, 9, 338.
- Adkinson, J.M.; Chung, K.C. Advances in Small Joint Arthroplasty of the Hand. Plast. Reconstr. Surg. 2014, 134, 1260–1268.
- Miksovsky, J.; Voss, A.; Kozarova, R.; Kocourek, T.; Pisarik, P.; Ceccone, G.; Kulisch, W.; Jelinek, M.; Apostolova, M.D.; Reithmaier, J.P.; et al. Cell adhesion and growth on ultrananocrystalline diamond and diamond-like carbon films after different surface modifications. Appl. Surf. Sci. 2014, 297, 95–102.
- Granek, A.; Monika, M.; Ozimina, D. Diamond-like carbon films for use in medical implants. AIP Conf. Proc. 2018, 2017, 020006.
- Hajduga, M.B.; Bobiński, R. TiN, ZrN and DLC nanocoatings—A comparison of the effects on animals, in-vivo study. Mater. Sci. Eng. C Mater. 2019, 104, 109949.
- Hauert, R.; Thorwarth, K.; Thorwarth, G. An overview on diamond-like carbon coatings in medical applications. Surf. Coat. Technol. 2013, 233, 119–130.
- Chen, Y.; Nie, X.; Leyland, A.; Housden, J.; Matthews, A. Substrate and bonding layer effects on performance of DLC and TiN biomedical coatings in Hank’s solution under cyclic impact–sliding loads. Surf. Coat. Technol. 2013, 237, 219–229.
- Choudhury, D.; Lackner, J.; Fleming, R.A.; Goss, J.; Chen, J.; Zou, M. Diamond-like carbon coatings with zirconium-containing interlayers for orthopedic implants. J. Mech. Behav. Biomed. Mater. 2017, 68, 51–61.
- Yonezawa, K.; Kawaguchi, M.; Kaneuji, A.; Ichiseki, T.; Iinuma, Y.; Kawamura, K.; Shintani, K.; Oda, S.; Taki, M.; Kawahara, N. Evaluation of Antibacterial and Cytotoxic Properties of a Fluorinated Diamond-Like Carbon Coating for the Development of Antibacterial Medical Implants. Antibiotics 2020, 9, 495.
- Bociaga, D.; Sobczyk-Guzenda, A.; Komorowski, P.; Balcerzak, J.; Jastrzebski, K.; Przybyszewska, K.; Kaczmarek, A. Surface Characteristics and Biological Evaluation of Si-DLC Coatings Fabricated Using Magnetron Sputtering Method on Ti6Al7Nb Substrate. Nanomaterials 2019, 9, 812.
- Bociaga, D.; Sobczyk-Guzenda, A.; Szymanski, W.; Jedrzejczak, A.; Jastrzebska, A.; Olejnik, A.; Swiatek, L.; Jastrzebski, K. Diamond like carbon coatings doped by Si fabricated by a multi-target DC-RF magnetron sputtering method—Mechanical properties, chemical analysis and biological evaluation. Vacuum 2017, 143, 395–406.
- Wachesk, C.C.; Seabra, S.H.; Dos Santos, T.A.T.; Trava-Airoldi, V.J.; Lobo, A.O.; Marciano, F.R. In vivo biocompatibility of diamond-like carbon films containing TiO2 nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2021, 32, 1–10.
- Park, S.J.; Lee, K.-R.; Ahn, S.-H.; Kim, J.-G. Instability of diamond-like carbon (DLC) films during sliding in aqueous environment. Diam. Relat. Mater. 2008, 17, 247–251.
- Van Der Laan, K.J.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds for In Vivo Applications. Small 2018, 14, e1703838.
- Xue, Y.; Feng, X.; Roberts, S.C.; Chen, X. Diamond and carbon nanostructures for biomedical applications. Funct. Diam. 2021, 1, 221–242.
- Nistor, P.A.; May, P.W. Diamond thin films: Giving biomedical applications a new shine. J. R. Soc. Interface 2017, 14, 20170382.
- Ivanova, L.; Popov, C.; Kolev, I.; Shivachev, B.; Karadjov, J.; Tarassov, M.; Kulisch, W.; Reithmaier, J.P.; Apostolova, M.D. Nanocrystalline diamond containing hydrogels and coatings for acceleration of osteogenesis. Diam. Relat. Mater. 2011, 20, 165–169.
- Pareta, R.; Yang, L.; Kothari, A.; Sirinrath, S.; Xiao, X.; Sheldon, B.W.; Webster, T.J. Tailoring nanocrystalline diamond coated on titanium for osteoblast adhesion. J. Biomed. Mater. Res. Part A 2010, 95A, 129–136.
- Merker, D.; Handzhiyski, Y.; Merz, R.; Kopnarski, M.; Reithmaier, J.P.; Popov, C.; Apostolova, M.D. Influence of surface termination of ultrananocrystalline diamond films coated on titanium on response of human osteoblast cells: A proteome study. Mater. Sci. Eng. C Mater. 2021, 128, 112289.
- Duailibi, S.E.; Duailibi, M.T.; Ferreira, L.M.; Salmazi, K.I.L.C.; Salvadori, M.C.; Teixeira, F.D.S.; Pasquarelli, A.; Vacanti, J.P.; Yelick, P.C. Tooth Tissue Engineering: The Influence of Hydrophilic Surface on Nanocrystalline Diamond Films for Human Dental Stem Cells. Tissue Eng. Part A 2013, 19, 2537–2543.
- Grausova, L.; Kromka, A.; Burdikova, Z.; Eckhardt, A.; Rezek, B.; Vacik, J.; Haenen, K.; Lisa, V.; Bacakova, L. Enhanced Growth and Osteogenic Differentiation of Human Osteoblast-Like Cells on Boron-Doped Nanocrystalline Diamond Thin Films. PLoS ONE 2011, 6, e20943.
- Hristova, K.; Pecheva, E.; Pramatarova, L.; Altankov, G. Improved interaction of osteoblast-like cells with apatite–nanodiamond coatings depends on fibronectin. J. Mater. Sci. Mater. Med. 2011, 22, 1891–1900.
- Medina, O.; Nocua, J.; Mendoza, F.; Gómez-Moreno, R.; Ávalos, J.; Rodríguez, C.; Morell, G. Bactericide and bacterial anti-adhesive properties of the nanocrystalline diamond surface. Diam. Relat. Mater. 2012, 22, 77–81.
- Liu, Y.; Dang, Z.; Wang, Y.; Huang, J.; Li, H. Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: Inherited nanostructures and enhanced properties. Carbon 2014, 67, 250–259.
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO). Graphene 2017, 6, 1–18.
- Khan, Z.U.; Kausar, A.; Ullah, H.; Badshah, A.; Khan, W.U. A review of graphene oxide, graphene buckypaper, and polymer/graphene composites: Properties and fabrication techniques. J. Plast. Film Sheeting 2016, 32, 336–379.
- Yao, J.; Wang, H.; Chen, M.; Yang, M. Recent advances in graphene-based nanomaterials: Properties, toxicity and applications in chemistry, biology and medicine. Microchim. Acta 2019, 186, 395.
- Malavika, C.; Thomas, P.; Jose, A.J. Graphene: Promising nanoplatform for biomedical applications. In Materials for Biomedical Engineering: Bioactive Materials, Properties, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–322.
- Asgar, H.; Deen, K.M.; Rahman, Z.U.; Shah, U.H.; Raza, M.A.; Haider, W. Functionalized graphene oxide coating on Ti6Al4V alloy for improved biocompatibility and corrosion resistance. Mater. Sci. Eng. C Mater. 2019, 94, 920–928.
- Catt, K.; Li, H.; Hoang, V.; Beard, R.; Cui, X.T. Self-powered therapeutic release from conducting polymer/graphene oxide films on magnesium. Nanomedicine: Nanotechnology, Biol. Med. 2018, 14, 2495–2503.
- Selim, M.S.; El-Safty, S.A.; Abbas, M.A.; Shenashen, M.A. Facile design of graphene oxide-ZnO nanorod-based ternary nanocomposite as a superhydrophobic and corrosion-barrier coating. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 611, 125793.
- Rashad, M.; Pan, F.; Tang, A.; Asif, M. Effect of Graphene Nanoplatelets addition on mechanical properties of pure aluminum using a semi-powder method. Prog. Nat. Sci. Mater. Int. 2014, 24, 101–108.
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 635–645.
- Nair, M.; Nancy, D.; Krishnan, A.G.; Anjusree, G.S.; Vadukumpully, S.; Nair, S.V. Graphene oxide nanoflakes incorporated gelatin–hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology 2015, 26, 161001.
- Agarwalla, S.V.; Ellepola, K.; da Costa, M.C.F.; Fechine, G.J.M.; Morin, J.L.P.; Neto, A.H.C.; Seneviratne, C.J.; Rosa, V. Hydrophobicity of graphene as a driving force for inhibiting biofilm formation of pathogenic bacteria and fungi. Dent. Mater. 2019, 35, 403–413.
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973.
- Arbo, M.D.; Altknecht, L.F.; Cattani, S.; Braga, W.V.; Peruzzi, C.P.; Cestonaro, L.V.; Göethel, G.; Durán, N.; Garcia, S.C. In vitro cardiotoxicity evaluation of graphene oxide. Mutat. Res. Gen. Toxicol. Environ. Mutagen. 2019, 841, 8–13.
- Liu, G.; Tang, S.; Li, D.; Hu, J. Self-adjustment of calcium phosphate coating on micro-arc oxidized magnesium and its influence on the corrosion behaviour in simulated body fluids. Corros. Sci. 2014, 79, 206–214.
- Kaliaraj, G.S.; Siva, T.; Ramadoss, A. Surface functionalized bioceramics coated on metallic implants for biomedical and anticorrosion performance—A review. J. Mater. Chem. B 2021, 9, 9433–9460.
- Al Amin, M.; Rani, A.M.A.; Aliyu, A.A.A.; Bryant, M.G.; Danish, M.; Ahmad, A. Bio-ceramic coatings adhesion and roughness of biomaterials through PM-EDM: A comprehensive review. Mater. Manuf. Proc. 2020, 35, 1157–1180.
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4.
- Kim, S.R.; Lee, J.H.; Kim, Y.T.; Riu, D.H.; Jung, S.J.; Lee, Y.J.; Chung, S.C.; Kim, Y.H. Synthesis of Si,Mg substituted hydroxyapatites and their sintering behaviors. Biomaterials 2003, 24, 1389–1398.
- Sankar, M.; Suwas, S.; Balasubramanian, S.; Manivasagam, G. Comparison of electrochemical behavior of hydroxyapatite coated onto WE43 Mg alloy by electrophoretic and pulsed laser deposition. Surf. Coat. Technol. 2017, 309, 840–848.
- Melero, H.C.; Sakai, R.T.; Vignatti, C.A.; Benedetti, A.V.; Fernández, J.; Guilemany, J.M.; Suegama, P.H. Corrosion Resistance Evaluation of HVOF Produced Hydroxyapatite and TiO2-hydroxyapatite Coatings in Hanks’ Solution. Mater. Res. 2018, 21, e20170210.
- Farrokhi-Rad, M. Electrophoretic deposition of fiber hydroxyapatite/titania nanocomposite coatings. Ceram. Int. 2018, 44, 622–630.
- Evcin, A.; Buyukleblebici, B. Ti6Al4V Coating with B2O3 and Al2O3 containing Hydroxyapatite by HVOF Technique. Sci. Iran. 2019, 26, 1980–1989.
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 1–16.
- Zhou, W.; Hu, Z.; Wang, T.; Yang, G.; Xi, W.; Gan, Y.; Lu, W.; Hu, J. Enhanced corrosion resistance and bioactivity of Mg alloy modified by Zn-doped nanowhisker hydroxyapatite coatings. Colloids Surfaces B Biointerfaces 2019, 186, 110710.
- Qiu, X.; Wan, P.; Tan, L.; Fan, X.; Yang, K. Preliminary research on a novel bioactive silicon doped calcium phosphate coating on AZ31 magnesium alloy via electrodeposition. Mater. Sci. Eng. C Mater. 2013, 36, 65–76.
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316.
- Cao, J.; Lian, R.; Jiang, X. Magnesium and fluoride doped hydroxyapatite coatings grown by pulsed laser deposition for promoting titanium implant cytocompatibility. Appl. Surf. Sci. 2020, 515, 146069.
- Blake, G.M.; Fogelman, I. Long-Term Effect of Strontium Ranelate Treatment on BMD. J. Bone Miner. Res. 2005, 20, 1901–1904.
- Shi, W.; Zhao, D.P.; Shang, P.; Nie, H.M.; Zhang, Y.; Tang, J.C. Strontium-doped Hydroxyapatite Coatings Deposited on Mg–4Zn Alloy: Physical-chemical Properties and in vitro Cell Response. Rare Metal Mater. Eng. 2018, 47, 2371–2380.
- Makkar, P.; Kang, H.J.; Padalhin, A.R.; Faruq, O.; Lee, B. In-vitro and in-vivo evaluation of strontium doped calcium phosphate coatings on biodegradable magnesium alloy for bone applications. Appl. Surf. Sci. 2020, 510, 145333.
- Batebi, K.; Khazaei, B.A.; Afshar, A. Characterization of sol-gel derived silver/fluor-hydroxyapatite composite coatings on titanium substrate. Surf. Coat. Technol. 2018, 352, 522–528.
- Zhang, L. Surface Modification of Titanium by Hydroxyapatite/CaSiO3/Chitosan Porous Bioceramic Coating. Int. J. Electrochem. Sci. 2020, 15, 3616–3626.
- Ciobanu, G.; Harja, M. Cerium-doped hydroxyapatite/collagen coatings on titanium for bone implants. Ceram. Int. 2019, 45, 2852–2857.
- ISO 13779-2; Implants for Surgery—Hydroxyapatite—Part 2: Thermally Sprayed Coatings of Hydroxyapatite. International Organization for Standardization: Geneva, Switzerland, 2018.
- Fiume, E.; Barberi, J.; Verne, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24.
- Cannio, M.; Bellucci, D.; Roether, J.A.; Boccaccini, D.N.; Cannillo, V. Bioactive Glass Applications: A Literature Review of Human Clinical Trials. Materials 2021, 14, 5440.
- Zhang, M.; Pu, X.; Chen, X.; Yin, G. In-vivo performance of plasma-sprayed CaO–MgO–SiO2-based bioactive glass-ceramic coating on Ti6Al4V alloy for bone regeneration. Heliyon 2019, 5, e02824.
- Rojas, O.; Prudent, M.; López, M.E.; Vargas, F.; Ageorges, H. Influence of Atmospheric Plasma Spraying Parameters on Porosity Formation in Coatings Manufactured from 45S5 Bioglass® powder. J. Therm. Spray Technol. 2020, 29, 185–198.
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/metal oxide nanocomposites for bactericidal effect: A review. Chemosphere 2021, 272, 128607.
- Kaviyarasu, K.; Geetha, N.; Kanimozhi, K.; Magdalane, C.M.; Sivaranjani, S.; Ayeshamariam, A.; Kennedy, J.; Maaza, M. In vitro cytotoxicity effect and antibacterial performance of human lung epithelial cells A549 activity of Zinc oxide doped TiO2 nanocrystals: Investigation of bio-medical application by chemical method. Mater. Sci. Eng. C Mater. 2017, 74, 325–333.
- Crush, J.; Hussain, A.; Seah, K.T.M.; Khan, W.S. Bioactive Glass: Methods for Assessing Angiogenesis and Osteogenesis. Front. Cell Dev. Biol. 2021, 9, 643781.
- Raja, C.A.; Balakumar, S.; Bargavi, P.; Rajashree, P.; Anandkumar, B.; George, R.P.; Mudali, U.K. Decoration of 1-D nano bioactive glass on reduced graphene oxide sheets: Strategies and in vitro bioactivity studies. Mater. Sci. Eng. C Mater. 2018, 90, 85–94.
- Raja, C.A.; Balakumar, S.; Anandkumar, B.; George, R.P.; Mudali, U.K. Formation of bioactive nano hybrid thin films on anodized titanium via electrophoretic deposition intended for biomedical applications. Mater. Today Commun. 2020, 25, 101666.
- Chalisgaonkar, V.; Das, M.; Balla, V.K. Laser processing of Ti composite coatings reinforced with hydroxyapatite and bioglass. Addit. Manuf. 2018, 20, 134–143.
- Say, Y.; Aksakal, B. Enhanced corrosion properties of biological NiTi alloy by hydroxyapatite and bioglass based biocomposite coatings. J. Mater. Res. Technol. 2020, 9, 1742–1749.
- Heise, S.; Höhlinger, M.; Hernández, Y.T.; Palacio, J.J.P.; Ortiz, J.A.R.; Wagener, V.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition and characterization of chitosan/bioactive glass composite coatings on Mg alloy substrates. Electrochim. Acta 2017, 232, 456–464.
- Pozzo, L.D.Y.; da Conceição, T.F.; Spinelli, A.; Scharnagl, N.; Pires, A.T.N. The influence of the crosslinking degree on the corrosion protection properties of chitosan coatings in simulated body fluid. Prog. Org. Coat. 2019, 137, 105328.
- Alaei, M.; Atapour, M.; Labbaf, S. Electrophoretic deposition of chitosan-bioactive glass nanocomposite coatings on AZ91 Mg alloy for biomedical applications. Prog. Org. Coatings 2020, 147, 105803.
- Singh, S.; Singh, G.; Bala, N. Characterization, electrochemical behavior and in vitro hemocompatibility of hydroxyapatite-bioglass-iron oxide-chitosan composite coating by electrophoretic deposition. Surf. Coat. Technol. 2021, 405, 126564.
- Hosseini, S.; Farnoush, H. Characterization and in vitro bioactivity of electrophoretically deposited Mn-modified bioglass-alginate nanostructured composite coatings. Mater. Res. Express 2019, 6, 025404.
- Mosas, K.K.A.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent Advancements in Materials and Coatings for Biomedical Implants. Gels 2022, 8, 323.
- Li, M.; Tian, W.; Zhang, Y.; Song, H.; Yu, Y.; Chen, X.; Yong, N.; Li, X.; Yin, Y.; Fan, Q.; et al. Enhanced Silk Fibroin/Sericin Composite Film: Preparation, Mechanical Properties and Mineralization Activity. Polymers 2022, 14, 2466.
- Szaraniec, B.; Pielichowska, K.; Pac, E.; Menaszek, E. Multifunctional polymer coatings for titanium implants. Mater. Sci. Eng. C Mater. 2018, 93, 950–957.
- Kolev, I.; Ivanova, L.; Markova, L.; Dimitrova, A.; Popov, C.; Apostolova, M.D. Osteoporosis: A Look at the Future. In Osteoporosis; IntechOpen: London, UK, 2012; pp. 667–694.
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705.
- Floroian, L.; Samoila, C.; Badea, M.; Munteanu, D.; Ristoscu, C.; Sima, F.; Negut, I.; Chifiriuc, M.C.; Mihailescu, I.N. Stainless steel surface biofunctionalization with PMMA-bioglass coatings: Compositional, electrochemical corrosion studies and microbiological assay. J. Mater. Sci. Mater. Electron. 2015, 26, 1–14.
- Li, X.; Zhitomirsky, I. Deposition of poly(methyl methacrylate) and composites containing bioceramics and bioglass by dip coating using isopropanol-water co-solvent. Prog. Org. Coat. 2020, 148, 105883.
- Huang, Z.-B.; Yin, G.-F.; Liao, X.-M.; Gu, J.-W. Conducting polypyrrole in tissue engineering applications. Front. Mater. Sci. 2014, 8, 39–45.
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353.
- Guo, Y.; Jia, S.; Qiao, L.; Su, Y.; Gu, R.; Li, G.; Lian, J. A multifunctional polypyrrole/zinc oxide composite coating on biodegradable magnesium alloys for orthopedic implants. Colloids Surfaces B Biointerfaces 2020, 194, 111186.
- Kumar, A.M.; Adesina, A.Y.; Hussein, M.; Umoren, S.A.; Ramakrishna, S.; Saravanan, S. Preparation and characterization of Pectin/Polypyrrole based multifunctional coatings on TiNbZr alloy for orthopaedic applications. Carbohydr. Polym. 2020, 242, 116285.
- Baştan, F.E.; Rehman, M.A.U.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surfaces B Biointerfaces 2018, 169, 176–182.
- Shafiee, B.M.; Torkaman, R.; Mahmoudi, M.; Emadi, R.; Derakhshan, M.; Karamian, E.; Tavangarian, F. Surface Modification of 316L SS Implants by Applying Bioglass/Gelatin/Polycaprolactone Composite Coatings for Biomedical Applications. Coatings 2020, 10, 1220.
- Leng, Y.; Yang, F.; Wang, Q.; Li, Z.; Yuan, B.; Peng, C.; Ren, G.; Wang, Z.; Cui, Y.; Wang, Y.; et al. Material-based therapy for bone nonunion. Mater. Des. 2019, 183, 108161.
- Sun, Y.; Zhao, Y.-Q.; Zeng, Q.; Wu, Y.-W.; Hu, Y.; Duan, S.; Tang, Z.; Xu, F.-J. Dual-Functional Implants with Antibacterial and Osteointegration-Promoting Performances. ACS Appl. Mater. Interfaces 2019, 11, 36449–36457.
- Gao, F.; Hu, Y.; Gong, Z.; Liu, T.; Gong, T.; Liu, S.; Zhang, C.; Quan, L.; Kaveendran, B.; Pan, C. Fabrication of chitosan/heparinized graphene oxide multilayer coating to improve corrosion resistance and biocompatibility of magnesium alloys. Mater. Sci. Eng. C Mater. 2019, 104, 109947.
- Guo, Y.; Yu, Y.; Han, L.; Ma, S.; Zhao, J.; Chen, H.; Yang, Z.; Zhang, F.; Xia, Y.; Zhou, Y. Biocompatibility and osteogenic activity of guided bone regeneration membrane based on chitosan-coated magnesium alloy. Mater. Sci. Eng. C Mater. 2019, 100, 226–235.
- Huang, L.; Yi, J.; Gao, Q.; Wang, X.; Chen, Y.; Liu, P. Carboxymethyl chitosan functionalization of CPED-treated magnesium alloy via polydopamine as intermediate layer. Surf. Coat. Technol. 2014, 258, 664–671.
- López, A.D.L.R.; Lee, M.-R.; Ortiz, B.J.; Gastfriend, B.D.; Whitehead, R.; Lynn, D.M.; Palecek, S.P. Preventing, S. aureus biofilm formation on titanium surfaces by the release of antimicrobial β-peptides from polyelectrolyte multilayers. Acta Biomater. 2019, 93, 50–62.
- Kowalski, P.; Łosiewicz, B.; Goryczka, T. Deposition of Chitosan Layers on NiTi Shape Memory Alloy. Arch. Metall. Mater. 2015, 60, 171–176.
- Ball, V. Polydopamine Nanomaterials: Recent Advances in Synthesis Methods and Applications. Front. Bioeng. Biotechnol. 2018, 6, 109.
- Kaushik, N.; Nguyen, L.N.; Kim, J.H.; Choi, E.H.; Kaushik, N.K. Strategies for Using Polydopamine to Induce Biomineralization of Hydroxyapatite on Implant Materials for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 6544.
- Peng, F.; Cheng, S.; Zhang, R.; Li, M.; Zhou, J.; Wang, D.; Zhang, Y. Zn-contained mussel-inspired film on Mg alloy for inhibiting bacterial infection and promoting bone regeneration. Regen. Biomater. 2021, 8, rbaa044.
- Li, J.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Balancing Bacteria–Osteoblast Competition through Selective Physical Puncture and Biofunctionalization of ZnO/Polydopamine/Arginine-Glycine-Aspartic Acid-Cysteine Nanorods. ACS Nano 2017, 11, 11250–11263.
- Long, X.; Xu, H.; Zhang, D.; Li, J. Bioinspired by both mussel foot protein and bone sialoprotein: Universal adhesive coatings for the promotion of mineralization and osteogenic differentiation. Polym. Chem. 2020, 11, 4995–5004.
- Wang, X.; Xu, K.; Cui, W.; Yang, X.; Maitz, M.F.; Li, W.; Li, X.; Chen, J. Controlled synthesis of mussel-inspired Ag nanoparticle coatings with demonstrated in vitro and in vivo antibacterial properties. Mater. Des. 2021, 208, 109944.
- Guo, C.; Cui, W.; Wang, X.; Lu, X.; Zhang, L.; Li, X.; Li, W.; Zhang, W.; Chen, J. Poly-l-lysine/Sodium Alginate Coating Loading Nanosilver for Improving the Antibacterial Effect and Inducing Mineralization of Dental Implants. ACS Omega 2020, 5, 10562–10571.
- Xie, K.; Zhou, Z.; Guo, Y.; Wang, L.; Li, G.; Zhao, S.; Liu, X.; Li, J.; Jiang, W.; Wu, S.; et al. Long-Term Prevention of Bacterial Infection and Enhanced Osteoinductivity of a Hybrid Coating with Selective Silver Toxicity. Adv. Healthc. Mater. 2019, 8, e1801465.
- Zuluaga-Vélez, A.; Quintero-Martinez, A.; Orozco, L.M.; Sepúlveda-Arias, J.C. Silk fibroin nanocomposites as tissue engineering scaffolds—A systematic review. Biomed. Pharmacother. 2021, 141, 111924.
- Lin, K.; Wu, H.; Zhao, C.; Wang, X. Silk fibroin scaffolds: A promising candidate for bone regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1054379.
- Rahman, M.; Dutta, N.K.; Choudhury, N.R.; Rahman, M.; Dutta, N.K.; Choudhury, N.R. Microroughness induced biomimetic coating for biodegradation control of magnesium. Mater. Sci. Eng. C Mater. 2020, 121, 111811.
- Jabbari, F.; Hesaraki, S.; Houshmand, B.; Jabari, F. The physical, mechanical, and biological properties of silk fibroin/chitosan/reduced graphene oxide composite membranes for guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1779–1802.
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42.
- Mushahary, D.; Wen, C.; Kumar, J.M.; Lin, J.; Harishankar, N.; Hodgson, P.; Pande, G.; Li, Y. Collagen type-I leads to in vivo matrix mineralization and secondary stabilization of Mg–Zr–Ca alloy implants. Colloids Surfaces B Biointerfaces 2014, 122, 719–728.
- Atefyekta, S.; Pihl, M.; Lindsay, C.; Heilshorn, S.C.; Andersson, M. Antibiofilm elastin-like polypeptide coatings: Functionality, stability, and selectivity. Acta Biomater. 2019, 83, 245–256.
- Wisdom, E.C.; Zhou, Y.; Chen, C.C.; Tamerler, C.; Snead, M.L. Mitigation of Peri-implantitis by Rational Design of Bifunctional Peptides with Antimicrobial Properties. ACS Biomater. Sci. Eng. 2020, 6, 2682–2695.
- Zhang, X.; Geng, H.; Gong, L.; Zhang, Q.; Li, H.; Zhang, X.; Wang, Y.; Gao, P. Modification of the surface of titanium with multifunctional chimeric peptides to prevent biofilm formation via inhibition of initial colonizers. Int. J. Nanomed. 2018, 13, 5361–5375.
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional Coatings of Titanium Implants Toward Promoting Osseointegration and Preventing Infection: Recent Developments. Front. Bioeng. Biotechnol. 2021, 9, 783816.
- Chen, L.; Mou, S.; Hou, J.; Fang, H.; Zeng, Y.; Sun, J.; Wang, Z. Simple application of adipose-derived stem cell-derived extracellular vesicles coating enhances cytocompatibility and osteoinductivity of titanium implant. Regen. Biomater. 2021, 8, rbaa038.
- Tan, L.; Fu, J.; Feng, F.; Liu, X.; Cui, Z.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, Z.; et al. Engineered probiotics biofilm enhances osseointegration via immunoregulation and anti-infection. Sci. Adv. 2020, 6, eaba5723.
- Montazerian, M.; Hosseinzadeh, F.; Migneco, C.; Fook, M.V.L.; Baino, F. Bioceramic coatings on metallic implants: An overview. Ceram. Int. 2022, 48, 8987–9005.
- Filip, N.; Radu, I.; Veliceasa, B.; Filip, C.; Pertea, M.; Clim, A.; Pinzariu, A.C.; Drochioi, I.C.; Hilitanu, R.L.; Serban, I.L. Biomaterials in Orthopedic Devices: Current Issues and Future Perspectives. Coatings 2022, 12, 1544.
- Kates, S.L.; Ackert-Bicknell, C.L. How do bisphosphonates affect fracture healing? Injury 2016, 47, S65–S68.
- Li, M.; Wan, P.; Wang, W.; Yang, K.; Zhang, Y.; Han, Y. Regulation of osteogenesis and osteoclastogenesis by zoledronic acid loaded on biodegradable magnesium-strontium alloy. Sci. Rep. 2019, 9, 1–12.
- Lu, Y.; Li, M.; Li, L.; Wei, S.; Hu, X.; Wang, X.; Shan, G.; Zhang, Y.; Xia, H.; Yin, Q. High-activity chitosan/nano hydroxyapatite/zoledronic acid scaffolds for simultaneous tumor inhibition, bone repair and infection eradication. Mater. Sci. Eng. C Mater. 2018, 82, 225–233.
- Moshiri, A.; Sharifi, A.M.; Oryan, A. Role of Simvastatin on fracture healing and osteoporosis: A systematic review on in vivo investigations. Clin. Exp. Pharmacol. Physiol. 2016, 43, 659–684.
- Qi, H.; Heise, S.; Zhou, J.; Schuhladen, K.; Yang, Y.; Cui, N.; Dong, R.; Virtanen, S.; Chen, Q.; Boccaccini, A.R.; et al. Electrophoretic Deposition of Bioadaptive Drug Delivery Coatings on Magnesium Alloy for Bone Repair. ACS Appl. Mater. Interfaces 2019, 11, 8625–8634.
- Nyan, M.; Sato, D.; Kihara, H.; Machida, T.; Ohya, K.; Kasugai, S. Effects of the combination with α-tricalcium phosphate and simvastatin on bone regeneration. Clin. Oral Implants Res. 2009, 20, 280–287.
- Chissas, D.; Stamatopoulos, G.; Verettas, D.; Kazakos, K.; Papalois, A.; Agrogiannis, G.; Papaeliou, A.; Agapitos, E.; Balanika, A.; Papadopoulou, E.; et al. Can low doses of simvastatin enhance fracture healing? An experimental study in rabbits. Injury 2010, 41, 687–692.
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol. Lett. 2009, 31, 1825–1835.
- Teng, F.-Y.; Tai, I.-C.; Ho, M.-L.; Wang, J.-W.; Weng, L.W.; Wang, Y.J.; Wang, M.-W.; Tseng, C.-C. Controlled release of BMP-2 from titanium with electrodeposition modification enhancing critical size bone formation. Mater. Sci. Eng. C Mater. 2019, 105, 109879.
- Kim, S.-Y.; Kim, Y.-K.; Kim, K.-S.; Lee, K.-B.; Lee, M.-H. Enhancement of bone formation on LBL-coated Mg alloy depending on the different concentration of BMP-2. Colloids Surfaces B Biointerfaces 2019, 173, 437–446.
- Zhu, Z.; Li, X.; Li, Y.; Zhu, L.; Zhu, C.; Che, Z.; Huang, L. Three-dimensionally printed porous biomimetic composite for sustained release of recombinant human bone morphogenetic protein 9 to promote osteointegration. Mater. Des. 2021, 208, 109882.
- Liu, C.; Wang, J.; Gao, C.; Wang, Z.; Zhou, X.; Tang, M.; Yu, K.; Deng, Y. Enhanced osteoinductivity and corrosion resistance of dopamine/gelatin/rhBMP-2–coated β-TCP/Mg-Zn orthopedic implants: An in vitro and in vivo study. PLoS ONE 2020, 15, e0228247.
- Jiang, Y.; Wang, B.; Jia, Z.; Lu, X.; Fang, L.; Wang, K.; Ren, F. Polydopamine mediated assembly of hydroxyapatite nanoparticles and bone morphogenetic protein-2 on magnesium alloys for enhanced corrosion resistance and bone regeneration. J. Biomed. Mater. Res. Part A 2017, 105, 2750–2761.
- James, A.W.; Lachaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B: Rev. 2016, 22, 284–297.
- Zhou, Z.; Shi, Q.; Wang, J.; Chen, X.; Hao, Y.; Zhang, Y.; Wang, X. The unfavorable role of titanium particles released from dental implants. Nanotheranostics 2021, 5, 321–332.
- Li, Q.; Liang, B.; Wang, F.; Wang, Z. Delivery of Interleukin 4 from a Titanium Substrate Coated with Graphene Oxide for Enhanced Osseointegration by Regulating Macrophage Polarization. ACS Biomater. Sci. Eng. 2020, 6, 5215–5229.
- Liu, J.; Tang, Y.; Yang, W.; Tao, B.; He, Y.; Shen, X.; Shen, T.; Lin, C.; Cai, K. Functionalization of titanium substrate with multifunctional peptide OGP-NAC for the regulation of osteoimmunology. Biomater. Sci. 2019, 7, 1463–1476.
- Tsuchiya, H.; Shirai, T.; Nishida, H.; Murakami, H.; Kabata, T.; Yamamoto, N.; Watanabe, K.; Nakase, J. Innovative antimicrobial coating of titanium implants with iodine. J. Orthop. Sci. 2012, 17, 595–604.
- Kato, T.; Shirai, T.; Yamamoto, N.; Nishida, H.; Hayashi, K.; Takeuchi, A.; Miwa, S.; Ohtani, K.; Tsuchiya, H. Temporal Attenuation of Iodine Content and its Effect on the Antibacterial Activity of Iodine-Supported Titanium Implants. J. Microb. Biochem. Technol. 2016, 8, 285–289.
- Kim, T.-S.; Park, S.-H.; Park, D.; Lee, J.-H.; Kang, S. Surface immobilization of chlorhexidine on a reverse osmosis membrane for in-situ biofouling control. J. Membr. Sci. 2019, 576, 17–25.
- Wang, S.; Yang, Y.; Li, W.; Wu, Z.; Li, J.; Xu, K.; Zhang, W.; Zheng, X.; Chen, J. Study of the Relationship Between Chlorhexidine-Grafted Amount and Biological Performances of Micro/Nanoporous Titanium Surfaces. ACS Omega 2019, 4, 18370–18380.
- Zhou, W.; Peng, X.; Ma, Y.; Hu, Y.; Wu, Y.; Lan, F.; Weir, M.D.; Li, M.; Ren, B.; Oates, T.W.; et al. Two-staged time-dependent materials for the prevention of implant-related infections. Acta Biomater. 2020, 101, 128–140.
- Acosta, S.; Ibañez-Fonseca, A.; Aparicio, C.; Rodríguez-Cabello, J.C. Antibiofilm coatings based on protein-engineered polymers and antimicrobial peptides for preventing implant-associated infections. Biomater. Sci. 2020, 8, 2866–2877.
- He, Y.; Zhang, Y.; Shen, X.; Tao, B.; Liu, J.; Yuan, Z.; Cai, K. The fabrication and in vitro properties of antibacterial polydopamine-LL-37-POPC coatings on micro-arc oxidized titanium. Colloids Surfaces B Biointerfaces 2018, 170, 54–63.
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.; et al. Rapid Biofilm Eradication on Bone Implants Using Red Phosphorus and Near-Infrared Light. Adv. Mater. 2018, 30, e1801808.
- Yuan, Z.; Tao, B.; He, Y.; Mu, C.; Liu, G.; Zhang, J.; Liao, Q.; Liu, P.; Cai, K. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials 2019, 223, 119479.
- Song, J.; Liu, H.; Lei, M.; Tan, H.; Chen, Z.; Antoshin, A.; Payne, G.F.; Qu, X.; Liu, C. Redox-Channeling Polydopamine-Ferrocene (PDA-Fc) Coating to Confer Context-Dependent and Photothermal Antimicrobial Activities. ACS Appl. Mater. Interfaces 2020, 12, 8915–8928.
- Sang, S.; Guo, G.; Yu, J.; Zhang, X. Antibacterial application of gentamicin–silk protein coating with smart release function on titanium, polyethylene, and Al2O3 materials. Mater. Sci. Eng. C Mater. 2021, 124, 112069.
- Tao, B.; Lin, C.; He, Y.; Yuan, Z.; Chen, M.; Xu, K.; Li, K.; Guo, A.; Cai, K.; Chen, L. Osteoimmunomodulation mediating improved osteointegration by OGP-loaded cobalt-metal organic framework on titanium implants with antibacterial property. Chem. Eng. J. 2021, 423, 130176.
- Zhao, Y.; Wong, S.M.; Wong, H.M.; Wu, S.; Hu, T.; Yeung, K.W.K.; Chu, P.K. Effects of Carbon and Nitrogen Plasma Immersion Ion Implantation on In vitro and In vivo Biocompatibility of Titanium Alloy. ACS Appl. Mater. Interfaces 2013, 5, 1510–1516.
- González-Murguía, J.L.; Lucien, V.; Alpuche-Avilés, M. Electroless Deposits of ZnO and Hybrid ZnO/Ag Nanoparticles on Mg-Ca0.3 Alloy Surface: Multiscale Characterization. Coatings 2022, 12, 1109.
- Motola, M.; Capek, J.; Zazpe, R.; Bacova, J.; Hromadko, L.; Bruckova, L.; Ng, S.; Handl, J.; Spotz, Z.; Knotek, P.; et al. Thin TiO2 Coatings by ALD Enhance the Cell Growth on TiO2 Nanotubular and Flat Substrates. ACS Appl. Bio Mater. 2020, 3, 6447–6456.
- Nasiri, N.; Ceramidas, A.; Mukherjee, S.; Panneerselvan, A.; Nisbet, D.R.; Tricoli, A. Ultra-Porous Nanoparticle Networks: A Biomimetic Coating Morphology for Enhanced Cellular Response and Infiltration. Sci. Rep. 2016, 6, 24305.
- Onoki, T.; Hashida, T. New method for hydroxyapatite coating of titanium by the hydrothermal hot isostatic pressing technique. Surf. Coat. Technol. 2006, 200, 6801–6807.
- Ali, A.; Iqbal, F.; Ahmad, A.; Ikram, F.; Nawaz, A.; Chaudhry, A.A.; Siddiqi, S.A.; Rehman, I. Hydrothermal deposition of high strength calcium phosphate coatings on magnesium alloy for biomedical applications. Surf. Coat. Technol. 2019, 357, 716–727.
- Aboudzadeh, N.; Dehghanian, C.; Shokrgozar, M.A. Effect of electrodeposition parameters and substrate on morphology of Si-HA coating. Surf. Coat. Technol. 2019, 375, 341–351.
- Bargavi, P.; Chitra, S.; Durgalakshmi, D.; Radha, G.; Balakumar, S. Zirconia reinforced bio-active glass coating by spray pyrolysis: Structure, surface topography, in-vitro biological evaluation and antibacterial activities. Mater. Today Commun. 2020, 25, 101253.
- Bajda, S.; Liu, Y.; Tosi, R.; Cholewa-Kowalska, K.; Krzyzanowski, M.; Dziadek, M.; Kopyscianski, M.; Dymek, S.; Polyakov, A.V.; Semenova, I.P.; et al. Laser cladding of bioactive glass coating on pure titanium substrate with highly refined grain structure. J. Mech. Behav. Biomed. Mater. 2021, 119, 104519.
- Chouirfa, H.; Evans, M.D.M.; Castner, D.G.; Bean, P.; Mercier, D.; Galtayries, A.; Falentin-Daudré, C.; Migonney, V. Grafting of architecture controlled poly(styrene sodium sulfonate) onto titanium surfaces using bio-adhesive molecules: Surface characterization and biological properties. Biointerphases 2017, 12, 02C418.
- Horvat, G.; Xhanari, K.; Finšgar, M.; Gradišnik, L.; Maver, U.; Knez, Z.; Novak, Z. Novel ethanol-induced pectin–xanthan aerogel coatings for orthopedic applications. Carbohydr. Polym. 2017, 166, 365–376.
- Benea, L.; Celis, J.P. Reactivity of porous titanium oxide film and chitosan layer electrochemically formed on Ti-6Al-4V alloy in biological solution. Surf. Coat. Technol. 2018, 354, 145–152.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
04 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




